Abstract
BACKGROUND AND PURPOSE: No prior report has comprehensively discussed the intravertebral vacuum cleft sign and the fluid sign on MR images of vertebral osteonecrosis. The purpose of this study was to investigate MR images of osteonecrotic vertebral bodies and adjacent intervertebral disks and vertebral bodies.
METHODS: We retrospectively reviewed MR images of patients with vertebral osteonecrosis. Affected vertebral bodies with osteonecrosis were defined as an avascular area (nonenhanced area on enhanced T1-weighted images) with collections of intravertebral fluid (hyperintense signal on T2-weighted images), air (signal void on all images), or both. The degree of vertebral collapse was classified as mild (>50%) or severe (<50%) preserved vertebral height. Changes in adjacent intervertebral disks or vertebral bodies 2 above and 2 below the affected vertebrae were compared.
RESULTS: We enrolled 112 patients (30 men, 82 women; 121 vertebral bodies) in our study. Intravertebral air alone was observed in 48 involved levels (39.7%), intravertebral fluid alone was found in 47 (38.8%), and both coexisted in 26 (21.5%). Degree of vertebral collapse in affected vertebral bodies significantly differed with presence of air or fluid (P < .05). Vertebral compression fractures adjacent to the affected vertebral bodies were more common in those with intravertebral air alone than in those with intravertebral fluid alone (P < .05).
CONCLUSION: Vertebral collapse was more advanced and adjacent vertebral compression fractures were more frequent in patients with intravertebral air than in those with intravertebral fluid.
Osteonecrosis often occurs over the femoral and humeral heads. Vertebral osteonecrosis is an uncommon disease and is typically considered the result of nonunion due to ischemia after compression fracture. It often leads to a collection of intravertebral air and creates a linear or semilunar radiolucent shadow on radiographs. This is the intravertebral vacuum cleft sign.1 The vacuum cleft is usually in the central area or adjacent to the endplate of a collapsed vertebral body. The intravertebral vacuum cleft sign is more easily seen on CT scans, on which it has a heterogeneous distribution and an irregular shape, than on radiographs.2 On MR images, the vacuum generally has low signal intensity with all sequences, and it appears as a signal intensity void on gradient-echo images because of magnetic susceptibility effect.
Collection of intravertebral fluid is also described in osteonecrosis of the vertebral body, and histologic analysis shows marrow edema and reactive fibrosis.3,4 The fluid collection appears as a well-circumscribed area of low signal intensity on T1-weighted MR images, with high signal intensity on T2-weighted images.2,4 This finding is called the fluid sign.5
To our knowledge, no prior report has comprehensively discussed the intravertebral vacuum cleft sign and the fluid sign on MR images of vertebral osteonecrosis. Therefore, the purpose of our study was to retrospectively investigate the content of the avascular or necrotic area of osteonecrotic vertebral bodies and adjacent vertebrae or intervertebral disks on MR imaging.
Methods
Study Population
From February 1994 to November 2004, we collected the medical and radiologic reports and image studies of patients with vertebral osteonecrosis. This diagnosis was first suspected by MR image findings and was established by serial image follow-up and/or tissue proof from bone biopsy. All patients gave written consent for the MR imaging examination that was performed on the basis of accepted clinical indications. Among these patients, those with a history of underlying malignancy, chronic steroid administration, previous spinal surgery or vertebroplasty, or severe trauma (eg, due to a motor vehicle crash or a fall from a standing height) were excluded. Those with proved pathologic compression fractures, malignancy, or infection were also excluded.
We included patients with simple vertebral compression fracture with unique findings of osteonecrosis on MR images. Affected vertebral bodies with osteonecrosis were defined as an avascular area (nonenhanced area on enhanced T1-weighted images) that contained collections of either or both intravertebral fluid or air. The intravertebral air revealed signal intensity void on both T1- and T2-weighted images, and intravertebral fluid revealed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The posterior third of the affected vertebral body or remaining portion around the avascular cavity had higher intensity on short tau inversion recovery images and stronger enhancement on enhanced T1-weighted images when compared with the nonaffected levels. The final diagnosis of benign nature was confirmed by means of serial follow-up for at least 12 months. In 15 patients, CT-guided transpedicle bone biopsies were requested by the clinicians to exclude the possibility of underlying malignancy or infectious processes. Finally, we enrolled 112 patients (30 men, 82 women; mean age, 73.5 years; age range, 50–90 years).
