Abstract
BACKGROUND AND PURPOSE: MR imaging signal intensity abnormalities in the cerebellum, the pons, and the basal ganglia, compatible with a neurodegenerative process (ND) were reported in up to 10% of patients with Langerhans cell histiocytosis (LCH). Although the imaging features of ND-LCH have been extensively described, the temporal course of ND-LCH has not been assessed as of yet. The purpose of this study was to describe the long-term course of MR imaging signal intensity abnormalities in ND-LCH on T1- and T2-weighted images.
MATERIALS AND METHODS: In this retrospective study, 9 patients with ND-LCH with an observation time of at least 5 years were included. Three or more MR imaging studies per patient, performed in 3-year intervals (±11 months), were reviewed. Signal intensity abnormalities on T1- and T2-weighted images in the cerebellum, the pons, and basal ganglia were scored for their signal intensity quality and their extension. In addition, the severity of cerebellar atrophy was scored.
RESULTS: The signal intensity alterations were not resolved in any of the patients. Instead, a progression of the signal intensity alterations either in the cerebellum or basal ganglia was observed in all of the patients but did not correlate with a clinical deterioration. Overt and severe neurologic symptoms were reported in only 2 patients in whom some form of atrophy was noted.
CONCLUSIONS: ND-LCH appears to be a slowly progressive process. The increase of signal intensity abnormalities in the cerebellum and basal ganglia does not correlate with neurologic deterioration. MR imaging appears to be a sensitive technique to detect and monitor radiologic ND-LCH.
Langerhans cell histiocytosis (LCH) is a rare systemic granulomatous disease of the dendritic system with a variable clinical course that may be encountered at any age. The annual incidence of LCH in children younger than 10 years was reported to be 0.2–2 patients per 100,000 children.1 The incidence in adults remains to be determined. Almost all organs of the body can be affected by the disease.2 The typical LCH lesion is composed of a granulomatous infiltrate of a variable number of monoclonal Langerhans cells, T cells, and eosinophils.2
Central nervous system (CNS) involvement in LCH most frequently manifests itself in the hypothalamic pituitary region with the key symptom of diabetes insipidus.3–5 Less frequently, granulomatous lesions in the meninges, the choroid plexus, the pineal gland, or the cerebral parenchyma are encountered.6,7 In addition to granulomatous lesions, LCH-associated MR imaging signal intensity abnormalities of variable intensity in the cerebellum, the basal ganglia, the pons, and the supratentorial white matter have been described in a number of reports.6–9 The signal intensity abnormalities in the cerebellum were composed of symmetric hyperintense signal intensity alterations on T2-weighted images and hypointense or hyperintense signals on T1-weighted images involving the gray matter only or extending to the surrounding white matter, eventually resulting in CSF-intense “holes” on T1-weighted images. Pontine lesions have been reported as T2 hyperintense signal intensity abnormalities in the pontine tegmentum or symmetrical T2 hyperintensities in the pontine pyramidal tracts.9 In the basal ganglia, the abnormalities consisted of hyperintensities on T1-weighted images and variable signal intensities on T2-weighted images. These lesions did not show contrast enhancement or mass effect and inconsistently displayed calcifications.7,9
Results of histopathologic examination of tissue from cerebellar biopsies and autopsies of patients with such MR imaging changes revealed neuron loss and axonal degeneration along with a profound T-cell inflammation. Thus, the described MR imaging signal intensity abnormalities were interpreted as indicative for a neurodegenerative process (ND).5
MR imaging findings compatible with neurodegenerative disease (radiologic ND-LCH) are frequently detected in patients without suggestive clinical symptoms. Neurologic symptoms of ND-LCH (clinical ND-LCH) range from subtle deficits like reflex abnormalities, gait disturbance, and behavioral disturbances to profound ataxia, dysarthria, spastic diparesis, or psychiatric disease.10
Although the pattern of signal intensity changes on MR imaging in ND-LCH has been extensively described in a number of reports,6–9 the evolution of the MR imaging findings of ND-LCH over the years and their clinical impact has not been assessed so far. The purpose of this study was to describe the long-term course of MR imaging signal intensity abnormalities in ND-LCH on T1- and T2-weighted images.
Patients and Methods
Patients
At the LCH study center in Vienna, Austria, 89 patients with radiologic ND-LCH were registered. In this retrospective study, we included only patients with an MR imaging follow-up period of at least 5 years after the detection of radiologic ND-LCH and with at least 3 MR imaging studies available for review. Nine patients met these criteria. They were 6 male and 3 female patients with a median age at the time of diagnosis of LCH of 2 years and 7 months. The diagnosis of LCH was established from extracranial lesions according to standard criteria in all of the patients.11 All but 1 patient (patient 4) were enrolled onto 1 of the prospective LCH studies of the Histiocyte Society (DAL HX 83/90, LCH I or II).
