Abstract
SUMMARY: Patients with hemodynamic impairment ipsilateral to a carotid occlusion are at a high risk of subsequent stroke, and currently 2 surgical options have been studied: extracranial-to-intracranial bypass and direct thromboendarterectomy. We report the successful revascularization of 2 symptomatic chronically occluded carotid arteries with stenting and angioplasty.
It has been recently demonstrated that a high proportion of acute total carotid occlusions can be revascularized with stent placement and angioplasty.1 Although surgical revascularization with extracranial-to-intracranial bypass is being studied for patients deemed at a higher risk of stroke,2 little is known about the feasibility and safety of endovascular treatment (stent placement and angioplasty) of chronically occluded carotid arteries.3 We describe 2 patients with symptomatic chronic carotid occlusions with hemodynamic impairment who underwent successful revascularization of a chronic carotid occlusion with stent placement and angioplasty.
Case Reports
Patient 1
A 60-year-old man with a history of coronary artery disease, mitral regurgitation, prior left carotid endarterectomy, and known right internal carotid artery (ICA) occlusion by a carotid sonography and MR angiography (MRA) 6 months earlier presented with 2 episodes of left-sided hemiparesis. Brain MR imaging/MRA confirmed the right ICA occlusion as well as a linear pattern of increased signal intensity in the internal border zone region of the right hemisphere on diffusion-weighted imaging (DWI) (Fig 1A) and an old right parietal infarct on fluid-attenuated inversion revovery imaging (Fig 1B). A perfusion-weighted MR image (PWI) revealed a large area of delayed time to peak in the right hemisphere. This pattern of infarction in conjunction with the findings on PWI was suggestive of significant hemodynamic impairment of large areas in the right hemisphere. Additionally, a xenon CT scan with acetazolamide was performed that confirmed impaired cerebral vasoreactivity to the right hemisphere (Fig 1E). Given that the patient had severe coronary disease, it was thought that general anesthesia and a superficial temporal artery to middle cerebral artery bypass surgery would be high risk. Consequently, endovascular repair of the artery with stent placement and angioplasty was considered.
Fig 1.
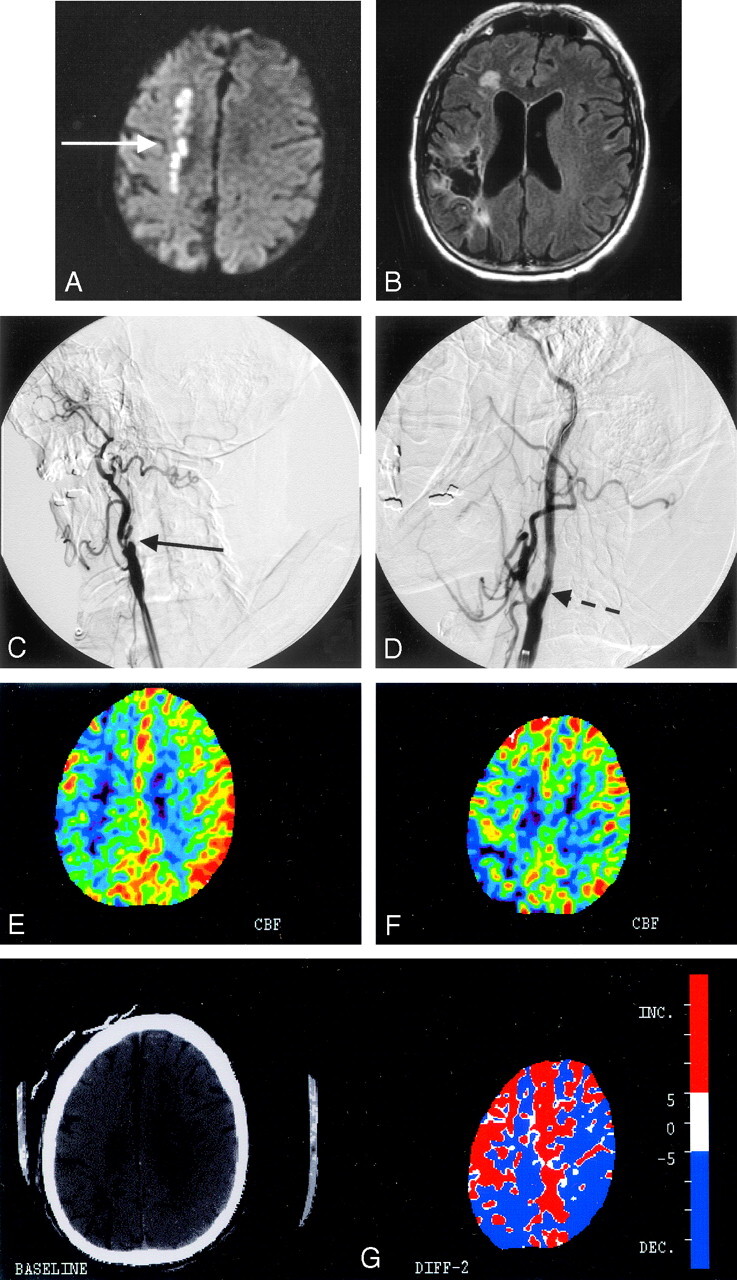
A, Diffusion-weighted MR imaging of patient 1 shows a linear pattern of increased signal intensity in the internal border zone region of the right hemisphere (white solid arrow). B, Fluid-attenuated inversion recovery image sequence shows an old right parietal infarct with encephalomalacia that was clinically silent before the procedure. C, Conventional angiography from the right common carotid artery confirms the presence of a total occlusion of the right internal carotid artery at the bifurcation (solid black arrow). D, Poststent placement and angioplasty of the occlusion show normal