Abstract
BACKGROUND AND PURPOSE: T2 mapping is useful for identifying and quantifying abnormalities of the hippocampus and amygdala. It is particularly useful in the presurgical evaluation of patients with temporal lobe epilepsy and for the identification of bilateral hippocampal sclerosis (HS). The purpose of this study was to implement and validate a dual-echo method for producing coronal T2 maps with complete coverage of the hippocampus and the rest of the brain on a 3T MR imaging scanner.
MATERIALS AND METHODS: T2 relaxation times were estimated on 10 occasions on 3 quality assessment Eurospin II (Diagnostic Sonar, Livingstone, Scotland) test objects with the use of conventional spin-echo (CSE), fast spin-echo, and fast recovery fast spin-echo (FRFSE) sequences on a 3T Excite MR imaging scanner (GE Healthcare, Milwaukee, Wis). Hippocampal T2 relaxation times were then measured in 15 healthy subjects and 20 subjects with clear-cut HS who were scanned at 1.5T with a previously validated dual-echo CSE sequence and 3T with an FRFSE sequence.
RESULTS: 3T FRFSE data were as reliable as CSE data at 1.5T. Reliability of hippocampal T2 measures was good on healthy volunteers and subjects with HS. FRFSE images were suitable for qualitative radiologic reporting and with complete brain coverage, so no additional T2-weighted sequences were required. There was good correlation between the 3T hippocampal T2 measurements and values obtained with the previously validated technique at 1.5T, with reliable identification of all of the subjects with HS.
CONCLUSIONS: T2 mapping with an FRFSE 30/80 sequence may be readily applied at 3T and can produce reliable T2 values in vivo with contiguous 5-mm sections and in a much reduced scan time of 3 minutes 1 second compared with 10 minutes 30 seconds for the CSE sequence at 1.5T.
Hippocampal sclerosis (HS) is the most common pathologic condition underlying intractable temporal lobe epilepsy; 70% of patients become seizure free with appropriate surgery. MR imaging is vital in the identification of HS and the determination of dual pathology or bilateral HS. Increased signal intensity on T2-weighted images is a feature of HS.1–3 Quantification is more sensitive and can determine whether the abnormality is unilateral or bilateral. Previously developed methods for in vivo measurement of T2 relaxation have been implemented on a 1.5T imager. A 16-echo sequence produced accurate T2 relaxation times but, because of the length of acquisition time, covered only a small area of the brain with a single 8-mm section through the hippocampus.4,5 A dual-echo conventional spin-echo (CSE) method with a slightly shorter acquisition time allowed whole brain coverage in 5-mm sections and provided a series of images that were acceptable for radiologic reporting, as well as the 2 data points necessary for a reproducible calculation of T2.6 T2 relaxometry can also be applied to the identification of neocortical abnormalities.7 In clinical practice, the important issue is reliable determination of T2 as normal or not in a reproducible manner. Our principal concern was to establish the normal range and reliability of the method of T2 measurement and that abnormalities detected on the previous method at 1.5T were detected by the new 3T method. Previous studies with dual-echo T2 have given good data for the evaluation of patients.8,9 More recently, multiecho T2 relaxometry techniques have been introduced at 3T.10
With the installation of a 3T Excite MR imaging scanner (GE Healthcare, Milwaukee, Wis), we decided to evaluate the improved fast spin-echo (FSE) options with respect to obtaining dual-echo data for the production of calculated T2 maps. Previous studies at 1.5T showed that the normal range and coefficient of variation of hippocampal T2 relaxation time were greater with the FSE sequences than with the CSE sequences.6 The use of an FSE sequence, however, would mean a shorter acquisition time, which would mean a shorter total examination time or would allow the addition of further imaging sequences to the routine epilepsy protocol.11