Clinical information on age, sex, extent of LCH, therapy, and disease course from all of the patients was collected with the use of standardized study questionnaires. Regular neuropsychologic examinations were not requested in the LCH clinical trial protocols. Information on the neuropsychologic status was thus taken from the medical reports. According to the information available from the patient medical charts, all of the patients were tested by their local physicians for the presence of neurologic or psychologic symptoms at the time of the last MR imaging follow-up. The various tests applied at the different institutions included a thorough neurologic examination and psychologic tests with particular focus on cerebellar-pontine symptoms, behavioral disturbances, concentration and memory, and cognitive deficits.
MR Imaging
The indications for the MR imaging examinations at the time of diagnosis of radiologic ND-LCH were diabetes insipidus in 4 patients, craniofacial bone lesions in 3 patients, follow-up of an intracerebral enhancing lesion in 1 patient, and neurologic symptoms (ataxia) in another patient.
Overall, 72 MR imaging studies were available from the 9 patients, each of whom had 3–13 MR imaging studies (median, 8 studies) performed in variable intervals. To investigate the patients in comparable intervals, we considered only those follow-up MR imaging studies that were performed in 3-year intervals (±11 months). Consequently, we included 3 MR imaging studies from 4 patients, 4 MR imaging studies from 3 patients, and 5 MR imaging studies from 2 patients in the study. The MR imaging studies were performed in 7 centers on various 1.5T MR scanners according to different protocols. For the evaluation of the course of radiologic ND-LCH and to facilitate the comparability of the patients, only T1 spin-echo-weighted sequences and T2-weighted sequences were considered in this study, because these sequences were available from all of the patients. All of the images were reviewed in consensus by 1 experienced pediatric neuroradiologist (D.P.) and 1 radiology resident with a special interest in LCH (H.P.). The images were evaluated according to a checklist for the presence of LCH-associated MR imaging abnormalities. The supratentorial and infratentorial regions were evaluated for alterations in signal intensity on T1- and T2-weighted images. The signal intensity abnormalities were characterized regarding their extent and anatomic location. Furthermore, signs of supratentorial (enlarged sulci and thinning of the corpus callosum) or cerebellar atrophy (enlarged fissures and thinning of the dentate nuclei) were recorded. In addition, the anatomic location and extent of intra-axial space-occupying lesions or space-occupying lesions in the hypothalamic pituitary region, the meninges, and craniofacial bone lesions were noted.
The signal intensity abnormalities in the cerebellum and basal ganglia were further subjectively scored for their signal intensity quality and the extent of the involvement into 3 categories: mild, moderate, and severe. Mild signal intensity abnormalities in the cerebellum were limited to the dentate nucleus and were hyperintense on T1-weighted images and isointense or hyperintense on T2-weighted images. Moderate changes included hyperintensities on T1-weighted images in the dentate nucleus with a hypointense core. On corresponding T2-weighted images, the dentate nucleus was isointense to hypointense with a hyperintense core. If severe, the dentate nucleus was hyperintense on T1-weighted images and hypointense on T2-weighted images. The white matter surrounding the dentate nucleus, as well as the middle cerebellar peduncles and the dorsal part of the pons, were patchy hyperintense on T2-weighted images and hypointense to isointense on T1-weighted images.
Basal ganglia involvement was graded mild (subtle signal intensity alterations limited to the pallidum), moderate (prominent signal intensity alterations limited to the pallidum), and severe (signal intensity alterations in the pallidum and the putamen). Signal intensity alterations in the anterior pons were patchy hyperintense on T2-weighted images and graded mild or prominent. Cerebellar atrophy was graded mild (enlarged fissures limited to the vermis), moderate (enlarged fissures involving more than the vermis), and severe (all folia shrunken and fissures enlarged).
CNS Biopsy
A small biopsy sample was available for central review from patient 4. The material was fixed in formalin and embedded in paraffin. In addition to hematoxylin-eosin stains, Luxol fast blue stain for myelin, Bielschowsky silver impregnation for axons, and immunocytochemistry were performed and reviewed by an experienced neuropathologist (Prof Hans Lassmann, Brain Research Institute, Medical University of Vienna, Vienna, Austria).
Results
The key features of patients with radiologic ND-LCH disease are summarized in Table 1.