antegrade flow distal to the previously occluded segment (dashed black arrow). E, A xenon CT postacetazolamide performed before stent placement shows lack of cerebral vasoreactivity in the right hemisphere in contrast to the left side. F, Poststenting and angioplasty xenon CT reveal augmentation of flow to the right hemisphere except for the known area of infarct in the right parietal lobe. G, Color maps demonstrating the differences in flows before (from Fig E) and after (from Fig F) the procedure show the improved augmented blood flow to the right hemisphere as demarcated by the red color.
Procedure
The patient was premedicated with clopidogrel (300 mg) and aspirin (325 mg) the night before the procedure. The procedure was performed under IV conscious sedation. A 5F diagnostic catheter was placed in the right ICA to confirm the presence of the occlusion (Fig 1C). The catheter was then placed in the external carotid artery and a 0.035-inch Amplatz Superstiff guidewire (Boston Scientific, Natick, Mass) was used to exchange a 7F Shuttle-SL guide sheath (Cook, Bloomington, Ind) into the right common carotid artery. The patient received a bolus of 5000 U of heparin after placement of the Shuttle sheath, and the activated clotting time was maintained above 250 seconds throughout the procedure. Under road-mapping guidance, we attempted to cross the occlusion successively with microwires ranging from 0.014 to 0.018 inch. These attempts were unsuccessful and thus a 0.035-inch Amplatz Superstiff guidewire was used to create a channel across the occluded segment. This wire was then removed and an 0.018-inch V-18 ControlWire guidewire (Boston Scientific) was navigated through the channel into the right ICA at the C1 level. A microcatheter was placed over the microwire, and control runs performed through the microcatheter confirmed that the occlusion was a focal segment at the bifurcation and that the wire was in the true arterial lumen.
Unsuccessful attempts were made to cross the lesion with a distal protection device by using the Accunet filter (Guidant, St. Paul, Minn). A 0.014-inch microwire was exchanged through the microcatheter. Predilation was performed with a 2.5 × 12-mm Voyager balloon (Guidant) over the 0.014-inch microwire. Two 6 × 40-mm Acculink stents (Guidant) were then placed across the lesion that was thought to measure 60 mm in total length. Both stents were postdilated with a 4.5 × 20-mm Viatrac balloon (Guidant). Postprocedure control runs demonstrated good antegrade flow through the previously occluded cervical carotid artery (Fig 1D). Xenon CT imaging with acetazolamide was performed before and after stenting and confirmed a marked increase in the augmentation of blood flow to the right hemisphere (Fig 1E–G). The patient was placed on aspirin (325 mg a day) indefinitely and clopidogrel (75 mg a day) for 4 weeks. He was discharged to a rehabilitation facility posthospitalization, and at 30-day follow-up he continued to be free of symptoms.