The previous study at 1.5T had shown that the measurements obtained using the 30/120 TE protocol had a superior test-retest and inter-rater reliability compared with the 30/80 protocol. Given that T2 changes little with field, we felt that we should evaluate the late echo time of 120 ms, as has been recommended at 1.5T. However, at 3T, we observed that there is increased signal intensity dropout at 3T at this longer echo time, and so we evaluated the echo time of 80 ms as well.12
Materials and Methods
Studies on Test Objects
Three Eurospin II (Diagnostic Sonar, Livingstone, Scotland) MR quality assessment test objects with nominal T2 relaxation times of 103, 121, and 203 ms were studied on 10 separate occasions over a 6-week period. The section thickness was 5 mm with no section gap. Six sequences were evaluated: a CSE sequence at 2000 ms/30, 120 ms (TR/TE), a CSE sequence at 2000 ms/30, 80 ms (TR/TE), an FSE sequence at 2000 ms/30, 120 ms (TR/TE), an FSE sequence at 2000 ms/30, 120 ms and 2000 ms/30, 80 ms (TR/TE), and a fast recovery FSE (FRFSE) at 2000 ms/30, 120 ms and 2000 ms/30, 80 ms (TR/TE). The CSE sequences had a matrix of 256 × 192 with full-phase FOV and NEX = 1. The FSE and FRFSE sequences had a matrix of 256 × 256 with a 75% FOV. To obtain test-retest reliability, we measured T2 twice in 12 MR quality assessment test objects with nominal T2 values with a range of 52–390 ms.13
Image data were transferred to a Sun Microsystems (Mountain View, Calif) workstation and converted to a variant of the University of North Carolina “/usr/image” format. Pixel-by-pixel T2 maps were calculated from the images by using the expression T2 = (TE2 − TE1)/[ln(S1/S2)], with TE1 and TE2, respectively.6,14
Evaluation of Measures of Hippocampal T2 Relaxation Time in a Control Population
Fifteen healthy subjects with no history of neurologic disease were scanned at 1.5T and 3T (7 women; age range, 26–64 years; median age, 44 years). Visual inspection of the MR images obtained in all of the subjects showed no indication of structural abnormalities; data were acquired from 30 hippocampi.
The sequence used at 3T had a 5-mm-section thickness and no section gap, obtained by interleaving 2 acquisitions to cover the entire brain with a 24-cm FOV. Scans were oriented in the oblique coronal plane in the same axis as the brain stem, orthogonal to the hippocampus, so as to minimize partial volume effects.4 All of the scans were performed with the middle section of the acquisition placed at the anterior border of the brain stem. This section positioning method was also used at 1.5T, which allowed for similar sections to be measured at both field strengths. The sequence used was an FRFSE 24 echo-train length 2000/30, 80 sequence, NEX = 1, 256 × 256 matrix with a 75% FOV giving a scan time of 3 minutes 1 second.
All of the control subjects had previously been scanned on a 1.5T scanner (EchoSpeed; GE Healthcare) with a CSE 2000/30, 120 sequence, NEX = 1, 192 × 256 matrix with a full FOV, for which the scan time was 10 minutes and 30 seconds.6
The median interscan duration was 5 months with a range of 4–6 months.
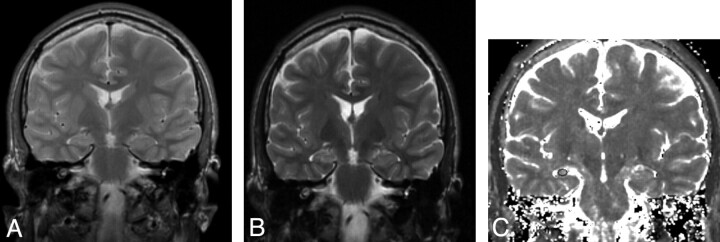
A calculated T2 map in the same orientation as the original scans was obtained in the same manner as the test objects. T2 maps were presented and regions of interest drawn with the Dispimage (University of London, London, UK) image display program.13 Regions were placed separately by 2 observers to establish inter-rater reliability and by 1 observer on 2 occasions to establish intrarater reliability. Elliptical regions of interest were placed within the hippocampus and were as large as the hippocampal anatomy would allow, typically 20 mm2. Careful attention was paid to avoid boundaries that would give rise to partial volume effects with CSF (Fig 1). T2 values were obtained in the 4 consecutive 5-mm coronal sections covering the hippocampi with the most posterior section being at the level of the posterior brain stem and in consecutive sections anterior to this. Data were averaged for each hippocampus. The whole process to obtain the T2 measurements took less than 5 minutes per case subject.