Table 1:
Key features of 9 patients with radiologic ND-LCH disease
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Female | Male | Male | Male | Male | Female | Female |
| Age at diagnosis of LCH | 1 y 10 mo | 2 y 9 mo | 1 y 10 mo | 3 y 4 mo | 2 y | 2 y 8 mo | 16 y 10 mo | 2 y 10 mo | 6 mo |
| Organs involved at diagnosis of LCH | Bone, skin, liver, lung, spleen, hematopoietic system | Bone, skin | Bone, skin, liver | Bone, skin, DI | Bone, skin | Bone, skin, liver, spleen | Bone, skin, DI | Bone, skin, hematopoietic system, DI | Skin, liver |
| Course of LCH (outside the CNS) | Chronic active | 3 RA | 2 RA | no RA | 4 RA | 1 RA | 2 RA | 2 RA | Chronic active |
| Age at diagnosis of ND-LCH | 6 y 6 mo | 7 y 3 mo | 5 y 7 mo | 4 y 5 mo | 2 y 6 mo | 8 y 2 mo | 17 y 11 mo | 4 y 3 mo | 4 y 3 mo |
| Interval diagnosis LCH to the diagnosis of ND | 4 y 7 mo | 4 y 7 mo | 3 y 10 mo | 1 y 1 mo | 5 mo | 5 y 7 mo | 1y | 1 y 5 mo | 3 y 9 mo |
| MR imaging follow-up period | 7 y 5 mo | 10 y 3 mo | 5 y 2 mo | 7 y 6 mo | 11 y | 7 y 3 mo | 5 y | 11 y 8 mo | 6 y 8 mo |
| No. of evaluated MR imaging studies | 4 | 4 | 3 | 3 | 5 | 4 | 3 | 5 | 3 |
| Age at last MR imaging | 13 y 10 mo | 17 y 6 mo | 10 y 10 mo | 11 y 8 mo | 13 y 1 mo | 15 y 5 mo | 22 y 11 mo | 15 y 10 mo | 10 y 10 mo |
| Additional intracranial abnormalities | |||||||||
| HPR abnormalities** | −/+ | +/+ | +/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ |
| Space-occupying meningeal lesions** | −/− | −/+ | −/− | −/− | −/+ | −/− | −/− | +/+ | −/− |
| Craniofacial bone lesions** | +/+ | +/+ | +/+ | +/− | +/+ | +/− | +/+ | +/+ | −/− |
| Space-occupying intraparenchymal lesions** | −/− | −/− | −/− | −/− | −/− | −/− | +/+ | −/− | −/− |
| DI* | −/+ | +/+ | −/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ |
| Growth hormone deficiency* | −/+ | +/+ | −/+ | −/− | −/− | −/− | +/+ | −/+ | −/− |
| Neurologic symptoms* | −/− | −/− | −/− | Ataxia/severe cerebellar symptoms | −/− | −/− | −/− | −/ataxia, spastic diplegia | −/− |
Note:—ND indicates neurodegeneration; LCH, Langerhans cell histiocytosis; y, year; mo, month; DI, diabetes insipidus; RA, reactivation; HPR, hypothalamic pituitary region; −, absent; +, present.
At diagnosis/at the last follow-up.
before or at the diagnosis of ND-LCH/after the diagnosis of ND-LCH.
Infratentorial Signal Intensity Abnormalities
Cerebellar signal intensity abnormalities were found in all of the patients. The course of cerebellar signal intensity abnormalities is summarized in Table 2. In 7 patients, the signal intensity alterations were limited to the dentate nucleus at the time of diagnosis of ND-LCH (4 mild and 3 moderate). In 2 patients, severe cerebellar signal intensity alterations, including the dentate nucleus, the cerebellar white matter, and the dorsal pons, were already present at the diagnosis of radiologic ND-LCH. In 2 patients (patients 3 and 7) who presented with mild neurodegenerative signal intensity alteration in the dentate nucleus, no progression could be observed over an observation time of 5 years. In another 2 patients who presented with moderate cerebellar signal intensity abnormalities, no progression was observed (observation time, 7 years and 10 years, respectively). In the other 3 patients, a progression of the cerebellar signal intensity abnormalities was observed.
Table 2:
Course of radiologic ND-LCH in the cerebellum, anterior pons, and basal ganglia in 9 patients
| Patient | Follow-Up |
||||
|---|---|---|---|---|---|
| At Diagnosis | 3 years | 6 years | 9 years | 12 years | |
| 1 | |||||
| Cerebellum | ++ | ++ | ++ | ++ | |
| Basal ganglia | − | + | ++ | ++ | |
| Anterior pons | − | − | − | − | |
| 2 | |||||
| Cerebellum | ++ | ++ | ++ | ++ | |
| Basal ganglia | + | + | ++ | ++ | |
| Anterior pons | − | − | − | − | |
| 3 | |||||
| Cerebellum | + | + | + | ||
| Basal ganglia | + | + | ++ | ||
| Anterior pons | − | − | − | ||
| 4 | |||||
| Cerebellum | +++* | +++* | +++* | ||
| Basal ganglia | +* | ++* | ++* | ||
| Anterior pons | +* | +* | +* | ||
| 5 | |||||
| Cerebellum | + | ++ | +++ | +++ | +++ |
| Basal ganglia | − | + | + | + | + |
| Anterior pons | − | − | + | + | + |
| 6 | |||||
| Cerebellum | +++ | +++ | +++ | +++ | |
| Basal ganglia | + | +++ | +++ | +++ | |
| Anterior pons | − | − | − | − | |
| 7 | |||||
| Cerebellum | + | + | + | ||
| Basal ganglia | − | + | + | ||
| Anterior pons | − | − | − | ||
| 8 | |||||
| Cerebellum | + | +++ | +++* | +++* | +++* |
| Basal ganglia | − | + | ++* | ++* | ++* |
| Anterior pons | − | + | ++* | ++* | ++* |
| 9 | |||||
| Cerebellum | ++ | ++ | +++ | ||
| Basal ganglia | − | − | ++ | ||
| Anterior pons | − | − | − | ||
Note:—ND indicates neurodegeneration; LCH, Langerhans cell histiocytosis; Cerebellum: +, mild signal alterations limited to the dentate nucleus; ++, moderate signal alterations in dentate nucleus; +++, extensive signal alterations in the dentate nucleus, cerebellar white matter, middle cerebellar peduncles, and dorsal part of the pons. Anterior pons: −, normal; +, subtle signal alterations; ++, prominent signal alterations. Basal ganglia: −, normal; +, mild signal alterations limited to the pallidum; ++, moderate signal alterations limited to the pallidum; +++, extensive alterations involving the pallidum and the putamen.
