Abstract
SUMMARY: MRA is emerging as an alternative to conventional catheter based angiography for the assessment of aneurysms after endovascular treatment. Short TE and contrast enhanced MRA techniques can be applied to optimize image quality. We review the available data regarding the application of MR for the assessment of cerebral aneurysms after endovascular therapy.
MR angiography (MRA) and CT angiography (CTA) are routinely used to diagnose cerebral aneurysms.1–4 In many instances, these same noninvasive data can be used to plan subsequent treatment. After treatment has been completed, the application of these powerful noninvasive imaging techniques becomes much more challenging owing to the interaction of the various therapeutic devices (ie, aneurysm clips, embolic coils, and/or stents) with the proton relaxation signal intensity or photon flux in MR imaging and CT, respectively, in the region of the treated aneurysm. The standard of practice has been to use catheter-based angiography5,6 for the evaluation of treated aneurysms. However, recent studies have suggested that noninvasive imaging techniques can be used to reduce, or in some cases eliminate, the need for follow-up conventional angiography.7–12
Neurovascular Imaging Following Coil Embolization
Endovascular therapy is increasingly being used as the primary treatment of ruptured and unruptured intracranial aneurysms. Results of the International Subarachnoid Aneurysm Trial (ISAT)13 indicate that for patients with ruptured intracranial aneurysms, those treated with endovascular coiling are more likely to survive and live independently than those treated with surgical clipping.14 The same study also demonstrated a small increase in the risk of rebleed after coiling in comparison with surgical clipping. This potential for rebleeding represents the primary shortcoming of endovascular aneurysm therapy. Manabe et al15 reported that incomplete aneurysm embolization or aneurysm recurrence after complete embolization is associated with a continued potential for aneurysm rupture (or rerupture).
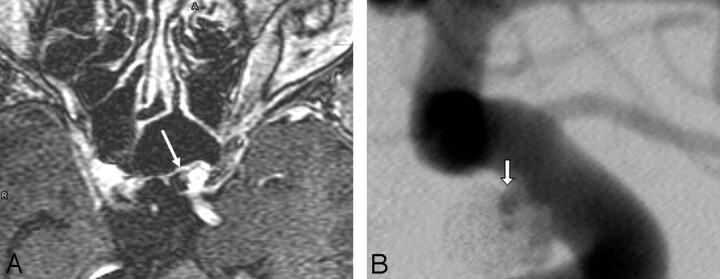
Aneurysm recurrence after coil embolization has been estimated to occur in anywhere from 10%–40% of patients and is dependent on multiple variables.16–22 Aneurysm recurrence refers to a new or increased area of patency within an aneurysm that has been previously treated. This process may be related to instability and subsequent compaction of the original coil mass or migration of the coil mass into intra-aneurysmal thrombus or into the fundus of a continually expanding aneurysmal sac (Fig 1). Occasionally, recurrence is the result of the growth of a new outpouching from a diseased parent vessel adjacent to the neck of the originally treated aneurysm with the recurrence projecting alongside the original coil mass. Although various factors have been associated with aneurysm recurrence, it is impossible to prospectively and accurately predict which aneurysms will recur and require retreatment.
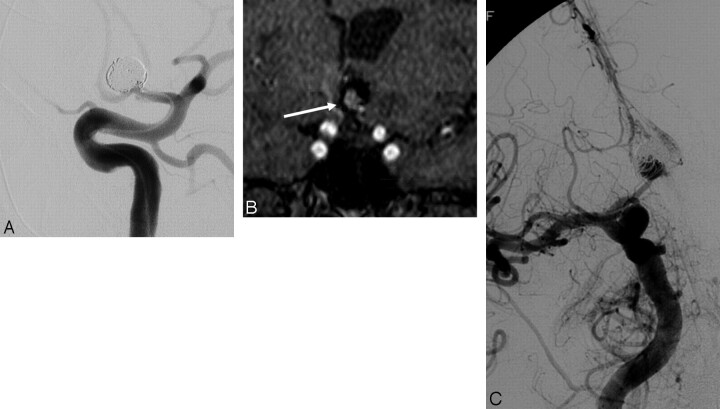
Fig 1.