Fig 1.
Subject with unilateral right-sided HS (left of image).
A, FRFSE 30/80 early echo.
B, FRFSE 30/80 late echo.
C, FRFSE 30/80 T2 calculated image showing placement of region of interest.
Comparison of Hippocampal T2 Relaxation Times in Patients with Data Acquired Using the Previously Established Dual-Echo CSE Protocol
Twenty patients with temporal lobe epilepsy and HS in whom hippocampal T2 relaxation times had been measured to be above the healthy range by the previously established method at 1.5T with the dual-echo CSE sequence were rescanned at 3T with FRFSE. The interscan duration was a median of 57 months, (range, 12–115 months) in the patient group. The restudied patients did not have any episodes of serial seizures or status epilepticus in the intervening time, and one would not expect to see a change in hippocampal T2 over this interval in this population of patients.15 All of the subjects gave informed consent, and these studies were approved by the National Hospital for Neurology and Neurosurgery and Institute of Neurology Joint Medical Ethics Committee.
Statistics
Hippocampal T2 data were analyzed using SPSS 11 (SPSS, Chicago, Ill) on a personal computer. Test-retest reliability and inter-rater reliability were assessed on 70 hippocampi (30 from healthy subjects and 40 from patients with temporal lobe epilepsy) by calculating the coefficient of reliability.13 The coefficient of reliability is a stringent test of repeatability and is calculated as 2 times SD of the mean of the difference between 2 measures divided by the mean of both measures. Scatterplots and the Pearson correlation coefficient were used to compare the CSE-derived hippocampal T2 times acquired at 1.5T and the FRFSE-derived data acquired at 3T.
Results
Studies on Eurospin II Test Objects
Four of the 6 sequences used (CSE 2000/30, 120/1 and 2000/30, 80/1 and FRFSE 2000/30, 120/1 and 2000/30, 80/1) produced measures of T2 that were lower than the reference values quoted by the manufacturer of the Eurospin II MR quality assessment objects, and the other 2 sequences (FSE 2000/30, 120/1 and 2000/30, 80/1) produced measures that were much higher than those quoted by the manufacturer. The FRFSE sequence produced the measures closest to the manufacturer's values (Table). The test-retest coefficient of reliability was 3.3% (±1 SD). Accordingly, we decided to use the FRFSE 30/80 sequence for in vivo evaluation in healthy control subjects and patients with HS.
Estimations of T2 relaxation time in 3 Eurospin II MR quality assessment objects using a range of MR acquisition protocols
| Nominal Values of T2 | CSE |
FSE |
FRFSE |
|||
|---|---|---|---|---|---|---|
| 2000, 30/120 | 2000, 30/80 | 2000, 30/120 | 2000, 30/80 | 2000, 30/120 | 2000, 30/80 | |
| 103 ms | 88.8 (2.7) | 82.3 (2.9) | 128.5 (3.0) | 138.7 (3.4) | 99.4 (2.6) | 94.6 (1.8) |
| 121 ms | 100.8 (2.4) | 92.4 (3.8) | 161 (2.8) | 158.8 (2.6) | 114.3 (2.3) | 106.4 (1.8) |
| 203 ms | 207.1 (4.3) | 174.6 (6.5) | 369.8 (6.5) | 370.2 (6.7) | 235 (6.4) | 212.8 (2.7) |
Note:—CSE indicates conventional spin-echo; FSE, fast spin-echo; FRFSE, fast recovery fast spin-echo. Values are presented as mean (SD).