Image Evaluation
All MR images were obtained with 1.5T MR units (Signa Advantage, GE Medical Systems, Milwaukee, Wis; Vision Plus, Magnetom or Sonata, Siemens, Erlangen, Germany). The imaging protocol included several pulse sequences: nonenhanced sagittal T1-weighted fast or turbo spin-echo (repetition time (ms)/echo time (ms) of 500–630/10–15, section thickness of 4 mm, 1 signal intensity or 2 signals acquired, FOV of 256 × 300 or 300 × 300, and a matrix size of 288 × 512 or 256 × 512); sagittal T2-weighted fast or turbo spin-echo (3300–4400/90–130, an echo-train length of 8, section thickness of 4 mm, 1 signal intensity acquired, FOV and matrix size as in sagittal T1-weighted fast or turbo spin-echo); transverse T2-weighted fast or turbo spin-echo (3300–4400/90–130, an echo-train length of 3, section thickness of 5 mm, FOV of 175 × 200 or 200 × 200, a matrix size of 184 × 256 or 256 × 512); and contrast-enhanced T1-weighted fast or turbo spin-echo in the sagittal plane with fat saturation (620–750/10–15, other parameters as in nonenhanced sagittal T1-weighted fast or turbo spin-echo) and in the axial plane without fat saturation (600–720/10–15, section thickness of 5 mm, FOV of 175 × 200 or 200 × 200, a matrix size of 184 × 256 or 256 × 512). Enhanced images were obtained after the administration of gadopentetate dimeglumine at a dose of 0.1 mmol/kg. Radiographs obtained within 2 weeks before or after MR imaging were also collected for evaluation.
Three musculoskeletal radiologists (T.T.-F.S., C.Y.H., C.-W.Y.) retrospectively and independently reviewed all MR images and radiographs, and any inconsistencies were resolved by consensus. The content of the affected vertebral bodies was evaluated for the intravertebral air, intravertebral fluid, or both. Intravertebral air was defined as an area of hypointensity or signal intensity void on T1- and T2-weighted images and as a lack of enhancement on enhanced T1-weighted images. Intravertebral fluid was an area of hypointensity on T1-weighted images and hyperintensity on T2-weighted images, with no enhancement on enhanced T1-weighted images. For comparison, patients were categorized as having only intravertebral air (air only), only intravertebral fluid (fluid only), or both intravertebral air and intravertebral fluid (fluid with air).
The degree of collapse of the affected vertebral bodies was assessed according to the preservation of vertebral height. The height of the affected vertebral body was compared with that of noncollapsed vertebrae in the same spinal segment (thoracic or lumbar). Vertebrae with more than 50% preserved height were mildly collapsed, and those with less than 50% preserved height were severely collapsed.
The content of the 2 disks above and the 2 disks below the affected vertebrae were evaluated for intradiskal air. This air appeared as a signal intensity voids on both T1- and T2-weighted images.
Vertebral collapse was evaluated in the 2 levels above and the 2 levels below the affected vertebrae. Collapse was defined as a loss of vertebral height of more than 20% the height of noncollapsed vertebrae in the same spinal segment (thoracic or lumbar).
Statistical Analysis
We analyzed the data in cross-classification tables and used the χ2 or Fisher exact test to determine any associations. Statistical significance was assumed at a P value less than 0.05.
Results
Our study included 121 affected vertebral bodies in 112 patients. Table 1 shows the patients’ sex and age distributions. In 8 of the 112 patients, vertebral osteonecrosis was diagnosed in more than one vertebral body. Among these patients, 7 patients had 2 involved vertebrae and 1 patient had 3 involved vertebrae.