Patient 2
A 70-year-old man with a history of coronary artery disease, left carotid endarterectomy, and severe chronic obstructive pulmonary disease was admitted with an episode of difficulties in speaking. He had an episode of left hemiparesis 4 weeks earlier and was found to have an occluded right ICA on carotid sonography and CT angiography. At the time of admission, he underwent MR imaging/MRA of the brain that revealed DWI hyperintensity in the internal borderzone area of the right hemisphere (Fig 2A) and a large PWI-DWI mismatch. A xenon CT performed with acetazolamide confirmed impaired vasoreactivity to the right hemisphere, as was also seen in patient 1 (Fig 2E). Given that the patient had 2 recent clinical events along with the DWI pattern of infarction highly suggestive of hemodynamic failure, the patient was taken to angiography to revascularize the occluded right ICA.
Fig 2.
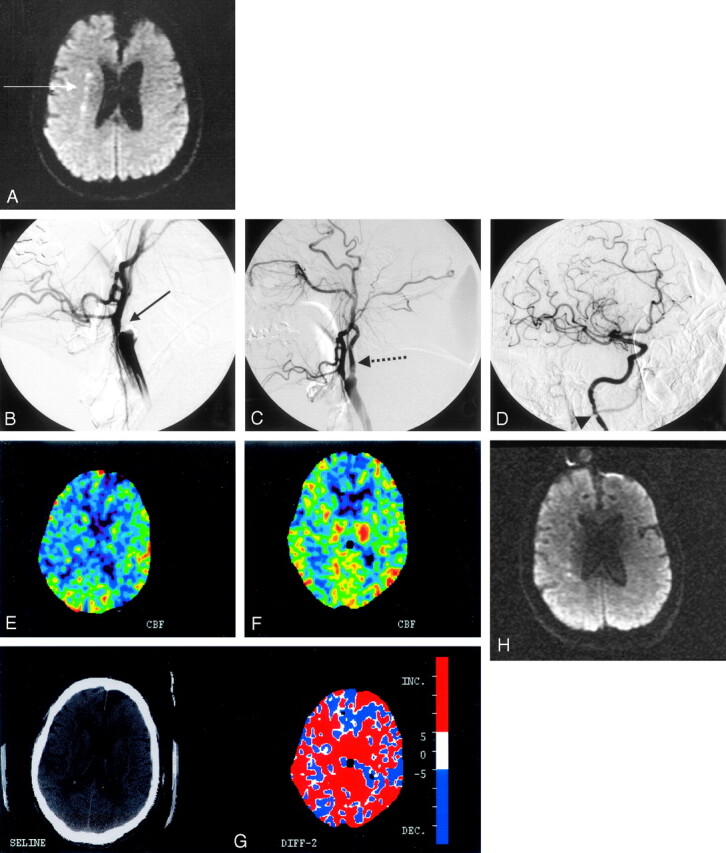
A, Diffusion-weighted MR imaging of patient 2 shows a linear pattern of increased signal intensity in the internal borderzone region of the right hemisphere (white arrow). B, Conventional angiography confirms the presence of a heavily calcified bulb with total occlusion of the internal carotid artery at the bifurcation (solid black arrow). C, Poststent placement and angioplasty show the antegrade flow across the occluded segment distally (dashed black arrow). D, The patient also had an atherosclerotic stenosis evident at the C1 level of the extracranial internal carotid artery that was not revascularized (black arrowhead). There is normal filling of the intracranial vessels. E, A xenon CT after preprocedure acetazolamide administration demonstrates poor cerebral vasoreactivity to the right hemisphere. F, A xenon CT after postprocedure acetazolamide administration demonstrates robust augmentation of blood flow to the right hemisphere. G, The color map demonstrates the difference in blood flows between Figures E and F, with red demarcating an increase in flow postprocedure. H, Diffusion-weighted MR imaging after stent placement revealed no additional acute infarcts as a result of the procedure.