Test-Retest and Inter-Rater Repeatability Measures of Repeated Data Analysis with 30/80 FRFSE Sequence in Healthy Volunteers and Patients with Temporal Lobe Epilepsy
Data were available from 70 hippocampi, composed of left and right hippocampi in 15 healthy subjects and 20 patients with temporal lobe epilepsy and HS (9 left, 6 right, and 5 bilateral), all of whom had clear evidence of prolongation of hippocampal T2 demonstrated previously in the sclerotic hippocampi with a dual-echo CSE sequence at 1.5T. The mean (SD) for normal average hippocampal T2 times in healthy subjects using the 30/80 FRFSE sequence was 113 ms (1 ms). The upper limit of normal hippocampal T2, defined as the mean +2 SDs, was 115 ms; 118 ms was the highest recorded value in any individual 5-mm section. It was also noted that there is an anteroposterior gradient of T2 in control subjects and patients; T2 values were higher anteriorly than posteriorly, as had been shown at 1.5T.8
For intrarater reliability in healthy volunteers, the coefficient of reliability was 2.8% with the limit of agreement at 6 ms. The coefficient for reliability for inter-rater reliability was 3.8%, and the limit of agreement was 8.2 ms (2 SD of the mean difference).
In patients with temporal lobe epilepsy, the coefficient of reliability for test-retest was 3%, with a limit of agreement of 7.4 ms. For inter-rater reliability, the coefficient of reliability was 3%, with a limit of agreement of 7.6 ms.
Comparison of FRFSE Hippocampal T2 Relaxation Time Measurements Acquired at 3T with Data Acquired with a Dual-Echo CSE Sequence at 1.5T in Patients with Temporal Lobe Epilepsy
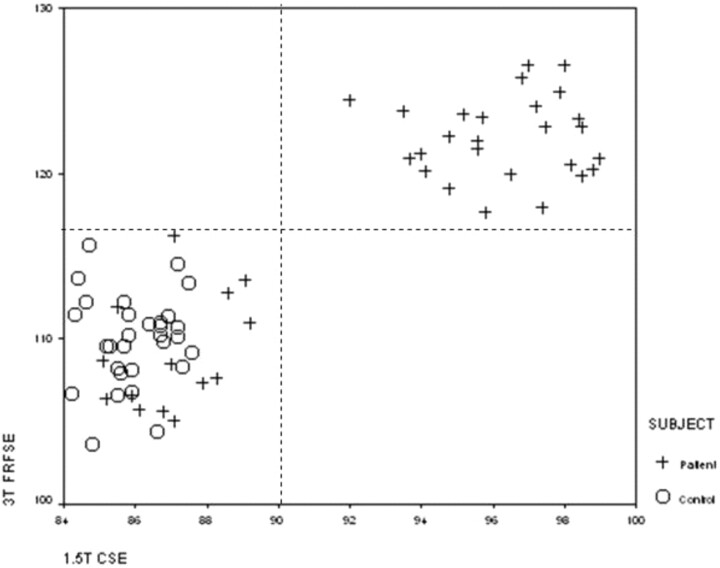
In 20 patients (40 hippocampi) with clear-cut HS, hippocampal T2 relaxation data were available from the dual-echo CSE sequence used previously at 1.5T and from the 30/80 FRFSE sequence at 3T. Normal ranges for the CSE sequence at 1.5T were a mean of 85.6 ms (SD, ±1.8 ms), with an upper limit of normal of 90 ms.6 The normal ranges for the sequences at 3T were clearly different. The FRFSE 30/80 T2 data were strongly correlated with the CSE data, with a Pearson correlation coefficient of 89.9% (P < .01; Fig 2).
Fig 2.
Scatterplot of calculated hippocampal T2 relaxation times obtained with 3T FRFSE 2000/30, 80 sequence (y-axis), and 1.5T CSE 2000/30,120 sequence (x-axis), in 15 healthy control subjects (30 hippocampi) and 20 patients with hippocampal sclerosis (40 hippocampi).