Table 1:
Distribution of sex and age of patients with vertebral osteonecrosis
| Age (y) | Men | Women | Total |
|---|---|---|---|
| 51–60 | 1 | 5 | 6 |
| 61–70 | 8 | 28 | 36 |
| 71–80 | 13 | 36 | 49 |
| 81–90 | 8 | 13 | 21 |
| Total | 30 | 82 | 112 |
Intravertebral Air and Fluid
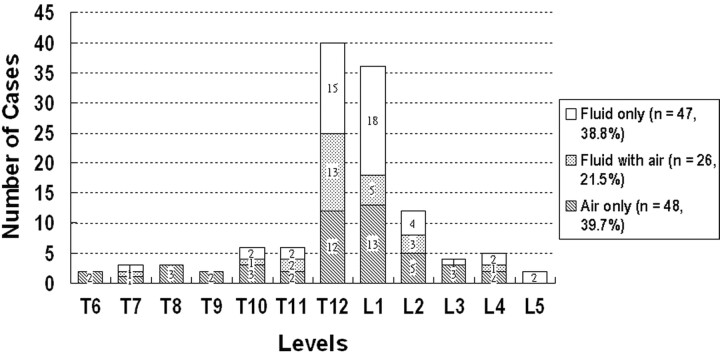
Figure 1 shows changes in the affected vertebral bodies at different levels. Vertebral bodies at T12 (n = 40) and L1 (n = 36) were most often involved and together accounted for about two thirds of all affected vertebral bodies. Of 121 osteonecrotic vertebral bodies, 48 (39.7%) appeared with only intravertebral air (air only, Fig 2), 47 (38.8%) appeared with only intravertebral fluid (fluid only, Fig 3), and 26 (21.5%) appeared with both air and fluid (fluid with air, Fig 4).
Fig 1.
Chart showing the distribution of vertebral osteonecrosis at different levels. Changes of the content of affected vertebral bodies, intravertebral fluid only (blank bars), coexistence of both fluid and air (dot bars), and intravertebral air only (oblique line bars) are also shown. T12 (n = 40) and L1 (n = 36) were most often involved. Of 121 osteonecrotic vertebral bodies, 48 (39.7%) appeared with only intravertebral air, 47 (38.8%) appeared with only intravertebral fluid, and 26 (21.5%) appeared with both air and fluid.
Fig 2.
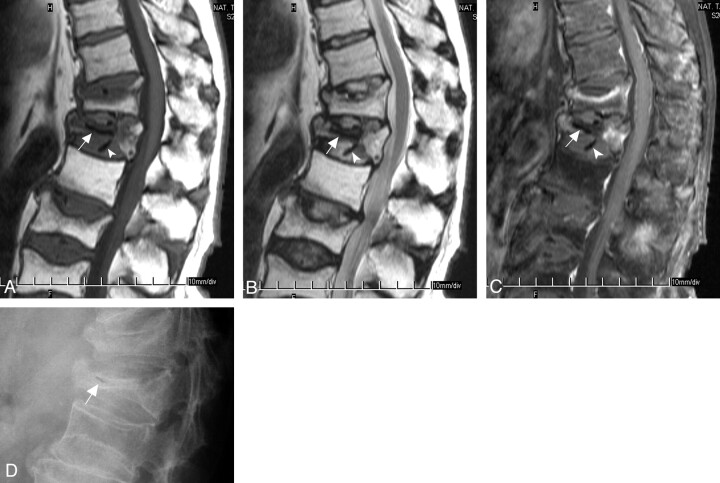
MR images and plain radiograph of a 73-year-old man who had compression fractures at T12, L1, L3, and L4 vertebral bodies and osteonecrosis at L1 vertebral body. There is intravertebral air in the severely collapsed T12 vertebral body and also intradiskal air in the adjacent T11–T12, T12–L1, and L1–L2 disks. In the center of the collapsed vertebral body is a horizontally oriented signal intensity void line (white arrows, panels A–C) on all pulse sequences. Also, several signal intensity void dots or rods in the adjacent disks (above and below) are shown on all pulse sequences (white arrowheads, panels A–C). A, Sagittal T1-weighted turbo spin-echo image (600/12, 4-mm section thickness) shows hypointensity in the most of the vertebral body; only the posterior fifth is relatively spared. B, Sagittal T2-weighted turbo spin-echo image (4000/120) shows some hypointensity at anterior four fifths of the collapsed vertebral body. C, Sagittal contrast-enhanced T1-weighted, fat-suppressed turbo spin-echo image (690/12, 4-mm section thickness) shows nonenhancement portion at middle three fifths of the collapsed vertebral body. D, Lateral view of plain radiograph shows severely collapsed vertebral body with a short and horizontally oriented air cleft at the anterior second fifth of the vertebral body (white arrow).
Fig 3.