A, Internal carotid angiogram demonstrates near-complete coil embolization of a large anterior communicating artery aneurysm. Follow-up short TE MRA was performed with MPR and MIP postprocessing. B, An image from the coronal MPR demonstrates a large recurrence (arrow) within the central aspect of the coil mass. A black signal-intensity void is distributed about the periphery of this central recanalization, indicative of the displaced and compacted coil mass that is now draped around the periphery of the recanalized pocket of central flow. Signal-intensity voids running though the central area of flow-related enhancement correspond to individual coil strands that bridge the recanalization. C, Correlative follow-up right internal carotid angiogram in a projection performed to replicate the coronal MPR confirms the large central recanalization with the coil mass displaced into the periphery of the fundus and a few individual coil strands bridging the recurrent aneurysm.
Although there is no established estimate of the risk associated with aneurysm recurrence or incomplete embolization and no accepted threshold for the severity of aneurysm recurrence that warrants retreatment, continued surveillance of aneurysms after coil embolization has become standard practice.23–26 Some investigators have recommended that at least 2 follow-up examinations be performed within the first year after treatment.17 Catheter-based digital subtraction angiography (DSA) is the most common technique used for this purpose. However, DSA is invasive and carries a small yet significant risk of neurologic morbidity.27–31 Serial follow-up DSA examinations could conceivably result in a cumulative morbidity and with time could erode the advantages of endovascular treatment. Minimizing morbidity related to angiographic surveillance is particularly important when analyzed within the context of the very low risk of rebleeding after successful coil embolization—estimated to be 0.11% per year in the recent Cerebral Aneurysm Rerupture After Treatment study32 and 0.21% per year in the ISAT study.14 Primarily for the aforementioned safety reasons but also for patient comfort, convenience, and overall cost containment, it would be optimal if surveillance imaging could be performed by using a noninvasive technique, rather than conventional angiography. MR imaging is particularly attractive for this purpose because it does not involve exposure to ionizing radiation and resultant images are minimally impacted by coil-induced artifact.
Available Noninvasive Imaging Techniques and Their Utility in the Assessment of Coiled Aneurysms
Platinum alloy coils are designed to allow optimum visibility during endovascular treatments performed under fluoroscopic control. However, the high attenuation of the subsequent endovascular coil mass causes marked beam hardening and streak artifact on CT and CTA, which typically obscures not only the aneurysm but also the adjacent parent and branch vessels as well as the surrounding brain parenchyma.33 On the other hand, these platinum alloys create relatively little distortion of the local magnetic field and, therefore, cause a much less dramatic disruption of the MR imaging/MRA signal intensity. In fact, it is highly unusual for a coil mass to cause any significant degradation of the MR imaging signal intensity from the brain parenchyma immediately adjacent to a treated aneurysm. As such, noninvasive imaging surveillance after coil embolization has been essentially limited to MRA techniques.