There was good agreement between the normal and abnormal data for the CSE 30/120 at 1.5T and the FRFSE 30/80 at 3T. If the 1.5T CSE hippocampal T2 relaxation times were abnormal (>90 ms), the 3T FRFSE T2 relaxation times were always abnormal (>118 ms).
Discussion
As in the previous dual-echo CSE study at 1.5T,6 the absolute accuracy of T2 measures was not a prime consideration. This was considered to be less important than the production of reliable and reproducible data that differentiated normal from abnormal hippocampi.
T2 relaxometry at 3T encounters some of the problems and limitations typically posed by imaging at this higher field strength. The high radio frequency (RF) power deposition rate of multisection FSE sequences can cause them to be limited by scanner safety features. We found that all of the sequences used in this study were not limited in this way and gave whole brain coverage. Another problem with 3T imaging is that the transmit RF field may not be as uniform as at 1.5T. We circumvented most of this problem by using the body coil of the scanner to provide the transmitted RF; some field-subject interaction may remain, but there is little evidence of this in our images. The 8-element array coil used to receive the signal intensity is rather less homogeneous, but receiver coil inhomogeneities will only be reflected in the S0 map (which is unused) and not in the T2 map.16
The sequences used a variety of TEs and echo trains, each of which will have its own potential for stimulated echoes. The purpose of this article was not to study all of these effects in detail but to find a sequence that provided reproducible estimates of T2. Modern scanners, such as our new 3T GE Healthcare machine, incorporate several enhancements to FSE train stability that were not available a decade ago when it was found that the CSE sequence gave more reliable T2 values; we suggest that this is one reason why it is now possible to use an FRFSE sequence to obtain reliable T2 maps. Note that RF pulses and sequences will vary from one scanner manufacturer to another, so the procedures described in this article should be followed to evaluate the best T2 measurement sequences on other scanners.
With the improved pulse sequences and signal to noise afforded by the increase in field strength to 3T, it was possible to produce data with a similar coefficient of variation of hippocampal T2 relaxation times by using an FSE technique rather than the CSE technique used at 1.5T. This had the advantage of a shorter acquisition time. The total acquisition time for CSE 30, 120 at 1.5T was 10 minutes 42 seconds and allowed only 28 5-mm sections, which did not always provide for complete brain coverage. The total acquisition time for the 30,80 FRFSE at 3T was 3 minutes 1 second and allowed for 32 5-mm sections, thus providing complete brain coverage in all of the subjects The FRFSE sequence uses a restoring 90° pulse (ie, one of opposite phase to the excitation pulse) to return the magnetization to the z-axis at the end of each TR. This greatly reduces the effect of T1 weighting on the sequence, allowing the use of a TR of 2 s, which is shorter than would normally be used at 3T, and giving the FRFSE sequence an advantage that the other sequences do not have. The FRFSE images were suitable for qualitative neuroradiologic interpretation, so no additional sequences were needed to quantify the hippocampal T2 signal intensity.
We were able to implement and validate a method for obtaining reliable quantitative T2 data by using a standard dual-echo FRFSE sequence on a standard 3T Excite MR scanner. Studies on standard test objects showed that the FSE sequences gave T2 measurements that were much higher than the expected values, but the FRFSE sequences gave values that were close to the expected values. The probable explanation for this is that the FRFSE returns the spin magnetization back to a near-equilibrium state, unlike the FSE, in which magnetization remains in the transverse plane. This residual magnetization can then cause cross-talk between the neighboring sections in regions of high T1, altering the observed T2 measurement. In vivo, this error could result in erroneous values in areas where there was significant partial volume with CSF.
The hippocampal T2 measures derived from FRFSE sequences showed good test-retest reliability in both patients and control subjects. Although the absolute values of hippocampal T2 measured with FRFSE at 3T were different from those obtained using CSE at 1.5T, there was an excellent correlation, and, most importantly, there was concordance of whether values were normal or abnormal, which is the prime consideration when using data clinically in an epilepsy surgery program to be able to identify subtle HS and bilateral HS.