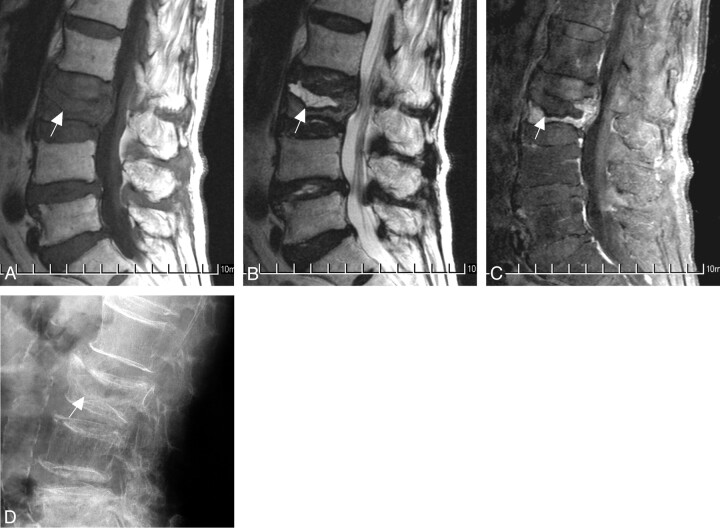
MR images and plain radiograph of an 82-year-old woman who had compression fractures and osteonecrosis at the L3 vertebral body. There is only intravertebral fluid in the mildly collapsed vertebral body. A, Sagittal T1-weighted turbo spin-echo image (600/12, 4-mm section thickness) shows complete bone marrow replacement by low signal intensity (arrow). B, Sagittal T2-weighted turbo spin-echo image (4000/128) shows homogeneous hyperintensity at the anterior superior portion of the vertebral body (arrow). The margin of the hyperintense area is well demarcated. C, Sagittal contrast-enhanced T1-weighted fat-suppressed turbo spin-echo image (700/12, 4-mm section thickness) shows nonenhancement of the anterior and superior portions of the vertebral body (arrow). The nonenhancing area corresponds to the hyperintense area of the T2WI in panel B. The remaining portion of this vertebral body had faint enhancement. D, Lateral view of plain radiograph shows faint radiolucent area at anterior superior portion of the vertebral body (arrow). The radiolucent area corresponds to the T2 hyperintnese area in the panel B.
Fig 4.
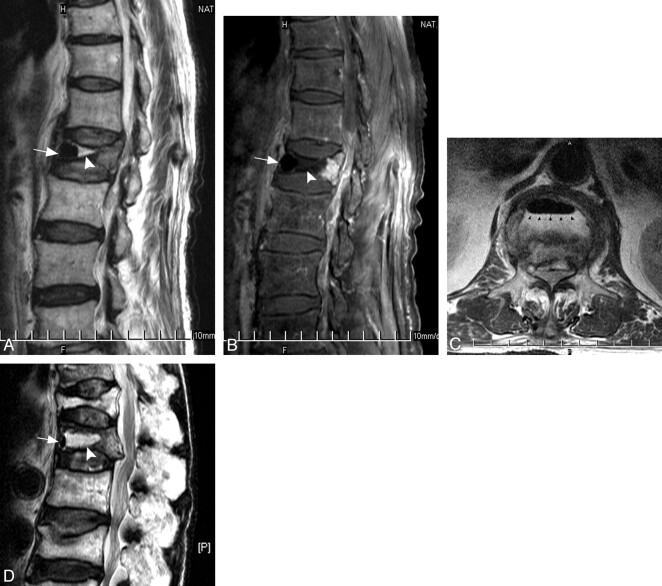
A–D, MR images of an 83-year-old man who was diagnosed with osteonecrosis at the L1 vertebral body. There coexists both intravertebral fluid and air in the affected vertebral body. A, Sagittal T2-weighted turbo spin-echo image (4,000/110) shows hyperintensity at middle third (arrowhead) and signal intensity void at anterior third (arrow) of the vertebral body. B, Sagittal contrast-enhanced T1-weighted, fat-suppressed turbo spin-echo image (550/12, 4-mm section thickness) shows nonenhancement of the anterior two thirds of the vertebral body (arrow and arrowhead). Only the posterior one third of vertebra had enhancement. C, Transverse T2-weighted turbo spin-echo image (4000/110) shows an ellipsoidal signal intensity void area (arrow) anterior to the hyperintensity within the vertebral body. The interface between these 2 components revealed an air–fluid level (small black arrowheads). D, Sagittal T2-weighted turbo spin-echo MR image of another 76-year-old man who was diagnosed with osteonecrosis at T12 vertebral body. There coexists both intravertebral fluid (most, arrowhead) and air (minority, arrow) in the affected vertebral body.
