Abstract
SUMMARY: Histologic findings in 71 elastase-induced rabbit aneurysms embolized with platinum coils were retrospectively reviewed. Mature bone formation was found in 2 aneurysms, one with coils implanted for 3 months and the other with coils implanted for 1 year. We present the histologic findings and offer potential explanations for these observations. These findings may be relevant in understanding mechanisms of aneurysm healing after coil embolization.
Since the development of the Guglielmi detachable coil (Boston Scientific, Natick, Mass), platinum coil embolization has become widely accepted as a valuable alternative to open surgery for the treatment of intracranial aneurysms. To date, however, only a few human histologic samples are available for the study of the healing processes associated with coil therapy.1–5 A case report has been published detailing the chondroid matrix within a rabbit aneurysm after endovascular therapy.6 Animal models are widely used for preclinical studies for advancing the efficacy of endovascular devices and exploring the histologic response to the coil embolization.
Our group has developed a modified histologic technique for processing metallic coil-bearing tissue,7 which has been used to explore the cellular healing mechanism in response to coil embolization in various species.1 In this report, we discuss a retrospective review of 71 rabbit aneurysms treated with platinum coils in which 2 aneurysms demonstrated unexpected, mature bone formation within the aneurysm cavity.
The institutional animal care and use committee at this institution approved all of the procedures before the start of the study. Elastase-induced, saccular aneurysms were created in New Zealand white rabbits. Detailed procedures for this model have been described elsewhere.8 Aneurysms were embolized with platinum coils as described.9 At sacrifice, the aneurysms were removed and immediately fixed in 10% neutral-buffered formalin and processed for histologic examination as described previously.7 Two sections from each block were stained with hematoxylin-eosin (H&E) and Masson Trichrome.
Bone metaplasia was noted in 2 (3%) of 71 aneurysms. Subject 1 was harvested 3 months after platinum coil embolization. Subject 2 was harvested 1 year after embolization. Gross pathology examination showed that coils within the aneurysms were visible through the thin aneurysm wall. The coil loops at neck orifices of the 2 subjects appeared to be embedded within the thin, translucent, smooth membranous tissue.
Case Reports
Subject 1
Light microscopic examination revealed that the aneurysm dome was primarily filled with hypocellular, loose connective tissue. Localized, irregular attenuated connective tissue in the center of the dome, as well as along the neck, was noted, which contained densely packed collagen fibers.
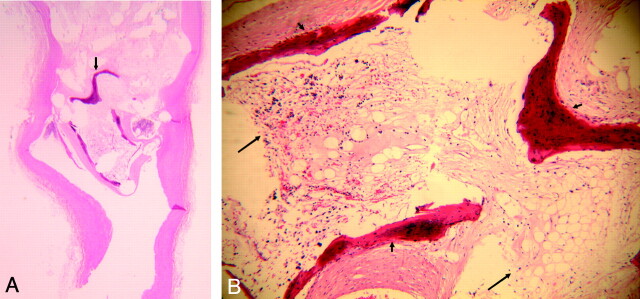
Near the neck area, several bony trabeculae were observed (Fig 1A, -B). A thin layer of flattened cells lined the bone trabeculae. Ovoid osteocytes in the cavelike lacunae were trapped within the formed bone matrix. A marrow cavity was also present, containing loose reticular connective tissue with various types of blood elements; single layers of cells lining thin-walled vessels; spindle-shaped stromal cells; and conspicuous, large adipose cells (Fig 1B). Dense connective tissue peripherally surrounding the bone trabeculae was in continuity with the periosteum and the marrow cavity.
Fig 1.
A, Lower-power view of H&E staining section of 3-month platinum coil embolized aneurysm, showing the mature bone formation near the neck area (arrow), (H&E, original magnification = ×30).
B, Higher-power view of formed bone in A, showing the bony trabeculae (short arrow) and bone marrow (long arrow), which contains loose reticular tissue with blood elements, thin-walled vessels, stromal cells, and adipose cells (H&E, original magnification = ×100).
Subject 2
Light microscopy showed that approximately two thirds of the aneurysm dome was filled with vascular, loose connective tissue. Dense, irregular connective tissue, which contained diffuse collagen deposition, occupied approximately another third of the dome.
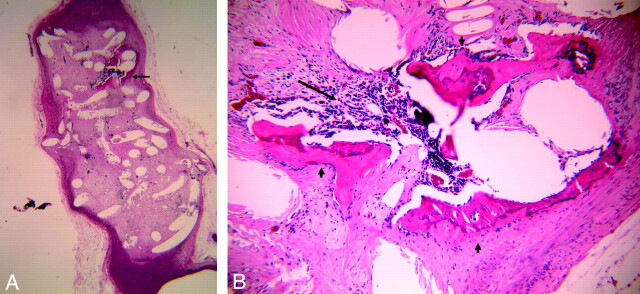
Localized bony trabeculae were noted within the aneurysm dome (Fig 2A, -B). The bony trabeculae were lined and covered with a thin layer of cells, probably representing osteoprogenitor cells (Fig 2B). Ovoid osteocytes in the cavelike lacunae were surrounded by bony matrix. One central canal with connective tissue was present within the bony matrix. A marrow cavity between trabeculae, containing various types of blood elements and blood sinusoids (Fig 2B), was also present. Dense connective tissue with abundant collagen fibers surrounded the bony trabecula. The connective tissue was in continuity with the marrow cavity and the periosteum.
Fig 2.