MRA Techniques for the Evaluation of Coiled Aneurysms: Efficacy
A number of small studies have investigated the utility of MR imaging for follow-up after coil embolization. Despite the application of different MRA techniques, most investigators have reported sensitivity and specificity rates for the detection of residual aneurysms between 90% and 100%, respectively.7–12,34 For example, Westerlaan et al34 reported a positive predictive value for demonstrating residual aneurysms of 89% and a negative predictive value for the exclusion of neck remnants and residual flow of 95% and 100%, respectively. In a series of 26 patients, Brunereau et al9 reported a positive predictive value of 100% and a negative predictive value of 96% for the detection of residual flow within the coil mass. Gaurvit et al35 reported that contrast-enhanced MRA (CE-MRA) successfully identified all recanalizations in a series of 48 aneurysms evaluated by both DSA and MRA during 12 months. Leclerc et al36 reported a 100% sensitivity of CE-MRA for the identification of residual aneurysm filling at 1 year. In most studies, regardless of the MRA technique used, the aneurysm remnants missed on MRA were very small (<2 mm) and in most cases would not necessarily have warranted retreatment.8,37 However, Farb et al38 reported that 3 larger aneurysm remnants measuring between 4 and 5 mm were missed when a time-of-flight (TOF)-MRA sequence was used alone. At the same time, all recurrences >3 mm were detected on a CE-MRA sequence used in the same study. Our experience would also be commensurate with the literature indicating that most treatable aneurysm remnants can be visualized with reasonable confidence by using high-resolution TOF-MRA or CE-MRA (Fig 2).
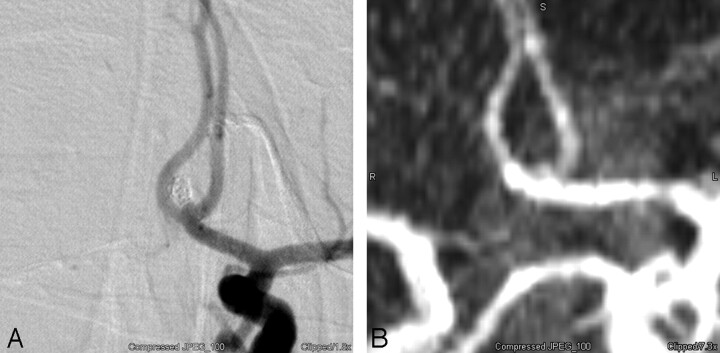
Fig 2.
A, Conventional left internal carotid angiogram demonstrates a tiny 1- to 2-mm residual neck at the base of a coiled anterior communicating artery aneurysm. B, Correlative MIP projection from a TOF-MRA accurately demonstrates this tiny residual.
In some series and in our own experience, MRA data were also found to be complimentary to those of DSA, demonstrating small aneurysm residuals that were not appreciated on the initial conventional angiographic views but were found after MR imaging correlation (Fig 3) and additional angiographic investigation/evaluation.12,38 When evaluating the various studies that have attempted an MRA-DSA comparison, one should consider that the gold standard of DSA is also an imperfect test. The radiopaque coil mass can, in some projections, completely obscure a small recanalization that is evident on MRA. This issue is particularly important if the working angles used for the original coiling procedure are not meticulously reproduced at follow-up angiography.
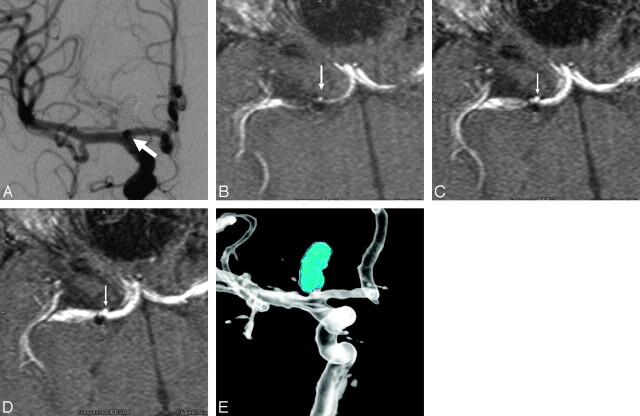
Fig 3.
A, Conventional right internal carotid angiogram depicts a coil mass within a right carotid terminus aneurysm. A tiny (2-mm) residual aneurysm (arrow) is evident only as a double attenuation overlapping the parent vessel. On the other A-plane angiographic views, this small residual projected over the coil mass and was obscured. On the lateral projections, the residual was obscured by overlying anterior cerebral artery and middle cerebral artery branches. B–D, Correlative TOF-MRA source images demonstrate that the tiny residual arises from the anterior aspect of the aneurysm neck and projects superiorly along the anterior aspect of the coil mass (arrows). In retrospect, it can be appreciated just how this small residual would be obscured by either the coil mass or adjacent vessels on the most angiographic projections. E, An image from a rotational angiogram with the coil mass attenuation mapped to a blue color provides additional evidence of the tiny residual at the base of the coil mass, projecting superiorly from the anterior aneurysm neck.