Bone biopsies were carried out on 15 vertebral bodies in 15 patients. Histologic analysis of biopsy bone specimen revealed small necrotic bone fragments with a fibrous stroma that is consistent with the phenomenon of osteonecrosis. Adjacent parts of the bone showed infiltration of osteoclasts and osteoblasts that are indicative of a high turnover status. In one case with intravertebral fluid, we advanced the biopsy needle into the necrotic cavity and aspirated a small amount of clear yellowish fluid. Interestingly, the biochemical analysis of this fluid revealed transudate rather than exudate.
Severity of Vertebral Collapse
Table 2 shows the severity of collapse in the affected vertebral bodies. Of 121 affected vertebral bodies, 67 were severely collapsed (Fig 1) and 54 were mildly collapsed (Fig 2). Severe collapse was more frequent in patients with only intravertebral air than in those with only intravertebral fluid (air only versus fluid only, 89.6% vs 27.7%, P < .05). Severe collapse was more common when intravertebral air was present (air only and fluid with air versus fluid only, 73.0% vs 27.7%, P < .05) than when intravertebral fluid was present (fluid only and fluid with air versus air only, 32.9% vs 89.6%, P < .05).
Table 2:
Severity of vertebral collapse with different internal contents
| Comparison in Categories | Severe* (n = 67) | Mild* (n = 54) | P Value |
|---|---|---|---|
| Air only (n = 48) | 43 (90) | 5 (10) | |
| Fluid only (n = 47) | 13 (28) | 34 (72) | <.05 |
| Presence of fluid† (n = 73) | 24 (33) | 49 (67) | |
| Absence of fluid (n = 48) | 43 (90) | 5 (10) | <.05 |
| Presence of air‡ (n = 74) | 54 (73) | 20 (27) | |
| Absence of air (n = 47) | 13 (28) | 34 (72) | <.05 |
Numbers in parentheses are percentages.
Vertebrae with >50% preserved height were categorized as mild collapse, and those with <50% preserved height were categorized as severe collapse.
Presence of fluid represents the sum of case numbers of fluid only and fluid with air.
Presence of air represents the sum of case numbers of air only and fluid with air.
Content of Adjacent Disks
Table 3 shows the content of disks adjacent to the affected vertebrae. Intradiskal air was not found in patients with only intravertebral fluid. Intradiskal air in adjacent disks was significantly more common in those with only intravertebral air than in those with both intravertebral air and fluid (air only versus fluid with air, 42.7% vs 21.2%, P < .05). When air in the disks next to the adjacent upper and lower disks was compared in patients with only intravertebral air and in those with both intravertebral air and fluid, the difference was not significant (air only versus fluid with air, 12.5% vs 5.8%, P = .195).
Table 3:
Changes of content of adjacent and next-to-adjacent disks according to different manifestation of vertebral osteonecrosis
| Changes of Adjacent Disks | Changes in Affected Vertebrae |
|||
|---|---|---|---|---|
| Air Only (n = 96) | Fluid with Air (n = 52) | Fluid Only (n = 94) | P Value | |
| Air in adjacent disks | 41 | 11 | <.05 | |
| Upper | 21 | 6 | 0 | |
| Lower | 20 | 5 | 0 | |
| Air in disks next to adjacent disks | 12 | 3 | <.05 | |
| Next to upper | 8 | 1 | 0 | |
| Next to lower | 4 | 2 | 0 | |
Collapse of Adjacent Vertebrae
Table 4 shows vertebral collapse in different groups of patients. We found 24 collapsed upper adjacent vertebrae and 18 collapsed lower adjacent vertebrae. Collapses were more frequent in patients with only intravertebral air than in those with only intravertebral fluid (air only versus fluid only, 25.0% vs 8.5%, P < .05). Collapse was more common when intravertebral air was present (air only and fluid with air versus fluid only, 12.3% vs 25.0%; P < .05) than when intravertebral fluid was present (fluid only and fluid with air versus air only, 23.0% vs 8.5%; P < .05). Collapses in vertebrae next to the adjacent upper and lower vertebrae did not differ significantly in groups (data not shown).