Furthermore, the FRFSE sequence gives data that are suitable for visual interpretation of the images, as well as for quantification of the hippocampal T2 relaxation time, so no extra sequences need to be acquired, and the acquisition time is only 3 minutes 1 second for complete brain coverage.
Acknowledgments
We are grateful to Drs John Stevens and Brian Kendall for expert neuroradiologic advice and qualitative review of images. Grants from the Big Lottery fund and the Wolfson Trust support the National Society for Epilepsy MRI scanner.
Footnotes
M.R.S. was supported by the Wellcome Trust. S.L.F. was supported by the Medical Research Council. The National Society for Epilepsy supported the NSE MR imaging unit.
References
- 1.Jackson GD, Berkovic SF, Tress BM, et al. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 1990;40:1869–75 [DOI] [PubMed] [Google Scholar]
- 2.Berkovic SF, Andemann F, Olivier A, et al. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol 1991;29:175–82 [DOI] [PubMed] [Google Scholar]
- 3.Jackson GD, Berkovic SF, Duncan JS, et al. Optimising the diagnosis of hippocampal sclerosis using magnetic resonance imaging. AJNR Am J Neuroradiol 1991;14:753–62 [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson GD, Connelly A, Duncan JS, et al. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative T2 relaxometry. Neurology 1993;43:1793–99 [DOI] [PubMed] [Google Scholar]
- 5.Grünewald RA, Jackson GD, Connelly A, et al. MRI detection of hippocampal pathology in epilepsy: factors influencing T2 relaxation time. AJNR Am J Neuroradiol 1994;15:1149–56 [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan JS, Bartlett PA, Barker GJ. Technique for measuring hippocampal T2 relaxation time. AJNR Am J Neuroradiol 1996;17:1805–10 [PMC free article] [PubMed] [Google Scholar]
- 7.Rugg-Gunn FJ, Boulby PA, Symms MR, et al. Whole-brain T2 mapping demonstrates occult abnormalities in focal epilepsy. Neurology 2005;64:318–25 [DOI] [PubMed] [Google Scholar]
- 8.Woermann FG, Barker GJ, Birnie KD, et al. Regional changes in hippocampal T2 relaxation and volume: a quantitative magnetic resonance imaging study of hippocampal sclerosis. J Neurol Neurosurg Psychiatry 1998;65:656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okujava M, Schulz R, Ebner A, et al. Measurement of temporal lobe T2 relaxation times using a routine diagnostic MR imaging protocol in epilepsy. Epilepsy Res 2002;48:131–42 [DOI] [PubMed] [Google Scholar]
- 10.Briellmann RS, Syngeniotis A, Fleming S, et al. Increased anterior temporal lobe T2 times in cases of hippocampal sclerosis: a multi-echo relaxometry study at 3T. AJNR Am J Neuroradiol 2004;25:389–94 [PMC free article] [PubMed] [Google Scholar]
- 11.von Oertzen J, Urbach H, Blumcke I, et al. Time-efficient T2 relaxometry of the entire hippocampus is feasible in temporal lobe epilepsy. Neurology 2002;58:257–64 [DOI] [PubMed] [Google Scholar]
- 12.Schenck JF, Zimmerman EA. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed 2004;17:433–45 [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;8:307–10 [PubMed] [Google Scholar]
- 14.Plummer DL. Dispimage: a display and analysis tool for medical images. Rev Neuroradiol 1992;5:489–95 [Google Scholar]
- 15.Liu RN, Lemieux L, Bell GS, et al. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia 2005;46:1482–94 [DOI] [PubMed] [Google Scholar]
- 16.Boulby PA, Rugg-Gunn FJ. T2: the transverse relaxation time. In: Tofts PS ed. Quantitative MRI of the Brain: Measuring Changes Caused by Disease. Chichester, UK: John Wiley and Sons Ltd;2003