MRA Techniques for the Evaluation of Coiled Aneurysms: Limitations and Technical Points
There are several challenges associated with the MRA follow-up of coiled aneurysms. These include the following: 1) artifacts related to distortion of the regional B0 field by the coil mass (ie, susceptibility artifacts); 2) dynamic eddy currents associated with the coil mass; 3) spin dephasing due to complex flow within the residual aneurysm and tortuous parent vascular segments; 4) slow flow within partially occluded aneurysm remnants with subsequent spin saturation; 5) long acquisition times (particularly for unenhanced 3D TOF scans), which increase the probability of patient motion artifacts; and 6) the recently described risk of nephrogenic systemic fibrosis associated with the administration of gadolinium in patients with compromised glomerular filtration rates.39
Even under optimal conditions, the evaluation of postcoiling MRA can be difficult, particularly for inexperienced readers. The coil mass appears as a region of signal-intensity void and can be difficult or impossible to perceive on the MR imaging source and reconstructed images. The anatomic relationship of the aneurysm with respect to the parent vessel may be complex and difficult to understand on MRA source images. Several procedural and technical aspects of imaging and image interpretation have evolved that may improve the utility of MRA for the follow-up of coiled aneurysms.
Image Interpretation.
An accurate interpretation of the posttreatment MR image requires a familiarity with the pretreatment and immediate postembolization conventional angiograms12 (Fig 4). A review of the images from the initial treatment provides the reader with a 2D projection of the 3D anatomy, demonstrating the orientation of the coil mass and aneurysm neck with respect to the parent vessel. Once this anatomy is understood on the angiograms, the interpreter can carefully scrutinize the MR imaging data with attention directed specifically to the aneurysm neck-parent vessel interface (the potential site of aneurysm residual or recurrence).
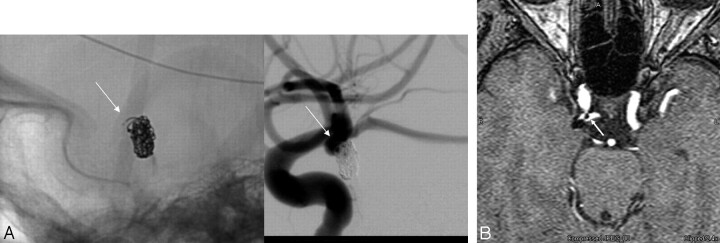
Fig 4.
A, Conventional angiogram demonstrates a small right posterior communicating artery aneurysm after coil embolization. The unsubtracted image (left) depicts a tail of coil projecting anteriorly (arrow). The subtracted image (right) demonstrates that this coil tail projects toward the parent artery from which the aneurysm arises. A small amount of filling between the interstices of the coil mass is also noted along the anterior aspect of the aneurysm neck. B, Correlative TOF-MRA source image demonstrates the coil mass as a signal-intensity void. A linear signal-intensity void (arrow) projects anteriorly into the parent posterior communicating artery corresponding to the coil tail visualized on the native image. The small pocket of residual filling is also appreciated along the lateral aspect of the coil mass. Reading the MRA without the posttreatment angiographic correlation could lead to confusion with respect to the interpretation of these structures in the region of the aneurysm neck. A correlation of the 2 techniques provides a detailed and complimentary understanding of the anatomy in this region.