Discussion
Vertebral osteonecrosis is an uncommon disease that occurs mostly in patients with a collapsed vertebral body. Vertebral osteonecrosis is thought to be the consequence of insult at the anterior segment of the vertebral body, with possible mechanisms being either traumatic or nontraumatic. The first mechanism is called Kummel disease and represents delayed vertebral collapse after major trauma.6,7 The second mechanism involves repeated microtrabecular fractures in a vertebral body that is weakened because of osteoporosis, replacement of marrow by abnormal cells, or long-term administration of glucocorticoids.8-10 In our study, we excluded cases with chronic steroid administration because insufficient data exist showing whether chronic steroid administration affects vertebral osteonecrosis. On the other hand, most of the steroid users often had more underlying medical diseases and/or unknown system diseases that might have caused uncertain influence to the bone marrow.
Vertebral osteonecrosis is usually suspected when a radiograph shows an abnormal radiolucent shadow in the collapsed vertebral body or when the intervertebral vacuum cleft sign is present. If vertebral osteonecrosis is present, subsequent CT will reveal air attenuation, whereas all MR imaging sequences will show a signal intensity void.1,2 Collection of intravertebral fluid has been described in cases of vertebral osteonecrosis, mainly on MR imaging.3-5 The fluid sign of vertrebral osteonecrosis is a circumscribed area of fluidlike hypointensity on T1-weighted images and hyperintensity on T2-weighted images. Histologic analysis will show reactive marrow fibrosis with high turnover rate, which indicates osteonecrosis. To our knowledge, our report is the first to describe a large-scale analysis of the intravertebral vacuum and intravertebral fluid in benign vertebral osteonecrosis on MR images.
Occurrences of air and fluid were approximately equal in vertebral bodies with benign osteonecrosis. The coexistence of both air and fluid in the same affected body was not rare and occurred in 21.5% of affected vertebrae. Our results suggest a high overall prevalence of intravertebral fluid in vertebral osteonecrosis. Additionally, although the data are not shown here, plain radiographs usually did not show obvious changes in the affected vertebral bodies with only intravertebral fluid. Thus, such cases may be underestimated if evaluations are based on only conventional radiography.
The severity of vertebral collapse differed significantly in the 3 groups of patients. Vertebral collapse was significantly more severe in those having only intravertebral air than in those having intravertebral fluid with or without air. Collapse was also significantly more severe when air was present than when fluid was present. Intravertebral air may represent an advanced stage of disease, whereas intravertebral fluid occurs at an earlier stage. In our patients with only intravertebral fluid, follow-up radiography and/or MR imaging ultimately revealed newly developing intravertebral air. On the contrary, none of the few available follow-up MR studies (n = 4) of patients with only intravertebral air depict intravertebral fluid. The sequential change from fluid to air may suggest that the stage of vertebral osteonecrosis is relatively late when intravertebral air appears. However, further study is warranted since a limited number of follow-up MR images were obtained for comparison.
With respect to the possible influence of position change of patients on vertebral height, the percentage of vertebral collapse would be quite different if measurements were done with weight-bearing radiographs. In our study, we calculated the percentage of vertebral collapse from MR images, which could only be obtained with patients in the supine position. We did not measure this vertebral height from the radiographs. We postulated that there would be more change of vertebral height in patients with intravertebral air than in those with intravertebral fluid after position change; however, this part of our assumption requires clarification with further study data.
In our study, intradiskal air in upper and lower disks adjacent to osteonecrotic vertebrae was not found in patients with intravertebral fluid and was significantly more common in patients with intravertebral air than in those with both intravertebral air and fluid. Previous findings have suggested that intradiskal gas may migrate into fractured vertebral bodies.11 Our results, however, suggest that the intradiskal air may come from intravertebral air. When we examined MR images in greater detail, we found some air beneath the endplate. We categorized such cases into an intradiskal air group because we presumed that such air came from the intravertebral location and migrated through a fractured endplate. In some circumstances, especially with more advanced collapsed vertebral bodies, there was some difficulty in differentiating between intradiskal (endplate) air and intravertebral air. In our experience, the air beneath the endplate often extended to the posterior third of the endplate (as present at the T11 lower endplate in Fig 2), whereas intravertebral air did not.