Radiologic ND-LCH associated with neurologic symptoms.
Signal intensity alterations in the dorsal pons occurred in all of the patients in whom signal intensity alterations in the cerebellar white matter were observed (graded as severe cerebellar signal intensity abnormalities). Patchy signal intensity alterations in the anterior pons were observed in 3 patients only (patients 4, 5, and 8). Signal intensity alterations in the anterior pons were invariably associated with severe cerebellar signal intensity abnormalities. In 2 patients (patients 4 and 5), the signal intensity alterations in the pons remained subtle. In patient 8, subtle signal intensity alterations in the anterior pons progressed to prominent signal intensity alterations (Table 2).
Supratentorial Signal Intensity Abnormalities
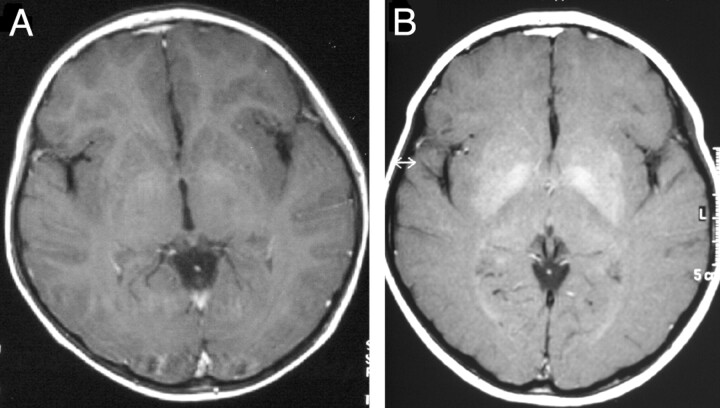
Signal intensity abnormalities of supratentorial white matter were not detected in any of the 9 patients. Signal hyperintensity abnormalities on T1-weighted images in the basal ganglia were present in all of the patients. In 2 patients (patients 5 and 6), the pallidum was also hyperintense on T2-weighted images. In all of the other patients, the pallidum was isointense to gray matter on T2-weighted images. The signal intensity alterations were already present at the time of diagnosis of radiologic ND-LCH in 4 patients. In the other 5 patients, signal intensity abnormalities developed during the course of the disease. The signal intensity alterations remained limited to the pallidum in all of the patients but patient 6, in whom the signal intensity alterations extended to the putamen (Fig 1).
Fig 1.
Course of signal intensity alteration in the basal ganglia in patient 6.
A, Axial T1-weighted images show mild hyperintense signal intensity alterations limited to the pallidum.
B, Three years later, prominent hyperintense signal intensity alterations involve the pallidum and the putamen.
Atrophy
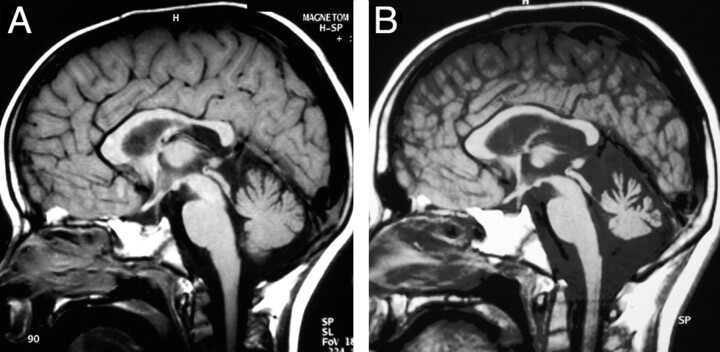
Cerebellar atrophy was observed in patients 4 and 8 only. In patient 4, mild cerebellar atrophy was observed at the time of diagnosis of radiologic ND-LCH and progressed to moderate cerebellar atrophy during the observation time of 7 years and 6 months (Fig 2). In patient 8, isolated atrophy of the dentate nucleus and a thinning of the corpus callosum was diagnosed 11 years after the diagnosis of radiologic ND-LCH (Fig 3). Supratentorial hemispheric atrophy was not observed in our patients.