The primary interpretation of the MRA should be based on the source images. Source data are complemented by multiplanar reformations (MPRs) and maximum intensity projection (MIP) images. Given the added value of data postprocessing, one should perform interpretation of these studies on a 3D workstation capable of real-time postprocessing by the interpreter. Depending on institutional workflow, this 3D workstation may be separate or embedded within the organizational PACS system. MIPs alone frequently lack the sensitivity required to demonstrate small aneurysm remnants, and they should not be interpreted in the absence of source data. At the same time, when large residuals are present, the optimal angiographic projections for retreatment can often be prospectively approximated by using the MIPs.
Short Echo Time Imaging.
The shortest possible echo time (TE) is a key parameter in reducing coil-associated artifact. Most intracranial MRA examinations use a TE in the range of 3–7 ms. MRA techniques that use TE values >5 ms have been shown to result in artifact of approximately 1–2 mm in the vicinity of the coil mass. Such artifacts are related to a distortion of the local B0 field (susceptibility artifact) plus dynamic eddy currents around the coil mass.40 Although this degree of artifact is not important with respect to the visualization of the surrounding brain parenchyma, any signal-intensity void could potentially obscure the identification of a small, but possibly significant, and potentially retreatable residual aneurysm. The use of short TEs (<2.5 ms) reduces the signal-intensity loss associated with the coil mass41,42 and thereby improves the visualization of any remaining intra-aneurysmal flow as well as flow within the adjacent parent vessel or branches.10,43 Gonner et al43 compared the artifacts generated by coil masses by using 2 different MRA protocols: 1 with a conventional TE (6 ms) and 1 with a short TE (2.4 ms). These investigators found that with the short TE sequences, overestimation of the diameter of the coil mass on MRA was significantly reduced, and in 36%, visualization of the adjacent parent vessel was improved. Walker et al44 found that increasing TE values created increasing volume overestimations at both 1.5T and 3T when imaging a coil mass within an in vitro model. They concluded that reducing the TE was the main factor in improving perianeurysmal visualization.
Readout Bandwidth.
At our institutions, we double the default receiver bandwidth for these studies. This modification reduces the amount of time during which echo sampling occurs, thus minimizing the spatial distortion attributable to susceptibility effects. However, this is achieved at the expense of a reduced signal-to-noise ratio. This trade-off may degrade image quality, particularly when a noncontrasted TOF-MRA technique is used.
Field Strength.
Although higher field strengths provide a better signal-to-noise ratio and background suppression, they also result in greater susceptibility artifact. Walker et al44 demonstrated that coil volume overestimation artifacts increased with imaging at 3T in comparison with 1.5T. At 3T, the impact of increased coil-packing attenuation on volume overestimation was also exacerbated. At the same time, these effects were most important when a 3D-TOF sequence was used and considerably less detrimental when a contrast-enhanced fast gradient-echo MRA technique was used (TE, 1.24 ms). The authors concluded from these data that a contrast-enhanced fast gradient technique at 3T might be optimal for posttreatment imaging.
At this time, very little clinical data exist regarding the use of 3T MRA for the evaluation of coiled aneurysms. Majoie et al45 evaluated 20 consecutive patients with 21 coiled aneurysms and found that 3T MRA depicted aneurysm remnants accurately and with minimal imaging artifacts. In this small series of patients, contrast administration provided no additional value.
CE-MRA.
Turbulent flow with intravoxel dephasing and/or slow flow with subsequent spin saturation can result in significant signal-intensity loss on TOF-MRA. Turbulence and complex flow patterns are not only a problem within the aneurysm but also within tortuous vascular segments, such as the cavernous and supraclinoid segments of the internal carotid artery (ICA). Aneurysms arising from these vascular segments can be particularly difficult to assess after treatment, given the additive effects of coil-induced susceptibility artifact and dephasing related to complex flow within both the aneurysm and adjacent parent vessel (Fig 5).
Fig 5.
A, Axial source image from a contrast-enhanced MRA unambiguously demonstrates a tiny (<2-mm) residual superior hypophyseal-region aneurysm (arrow). B, Correlative left internal carotid conventional angiogram shows that this tiny pocket of residual filling (arrow) is surrounded by strands of coil.