With regard to the adjacent levels evaluated, fractures in adjacent vertebrae were most frequent in those with only intravertebral air. The collapse of adjacent vertebral bodies may result from a relatively unstable condition. Our result that collapse was more severe with intravertebral air only than with fluid supports this possibility. In conjunction with our finding that vertebral collapse is significantly more severe in vertebrae having intravertebral air than in those having intravertebral fluid, aggressive treatment (instead of conservative observation) should be pursued as soon as intervertebral fluid is identified on MR images. Once such intravertebral fluid is identified, we should consider it as indicative of a cavity formation or gap between viable bones that would not heal spontaneously that would tend to become nonunited. Aggressive treatment (eg, vertebroplasty, interbody fusion, or posterior fixation) in such cases may prevent further collapse of affected vertebral bodies and adjacent levels. Further longitudinal comparisons, however, are warranted between different treatment groups (aggressive versus conservative) when intravertebral fluid appears.
Interestingly, the prevalence of benign vertebral osteonecrosis in our study was higher in women (n = 82) than in men (n = 30), though the mean age of patients did not significantly differ (women versus men, 73.1 versus 74.7 years). These data showed that the disease in our study group reflected mainly postmenopausal osteoporosis in women and senile osteoporosis in men.
Our study had limitations inherent in its design. In this retrospective study, bone mineral densities from 6 months before or after MRI were available in only 10 patients. Attenuations indicated osteoporosis in 9 patients and osteopenia in 1 patient. A prospective study that includes measurements of bone mineral densities may be necessary for further comparison of the data.
Second, different positioning of patients may have influenced changes in intravertebral content. One study has shown that progressive changes in the content of the cleft occur within an hour after patients are placed in a supine position.2 These changes included disappearance of the vacuum phenomenon on radiographs, as well as a fluidlike area of high signal intensity that appeared on T2- or T2*-weighted MR images. This result suggested that fluid or possible transudate with slow inflow was present in the intravertebral cleft after supine positioning. However, because our MRI studies were completed within 25–30 minutes, and delayed scanning was not performed in all cases, no fluid filling-in phenomenon was found between the first and latest sequences within 30 minutes in our cases.
In conclusion, our findings suggest that intravertebral fluid alone or in conjunction with air is present in cases of vertebral osteonecrosis. Our results also indicate that intravertebral fluid may represent a subsequent phase of vertebral osteonecrosis that precedes the development of intravertebral air. The stage of vertebral osteonecrosis is late when intravertebral air appears and indicates a tendency for vertebral collapse in adjacent levels for those without this air.
References
- 1.Theodorou DJ. The intravertebral vacuum cleft sign. Radiology 2001;21:787–88 [DOI] [PubMed] [Google Scholar]
- 2.Malghem J, Maldague B, Labaisse M. Intravertebral vacuum cleft: changes in content after supine positioning. Radiology 1993;187:483–87 [DOI] [PubMed] [Google Scholar]
- 3.Naul LG, Peet GJ, Maupin WB. Avascular necrosis of the vertebral body. Radiology 1989;172:219–22 [DOI] [PubMed] [Google Scholar]
- 4.Dupuy DE, Palmer WE, Rosenthal DI. Vertebral fluid collection associated with vertebral collapse. AJR Am J Roentgenol 1996;167:1535–38 [DOI] [PubMed] [Google Scholar]
- 5.Baur A, Stabler A, Arbogast S, et al. Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. Radiology 2002;225:730–35 [DOI] [PubMed] [Google Scholar]
- 6.Osterhouse MD, Kettner NW. Delayed posttraumatic vertebral collapse with intravertebral vacuum cleft. J Manipulative Physiol Ther 2002;25:270–75 [DOI] [PubMed] [Google Scholar]
- 7.Young WF, Brown D, Kendler A, et al. Delayed post-traumatic osteonecrosis of a vertebral body (Kummell’s disease). Acta Orthop Belg 2002;68:13–19 [PubMed] [Google Scholar]
- 8.Panow C, Valavanis A. A case of aseptic vertebral necrosis in the context of metastatic lumbar disease. Neuroradiology 2002;44:249–52 [DOI] [PubMed] [Google Scholar]
- 9.Weinstein RS, Jilka RL, Parfitt AM, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 1998;102:274–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldague B, Noel H, Malghem J. The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology 1978;129:23–29 [DOI] [PubMed] [Google Scholar]
- 11.Lafforgue P, Chagnaud C, Daumen-Legre V, et al. The intravertebral vacuum phenomenon (“vertebral osteonecrosis”). Migration of intradiscal gas in a fractured vertebral body? Spine 1997;22:1885–91 [DOI] [PubMed] [Google Scholar]