A, Lower-power view of H&E staining section of 1-year platinum coil embolized aneurysm, showing the localized formed bone and marrow within the aneurysm dome (arrow); (H&E, original magnification = ×15).
B, Higher-power view of formed bone in A, showing the bony trabeculae (short arrow) and bone marrow (long arrow), which contains various types of blood elements and blood sinusoids (H&E, original magnification = ×100).
Discussion
Platinum coils represent the first-generation materials for embolization of intracerebral aneurysms. Limited histopathologic data in coil-embolized aneurysms in humans and animal studies have suggested that platinum coils are biologically inert and fail to elicit a fibrotic response. This muted response has prompted numerous investigators to explore coil modifications. Histologic response to the coils has been the important parameter for evaluating the efficacy of new, modified endovascular devices.10 To date, thrombosis and loose or attenuated connective was the primary tissue type in all of the reports after coil embolization. Recently, Killer et al6 reported cartilage neoformation in aneurysms embolized with HydroCoils 1 year after coiling. To our knowledge, there are no reports demonstrating bone formation within aneurysm after coil embolization.
The current study found bone formation in 2 of 71 elastase-induced rabbit aneurysms after platinum coil embolization. Although a quantitative analysis comparing aneurysms with and without bone formation was not performed, it appears that the 2 cases with bony metaplasia also had markedly attenuated connective tissue within the aneurysm dome. This connective tissue was in continuity with the periosteum and the marrow cavity, indicating that the bone developed not from an endochondral ossification but rather from the connective tissue mesenchyme (intramembranous ossification). We hypothesize that the mesenchymal stem cells (MSCs) in the connective tissue differentiated into osteoprogenitor cells. These cells then differentiated into osteoblasts, which then secreted collagen and bony matrix.
The origin of these osteoprogenitor cells remains unclear. Improved understanding of the origin of these cells may be of fundamental importance in understanding the mechanism underlying the healing of aneurysms after coiling. Cells residing in aneurysm cavities after coiling may be derived from circulating progenitor cells trapped in the thrombus after embolization or they may be locally derived from the adjacent arterial wall. Recent studies suggest that MSCs may be found in numerous tissues beyond the bone marrow. These extramarrow MSCs are similar to bone marrow-derived stem cells, which can differentiate into different various cell lineages. MSCs may also be found in the arterial wall11 and may contribute to the bone formation in ectopic tissue. These MSCs also may enter the circulation, contributing to the population of circulating progenitor cells and engrafting other tissue.
The bone formation in one of our 2 cases was close to the aneurysm wall, whereas in the other case it was located at the neck area, close to the parent artery blood flow. It is difficult to determine the origin of these bone-forming cells (from the artery wall or from circulating cells) in the current study. Further study of the role of the progenitor cell in the aneurysm healing after coiling is ongoing our group.
Beyond understanding of cell lineage, these 2 cases of bone formation within aneurysms also raise the question about the role of cytokines in aneurysm healing. Numerous cytokines have been found to be the regulators of development and activation of bone cells during bone remodeling. An increase in the gene expression of tumor necrosis factor-α and osteopontin has been shown in the coiled aneurysm of our rabbit model (unpublished data). These data support the idea that these proteins may induce bone cell formation.
Footnotes
This work was supported by research grant NS42646 from the National Institutes of Health.
Data previously presented at: Annual Meeting of the American Society of Neuroradiology, May 1–5, 2006; San Diego, Calif.
References
- 1.Dai D, Ding Y, Danielson M, et al. Histopathologic and immunohistochemical comparison in human, rabbit, and swine aneurysms embolized with platinum coils. AJNR Am J Neuroradiol 2005;26:2560–68 [PMC free article] [PubMed] [Google Scholar]
- 2.Groden C, Hagel C, Delling G, et al. Histological findings in ruptured aneurysms treated with GDCs: six examples at varying times after treatment. AJNR Am J Neuroradiol 2003;24:579–84 [PMC free article] [PubMed] [Google Scholar]
- 3.Ishihara S, Mawad M, Ogata K, et al. Histopathologic findings in human cerebral aneurysms embolized with platinum coils: report of two cases and review of the literature. AJNR Am J Neuroradiol 2002;23:970–74 [PMC free article] [PubMed] [Google Scholar]
- 4.Castro E, Fortea F, Villoria F, et al. Long-term histopathologic findings in two cerebral aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:549–52 [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu S, Kurata A, Takano M, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. AJNR Am J Neuroradiol 1999;20:546–48 [PMC free article] [PubMed] [Google Scholar]
- 6.Killer M, Richling B, Minnich B, et al. Unexpected tissue engineering: cartilage neoformation in long-term HydroCoil®-occluded experimental aneurysms. Eur Cell Mater 2004;7:43 [Google Scholar]
- 7.Dai D, Ding Y, Danielson M, et al. Modified histologic technique for processing metallic coil-bearing tissue. AJNR Am J Neuroradiol 2005;26:1932–36 [PMC free article] [PubMed] [Google Scholar]
- 8.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol 2000;174:349–54 [DOI] [PubMed] [Google Scholar]
- 9.Kallmes D, Helm G, Hudson S, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217–22 [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Dai D, Lewis D, et al. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol 2005;26:1757–63 [PMC free article] [PubMed] [Google Scholar]
- 11.Abedin M, Tintut Y, Demer L. Mesenchymal stem cells and the artery wall. Circ Res 2004;95:671–76 [DOI] [PubMed] [Google Scholar]