Signal-intensity loss related to slow or complex flow can largely be overcome with a contrast-enhanced MRA technique. However, this is achieved at the expense of venous contamination and possibly diagnostic uncertainty related to T1-hyperintense thrombus and perianeurysmal enhancement. Uncertainty related to venous contamination can largely be mitigated by a detailed understanding of the anatomy of the aneurysm neck and its relationship to the parent vessel. This information can typically be gleaned from a review of the initial treatment angiograms. However, venous contamination remains a significant problem with aneurysms near the skull base, particularly those involving the cavernous and paraclinoid segments of the carotid artery.
In large or giant aneurysms, uncertainty related to intra-aneurysmal T1 hyperintense thrombus is a problem on both enhanced and unenhanced MRA sequences. This uncertainty can often be overcome to some degree by correlating the precontrast MR imaging with the CE-MRA. Although T1 shine through within regions of thrombosis often creates hyperintensity on TOF images, which could be confused with flow-related enhancement, a marked increase in intensity in the same region after contrast administration suggests that this signal intensity is related to true aneurysm filling rather than thrombus. Diffuse homogeneous enhancement of intra-aneurysmal thrombus, to the point that the thrombus becomes as hyperintense as the enhanced blood pool, is unusual. We perform both TOF-MRA and postcontrast imaging, and frequently, these issues of thrombus versus flow can be resolved by comparing the 2 sequences. Finally, correlation of the baseline posttreatment MRA examination with the immediate posttreatment DSA is frequently helpful in definitively proving that the T1 hyperintense region in question within the aneurysm demonstrated no filling with contrast on DSA.
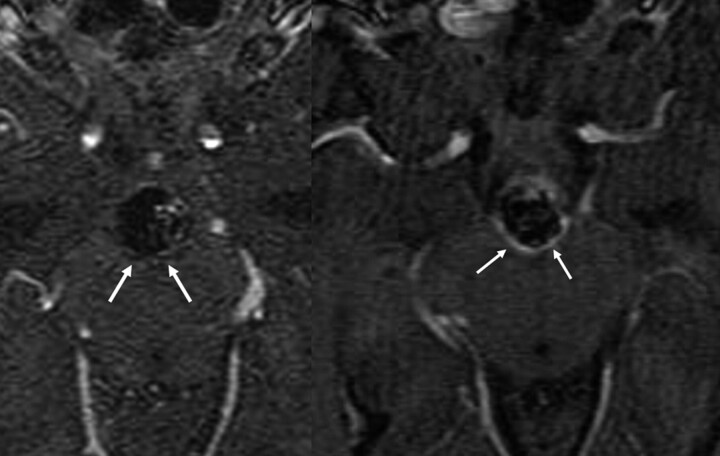
Contrast enhancement around the periphery of the aneurysm is thought to be attributable to some combination of organized peripherally distributed intra-aneurysmal thrombus, vasa vasorum within the adventitial layer of the aneurysm wall, and/or the ingrowth of vascularized tissue about the coil mass due to inflammation or healing.35 Thus, a thin linear rim of peripheral/circumferential contrast enhancement around the coil mass is commonly seen at CE-MRA follow-up and should be recognized as an expected finding (Fig 6). Aneurysm recanalization almost always occurs as a discrete pocket of flow-related enhancement or contrast enhancement in the region of the aneurysm neck projecting into or around 1 side of the coil mass. Circumferential enhancement around the entire coil mass would be a highly unusual appearance for aneurysm recanalization. In addition, correlation with a TOF-MRA typically verifies that this finding represents contrast enhancement rather than flow. Any persisting uncertainty should be further evaluated by using catheter-based angiography.
Fig 6.