Procedure
The patient was given clopidogrel (300 mg) and aspirin (325 mg) the night before the procedure. The procedure was performed with IV conscious sedation. A 7F Shuttle sheath was placed in the right common carotid artery as described in patient 1, and an activated clotting time of more than 250 seconds was maintained throughout the procedure. A control run through the Shuttle sheath confirmed the right ICA occlusion with a heavily calcified carotid bulb (Fig 2B). An 0.018 V-18 guidewire was navigated across the occluded segment, and a microcatheter was guided coaxially along the wire. Microcatheter injections confirmed the patency of the ICA distal to the occluded segment at the bifurcation. An attempt was made to navigate the Accunet distal protection device across the occluded segment with the 0.018-microwire in place, but the wire would not cross the lesion. Through the microcatheter a 0.014-inch microwire was exchanged and predilation was performed with a 3.0 × 12-mm Voyager balloon. After predilation, a 6–8 × 40-mm tapered Acculink stent was placed at the site of the occlusion and postdilated with a 5.5 × 20-mm Viatrac balloon. Poststent placement and angioplasty runs through the base catheter demonstrated the patency of the carotid artery throughout its course (Fig 1C), with antegrade filling of the middle cerebral and anterior cerebral arteries. There was atherosclerosis at the C2 level of the ICA that was not treated (Fig 1D).
The patient had no neurologic changes following the procedure. A postprocedure xenon CT with acetazolamide showed marked improvement in augmentation of blood flow to the right hemisphere (Fig 2F, -G) and no new infarcts on DWI MR imaging (Fig 2H). The patient was discharged on aspirin (325 mg a day) indefinitely and clopidogrel (75 mg a day) for 4 weeks. At 30-day follow-up the patient continued to be free of symptoms.
Discussion
This report suggests that endovascular revascularization can be performed in some patients with chronic carotid occlusions. The low number of patients presented herein does not permit conclusions with regard to rates of successful recanalization.
The literature for stent placement and angioplasty of a totally occluded subclavian artery suggests a successful revascularization rate of 50%–100% based on small case series.4,5 It remains to be determined if chronically occluded carotid arteries can be revascularized at similar rates. Although the diagnosis of ICA occlusion before our intervention was not made by the gold standard (ie, diagnostic angiography), it is very likely that the vessels were indeed occluded; that is, in both cases the prior diagnosis of occlusion was made by 2 concordant studies that are known to have a diagnostic accuracy higher than 90% for complete occlusion of the carotid artery.6 In both patients, filling of the occluded ICA was seen down to the base of the skull, which has been found to be a good prognostic sign for revascularization in prior reports of surgical revascularization.7 It is not clear whether endovascular revascularization of occluded carotid arteries should be attempted when no retrograde filling of the vessel down to the base of the skull is seen, but when this filling pattern is seen, successful revascularization in the appropriate patient may be considered.
Another critical factor to be considered when selecting patients for this therapy is the risk of subsequent stroke with medical therapy alone. The overall rate of subsequent stroke is 7% per year and 5.9% per year for ischemic stroke ipsilateral to the chronically occluded carotid artery.8 These risks persist despite standard medical therapy with antiplatelet agents or anticoagulants9 and are known to be higher (around 30% per year) in patients with hemodynamic impairment distal to the occluded artery.10,11 Clinically, the presence of limb-shaking transient ischemic attacks is specific to hemodynamic compromise,12 as is a linear pattern of white matter infarction with involvement of the centrum semiovale and corona radiata.13 Both of our patients had this linear pattern on DWI MR imaging as well as impaired cerebral vasoreactivity on xenon CT with acetazolamide.