Fig 2.
Course of cerebellar atrophy in patient 4. Sagittal T1-weighted images show mild cerebellar atrophy at the time of the diagnosis of ND-LCH (A). Seven years later the atrophy is more pronounced (B).
Fig 3.
Course of cerebellar MR imaging findings in patient 8. Left column, T1-weighted images (T1WI); right column, T2-weighted images (T2WI). The first signal intensity abnormalities in the cerebellum were detected at the age of 4 years (1994) and were composed of subtle increased signal intensities on T2WI limited to the region of the dentate nucleus. In 1997, the signal intensity abnormalities on T1- and T2WI involved the dentate nucleus, surrounding white matter, middle cerebellar peduncle, and pons. In 2001, the patient developed an obstructive hydrocephalus, a thinning of the corpus callosum, and progressive cerebellar neurologic symptoms. At the last follow-up (2005), the dentate nucleus appeared atrophic.
Additional MR Imaging Findings
At the diagnosis of radiologic ND-LCH, changes in the hypothalamic pituitary region associated with diabetes insipidus were observed in 7 patients (missing bright spot in 7 patients and hypothalamic tumorous lesions in 2 patients). In 3 patients, craniofacial bone lesions were detected. In patient 7, a small space-occupying lesion in the medulla oblongata was observed in addition to an hypothalamic tumor (10 cm). In patient 8, an obstructive hydrocephalus caused by membranous stenosis of the aqueductus and a thinning of the corpus callosum was observed 7 years after the diagnosis of radiologic ND-LCH. After endoscopic reopening of the aqueduct, the size of the supratentorial ventricles decreased and the corpus callosum remained thin.
Neurologic Symptoms
Patient 4 developed cerebellar symptoms with ataxia and gait disturbances 1 year after the diagnosis of LCH. The neurologic symptoms progressed continuously over the follow-up period of 7 years and 6 months after the MR imaging diagnosis of ND-LCH, despite therapy with diverse strategies, including steroids, indomethacin, retinoic acid, cyclosporine, and melatonin. At the last evaluation, the patient suffered from spastic diparesis, severe ataxia, dysarthria, and mental retardation.
Patient 8 developed mild cerebellar symptoms (ataxia and dysarthria) 7 years after the diagnosis of radiologic ND-LCH. At approximately the same time, an obstructive hydrocephalus and a thinning of the corpus callosum were diagnosed. Within 4 years, the symptoms progressed to spastic diplegia, severe ataxia, and dysdiadochokinesis.
In 7 patients, no overt neurologic symptoms were reported during a median observation period of 7 years after the detection of radiologic ND (range, 5–11 years).
CNS Biopsy
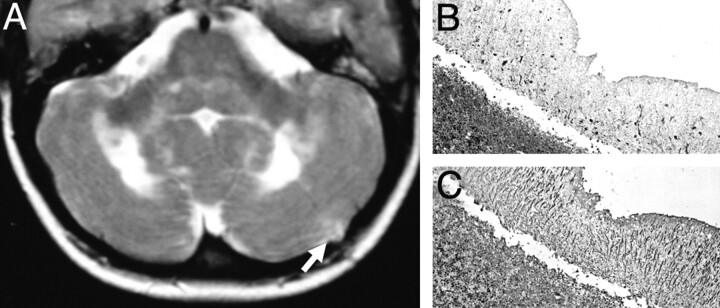
A biopsy of the cerebellar cortex was performed in patient 4, 2 years after the MR imaging diagnosis of ND-LCH (Fig 4). At the time of biopsy, the patient suffered from progressive ataxia. The biopsy revealed a loss of Purkinje cells and Purkinje cell dendrites along with Bergman gliosis and CD8+ T cells.
Fig 4.
(A) Axial T2-weighted image obtained 2 months after a biopsy of the left cerebellar cortex (arrow) shows extensive hyperintensities in the cerebellar white matter extending to the middle cerebellar peduncle and the dorsal pons.
B and C, Cerebellar biopsy: the cerebellar cortex shows diminished thickness of the molecular layer. Immunocytochemistry for microtubule associated protein II (MAP II) reveals a massive loss of Purkinje cells and their dendrites. (B; ×60), the adjacent sections, stained for glial fibrillary acidic protein, show massive astrocytic gliosis, reflected by thick radial glial cell processes in the molecular layer (C; ×60). (Courtesy of Prof Hans Lassmann, Brain Research Institute, Medical University of Vienna, Vienna, Austria).