The extracranial-to-intracranial bypass trial failed to show benefit with surgical revascularization when compared with medical therapy for symptomatic carotid artery occlusion.14 This may be explained partially by the fact that some of the higher risk patients with hemodynamic impairment were not enrolled in the trial. The ongoing Carotid Occlusion Surgery Study is comparing surgical revascularization with medical therapy on higher risk patients based on positron-emission tomography imaging criteria.2 We therefore think that endovascular approaches should only be considered in the patients exhibiting hemodynamic impairment, as these may represent the patients most likely to benefit from aggressive therapies.
Caution must be used when performing such procedures because distal embolization may occur in addition to dissection or perforation of the carotid artery when using stiffer wires to navigate across the occluded segment. Second, the restenosis rate is unknown after endovascular repair of an occluded carotid artery. Reports from stent placement and angioplasty of occluded subclavian arteries suggest that rates of restenosis may be low in larger high-flow vessels.15 Third, there is a risk of hyperperfusion syndrome in revascularization of high-grade carotid lesions; however, the risk is unknown with chronic occlusions. We maintained systolic blood pressures below 140 mmHg for 24 hours in both patients. Given that both patients had a significant coronary history along with radiographic imaging that supported hemodynamic failure, it was thought that revascularization would benefit these patients. Further studies are required to determine the safety and efficacy of performing endovascular repair of chronic occlusion of a carotid artery.
References
- 1.Jovin TG, Gupta R, Uchino K, et al. Emergent stenting of extracranial internal carotid artery occlusion in acute stroke has a high revascularization rate. Stroke 2005;36:2426–30 [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Powers WJ, Grubb RL, et al. Preview of a new trial of extracranial to intracranial arterial anastamosis: the carotid occlusion surgery study. Neurosurg Clin North Am 2001;12:613–24 [PubMed] [Google Scholar]
- 3.Terada T, Yamaga H, Tsumoto T, et al. Use of an embolic protection system during endovascular recanalization of a totally occluded cervical internal carotid artery at the chronic stage. Case report. J Neurosurg 2005;102:558–64 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez A, Gil-Peralta A, Gonzalez-Marcos JR, et al. Angioplasty and stenting for total symptomatic atherosclerotic occlusion of the subclavian or inominate arteries. Cerebrovasc Dis 2002;13:107–13 [DOI] [PubMed] [Google Scholar]
- 5.Henry M, Amor M, Henry I, et al. Percutaneous transluminal angioplasty of the subclavian arteries. J Endovasc Surg 1999;6:33–41 [DOI] [PubMed] [Google Scholar]
- 6.El-Saden SM, Grant EG, Hathout GM, et al. Imaging of the internal carotid artery: the dilemma of total versus near total occlusion. Radiology 2001;221:301–08 [DOI] [PubMed] [Google Scholar]
- 7.Greiner C, Wassmann H, Palkovic S, et al. Revascularization procedures in internal carotid artery pseudo-occlusion. Acta Neurochir (Wien)2004;146:237–43 [DOI] [PubMed] [Google Scholar]
- 8.Hankey GJ, Warlow CP. Prognosis of symptomatic carotid occlusion: an overview. Cerebrovasc Dis 1991;1:245–56 [Google Scholar]
- 9.Grubb RL Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–60 [DOI] [PubMed] [Google Scholar]
- 10.Yonas H, Smith HA, Durham SR, et al. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483–89 [DOI] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Grubb RL Jr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology 1999;53:251–59 [DOI] [PubMed] [Google Scholar]
- 12.Levine RL, Lagreze HL, Dobkin JA, et al. Cerebral vasocapacitance and TIAs. Neurology 1989;39:25–29 [DOI] [PubMed] [Google Scholar]
- 13.Waterston JA, Brown MM, Butler P, et al. Small deep cerebral infarcts associated with occlusive internal carotid artery disease. A hemodynamic phenomenon? Arch Neurol 1990;47:953–57 [DOI] [PubMed] [Google Scholar]
- 14.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med 1985;313:1191–200 [DOI] [PubMed] [Google Scholar]
- 15.Duber C, Klose KJ, Kopp H, et al. Percutaneous transluminal angioplasty for occlusion of the subclavian artery: short- and long-term results. Cardiovasc Intervent Radiol 1992;15:205–10 [DOI] [PubMed] [Google Scholar]