Clinical Course
During the observation period, 7 of 9 patients experienced a reactivating or chronic disease course. One patient had only 1 reactivation, and another patient remained free of active LCH since the initial episode. At the last follow-up examination, all of the patients were free of extracerebral disease activity. All of the patients had received systemic chemotherapy for their extracerebral LCH before the diagnosis of radiologic ND-LCH. Neurodegenerative signal intensity alterations on MR imaging were diagnosed 5 months to 5 years 7 months after the diagnosis of LCH (median, 3 years 9 months). All 9 of the patients had developed hypothalamic pituitary disease that was present before or at the diagnosis of radiologic ND-LCH in 7 patients. All had diabetes insipidus, and 5 also had growth hormone deficiency.
Six patients received systemic chemotherapy after the diagnosis of radiologic ND-LCH. Patient 5 received radiation therapy to the hypothalamic pituitary region for the treatment of an enhancing hypothalamic lesion (6 Gy) 2 years after the detection of radiologic ND-LCH.
Discussion
Neurodegenerative signal intensity alterations are a rare consequence of LCH. The prevalence of radiologic ND-LCH in pediatric patients with LCH is uncertain and ranges in different reports between 4% and 10%.1,8,12,13 Recent studies indicate that the radiologic ND-LCH is more frequently detected in patients with craniofacial lesions or neuroendocrine problems.14,15
The routine use of MR imaging in the evaluation of such patients led to an increasing number of reported cases. The wide range of radiologic findings in ND-LCH has been extensively described but not in the context of the course of the disease.6,7,9,10
The signal intensity abnormalities in radiologic ND-LCH are not pathognomonic for LCH. Similar findings can be observed in the cerebellum of patients with cerebrotendinous xanthomatosis, Erdheim-Chester disease, corticobasal degeneration, Machado disease, or toxic demyelination.16–19 Symmetric hyperintensities on T1-weighted images were also observed in the basal ganglia of patients with chronic liver failure and in patients with long-term parenteral nutrition.20,21 Although 4 of our LCH patients presented with initial LCH-liver involvement, liver failure was not observed in any of the patients, and none of our patients required parenteral nutrition.
In the setting of a history of known LCH, the pattern of radiologic ND-LCH is quite typical and distinct. However, little is known about the evolution of the disease and the clinical impact of the MR imaging findings.
This study investigated the long-term MR imaging and clinical course of ND over a period of at least 5 years. Because of the rarity of the disease and the retrospective and multicentric character of the study, the patients were evaluated according to different study protocols and at irregular intervals. To overcome this problem, we limited the evaluation to T1- and T2-weighted images, which were available in all of the patients. In addition, we included broad follow-up intervals, defined as 3 years ± 11 months. In keeping with these inclusion criteria, we could include 9 patients, well documented by several MR imaging studies. The diagnosis of ND-LCH was based on MR imaging findings only and lacked histopathologic confirmation in all but 1 patient. Because most of the patients were asymptomatic and the therapeutic consequence of a biopsy was uncertain, there was a general and understandable reluctance to perform biopsies in these patients. In a recent report on CNS neuropathology in LCH,5 describing 8 patients with neurodegenerative lesions, the hyperintense signal intensity alterations in the dentate nucleus and the basal ganglia could be correlated with loss of neurons, demyelination, gliosis, and inflammation. Accordingly, the signal intensity changes in the cerebellar gray and white matter seen in our patients and also described by others are compatible with neuronal loss along with wallerian degeneration, typical features of neurodegeneration.6,7
The neuropathologic observation that neurodegeneration may be observed in the contiguity of granulomas of the extra-axial spaces, the meninges, and the hypothalamic pituitary region raises questions on the role of such granulomas in the pathogenesis of neurodegenerative lesions.22 Indeed, granulomas in the meninges, the hypothalamic pituitary region, and/or the craniofacial bone lesions have been discovered at the diagnosis and during the course of ND-LCH in all of the patients in the present study. However, one must keep in mind that the evaluation of these lesions was the indication for MR imaging in all but 1 patient. It is noteworthy that craniofacial bone lesions with or without intracranial tumor extension and meningeal affection may be observed in 43% and hypothalamic lesions in 10% of LCH patients already at the diagnosis.22,23 In addition, neurodegenerative lesions may occur remote from obvious tumorous lesions and in the absence of detectable active LCH. Thus, (intra)cranial LCH granulomas may only be 1 factor contributing to the pathogenesis of ND-LCH. Strikingly, the ND signal intensity alterations did not resolve in any patients but progressed either in the infratentorial region or basal ganglia.
Interestingly, even severe infratentorial signal intensity abnormalities, including the dentate nuclei, the cerebellar white matter, cerebellar peduncles, and the dorsal and anterior pons, were not necessarily correlated to neurologic symptoms. Most of the patients with severe cerebellar signal intensity abnormalities were free of neurologic symptoms at the last available follow-up. This may indicate that a pronounced loss of neurons may be necessary to result in overt neurologic symptoms. Both symptomatic patients in our study presented with some form of atrophy compatible with a significant loss of neurons. This association of atrophy with neuronal symptoms in patients with LCH has also been noted in other reports.7,9,24,25 This observation is also in keeping with findings from multiple sclerosis where the progressive axonal loss remains clinically silent for many years until a threshold of axonal loss is reached, and the CNS is unable to compensate for the loss.26
With the limited number of patients followed for an observation time of between 5 and 11 years, it remains an open question whether the radiologic progression of ND on MR imaging will ensue in neurologic symptoms at a later stage. In a recent report on symptomatic patients with ND-LCH, the median age at presentation with neurologic symptoms was 24 years, which is much older than in our patient population.9 On the other hand, the process leading to the neurodegenerative MR changes could also arrest at some point and not progress to symptomatic tissue loss.
It also remains open whether our observation reflects the “natural” course of radiologic ND-LCH. The influence of therapy on the evolution and course of radiologic ND-LCH cannot be answered clearly. In our series, all of the patients had been treated with LCH-directed standard chemotherapy27–29 before the detection of ND. However, most patients with extensive multisystem LCH are treated with such chemotherapy, but only a minority of patients are diagnosed with neurodegenerative lesions. There are also a number of reports on patients in whom radiologic and or clinical ND-LCH was already present at the time of diagnosis of LCH before any treatment was given.6,10,30,31 In addition, the chemotherapeutic regimens used in LCH are very similar to treatment protocols for pediatric leukemia where such peculiar and permanent signal intensity abnormalities have never been described.6 Only 2 of our 9 patients did not receive chemotherapy after the diagnosis of radiologic ND-LCH. One of these 2 patients developed neurologic symptoms. In the other 7 patients, a progression of radiologic ND-LCH was observed, despite treatment with various chemotherapies or an immunosuppressive and immunomodulatory regimen. Accordingly, no conclusion should be drawn from these limited and heterogeneous data. Further long-term studies, including thorough clinical investigations, are needed to determine the clinical impact of radiologic ND-LCH.
Conclusions
ND-LCH appears to be a slowly progressing process. The increase of signal intensity abnormalities in the cerebellum and basal ganglia does not correlate with neurologic deterioration in all cases. MR imaging appears to be a sensitive technique to detect and monitor radiologic ND-LCH.
Acknowledgments
We thank Eve Laeverenz for proofreading our manuscript. We are indebted to Prof Hans Lassmann (Brain Research Institute, Medical University of Vienna, Vienna, Austria) for reviewing the biopsy sample.
Footnotes
This study was supported by a research grant of the Austrian National Bank (ONB-JBF 11561) and by the Phillips & House Group, Shelta, Austria.
Paper previously presented at: Annual Meeting of the Histiocyte Society, September 25–27, 2005; Vancouver, British Columbia, Canada.
References
- 1.The French Langerhans’ Cell Histiocytosis Study Group. A multicentre retrospective survey of Langerhans’ cell histiocytosis: 348 cases observed between 1983 and 1993. Arch Dis Child 1996;75:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arceci RJ. The histiocytoses: the fall of the Tower of Babel. Eur J Cancer 1999;35:747–67; discussion, 767–69 [DOI] [PubMed] [Google Scholar]
- 3.Tien RD, Newton TH, McDermott MW, et al. Thickened pituitary stalk on MR images in patients with diabetes insipidus and Langerhans cell histiocytosis. AJNR Am J Neuroradiol 1990;11:703–08 [PMC free article] [PubMed] [Google Scholar]
- 4.Czech T, Mazal PR, Schima W. Resection of a Langerhans cell histiocytosis granuloma of the hypothalamus: case report. Br J Neurosurg 1999;13:196–200 [DOI] [PubMed] [Google Scholar]
- 5.Grois N, Prayer D, Prosch H, et al. Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain 2005;128:829–38 [DOI] [PubMed] [Google Scholar]
- 6.Barthez MA, Araujo E, Donadieu J. Langerhans cell histiocytosis and the central nervous system in childhood: evolution and prognostic factors. Results of a collaborative study. J Child Neurol 2000;15:150–56 [DOI] [PubMed] [Google Scholar]
- 7.Prayer D, Grois N, Prosch H, et al. MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR Am J Neuroradiol 2004;25:880–91 [PMC free article] [PubMed] [Google Scholar]
- 8.Grois N, Barkovich AJ, Rosenau W, et al. Central nervous system disease associated with Langerhans’ cell histiocytosis. Am J Pediatr Hematol Oncol 1993;15:245–54 [DOI] [PubMed] [Google Scholar]
- 9.Martin-Duverneuil N, Idbaih A, Hoang-Xuan K, et al. MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol 2006;16:2074–82 [DOI] [PubMed] [Google Scholar]
- 10.Grois NG, Favara BE, Mostbeck GH, et al. Central nervous system disease in Langerhans cell histiocytosis. Hematol Oncol Clin North Am 1998;12:287–305 [DOI] [PubMed] [Google Scholar]
- 11.Broadbent V, Gadner H, Komp DM, et al. Histiocytosis syndromes in children: II. Approach to the clinical and laboratory evaluation of children with Langerhans cell histiocytosis. Clinical Writing Group of the Histiocyte Society. Med Pediatr Oncol 1989;17:492–95 [DOI] [PubMed] [Google Scholar]
- 12.Mittheisz E, Seidl R, Prayer D, et al. Central nervous system-related permanent consequences in patients with Langerhans cell histiocytosis. Pediatr Blood Cancer 2007;48:50–56 [DOI] [PubMed] [Google Scholar]
- 13.Poe LB, Dubowy RL, Hochhauser L, et al. Demyelinating and gliotic cerebellar lesions in Langerhans cell histiocytosis. AJNR Am J Neuroradiol 1994;15:1921–28 [PMC free article] [PubMed] [Google Scholar]
- 14.Donadieu J, Rolon MA, Thomas C, et al. Endocrine involvement in pediatric-onset Langerhans’ cell histiocytosis: a population-based study. J Pediatr 2004;144:344–50 [DOI] [PubMed] [Google Scholar]
- 15.Haupt R, Nanduri V, Calevo MG, et al. Permanent consequences in Langerhans cell histiocytosis patients: a pilot study from the Histiocyte Society-Late Effects Study Group. Pediatr Blood Cancer 2004;42:438–44 [DOI] [PubMed] [Google Scholar]
- 16.Bohlega S, Alwatban J, Tulbah A, et al. Cerebral manifestation of Erdheim-Chester disease: clinical and radiologic findings. Neurology 1997;49:1702–05 [DOI] [PubMed] [Google Scholar]
- 17.Barkhof F, Verrips A, Wesseling P, et al. Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology 2000;217:869–76 [DOI] [PubMed] [Google Scholar]
- 18.Su M, Yoshida Y, Hirata Y, et al. Degeneration of the cerebellar dentate nucleus in corticobasal degeneration: neuropathological and morphometric investigations. Acta Neuropathol (Berl) 2000;99:365–70 [DOI] [PubMed] [Google Scholar]
- 19.Kumada S, Hayashi M, Mizuguchi M, et al. Cerebellar degeneration in hereditary dentatorubral-pallidoluysian atrophy and Machado-Joseph disease. Acta Neuropathol (Berl) 2000;99:48–54 [DOI] [PubMed] [Google Scholar]
- 20.Klos KJ, Ahlskog JE, Josephs KA, et al. Neurologic spectrum of chronic liver failure and basal ganglia T1 hyperintensity on magnetic resonance imaging: probable manganese neurotoxicity. Arch Neurol 2005;62:1385–90 [DOI] [PubMed] [Google Scholar]
- 21.Fell JM, Reynolds AP, Meadows N, et al. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet 1996;347:1218–21 [DOI] [PubMed] [Google Scholar]
- 22.Grois N, Potschger U, Prosch H, et al. Risk factors for diabetes insipidus in Langerhans cell histiocytosis. Pediatr Blood Cancer 2006;46:228–33 [DOI] [PubMed] [Google Scholar]
- 23.Prosch H, Grois N, Prayer D, et al. Central diabetes insipidus as presenting symptom of Langerhans cell histiocytosis. Pediatr Blood Cancer 2004;43:594–99 [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum DC, Shields D, Lippe B, et al. Idiopathic central diabetes insipidus followed by progressive spastic cerebral ataxia. Report of four cases. Arch Neurol 1989;46:1001–03 [DOI] [PubMed] [Google Scholar]
- 25.Goldberg-Stern H, Weitz R, Zaizov R, et al. Progressive spinocerebellar degeneration “plus” associated with Langerhans cell histiocytosis: a new paraneoplastic syndrome? J Neurol Neurosurg Psychiatry 1995;58:180–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci 2003;206:165–71 [DOI] [PubMed] [Google Scholar]
- 27.Gadner H, Heitger A, Grois N, et al. Treatment strategy for disseminated Langerhans cell histiocytosis. DAL HX-83 Study Group. Med Pediatr Oncol 1994;23:72–80 [DOI] [PubMed] [Google Scholar]
- 28.Ladisch S, Gadner H, Arico M, et al. LCH-I: a randomized trial of etoposide vs. vinblastine in disseminated Langerhans cell histiocytosis. The Histiocyte Society. Med Pediatr Oncol 1994;23:107–10 [DOI] [PubMed] [Google Scholar]
- 29.Histiocyte Society. LCH-II: Treatment Protocol of the Second International Study 1996. (unpublished)
- 30.Polizzi A, Coghill S, McShane MA, et al. Acute ataxia complicating Langerhans cell histiocytosis. Arch Dis Child 2002;86:130–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Warrenburg BP, van der Heijden HF, Pieters G, et al. Langerhans’ cell histiocytosis presenting with progressive spinocerebellar ataxia. J Neurol 2003;250:1112–14 [DOI] [PubMed] [Google Scholar]