Axial source images from a contrast-enhanced MR image obtained immediately after treatment (left image) demonstrate no enhancement about the posterior aspect of a large basilar aneurysm coil mass (arrows). Follow-up imaging performed 6 weeks later (right image) demonstrates the interval development of a thin rim of enhancement marginating the posterior aspect of the coil mass (arrows). This pattern of enhancement is not representative of recanalization but more likely of mural enhancement as a sequela of the in-growth of granulation tissue about the periphery of the coil mass.
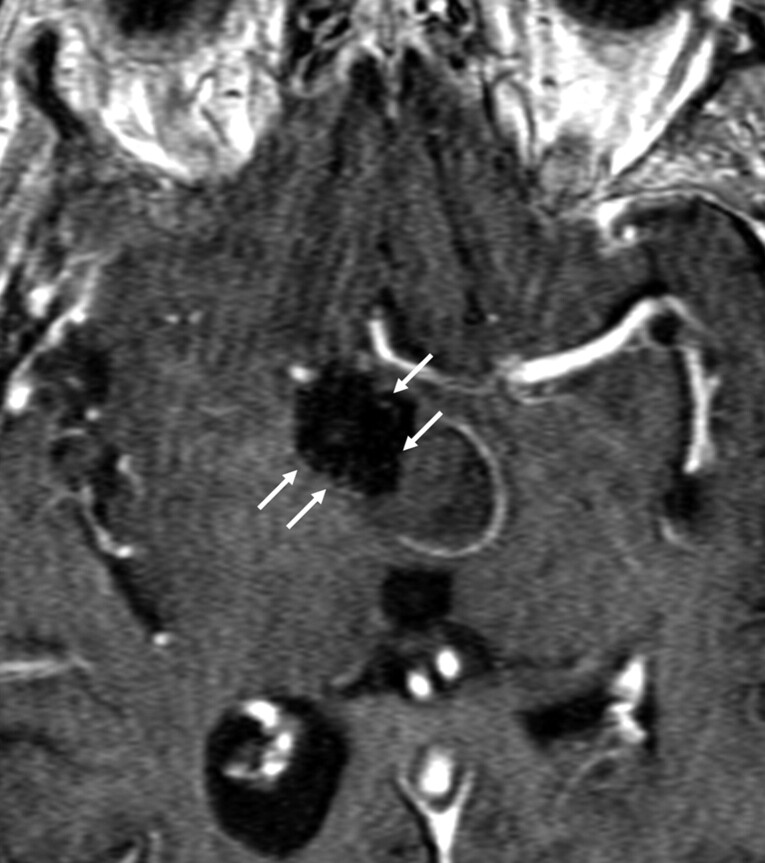
It is also common to observe multiple scattered hyperintense pixels, particularly on CE-MRA, distributed within the coil mass. Again, it is not clear whether these indicate flow within the interstices of the coil mass, small foci of T1 shinethrough from hyperintense thrombus, or enhancing scar tissue (Fig 7). The failure of conventional angiography to demonstrate correlative intra-aneurysmal flow in these cases could easily be secondary to subtraction artifact from the attenuated platinum coil mass, so it is difficult to draw conclusions as to the etiology of this finding when angiography is unrevealing. At the same time, the absence of a confluent pocket of residual filling precludes further treatment. Thus, prognostic value of these tiny foci of interstitial enhancement is currently unknown and probably has relatively little immediate clinical significance.
Fig 7.
CE-MRA image following coil embolization of a large right ICA posterior wall aneurysm. Arrows depict several scattered pixels of hyperintensity distributed within the signal-intensity void of the coil mass. Although commonly observed, the etiology and significance of these tiny foci of hyperintensity within the coil mass remain unclear.
Recently, the added concern of contrast-induced nephrogenic systemic fibrosis has introduced a mental pause in the carefree attitudes toward gadolinium contrast administration in patients with renal impairment. Conceivably, future use of high-field-strength 3T systems, incorporating short TEs, may reduce the need for contrast in the work-up of coiled aneurysms.
Contrast-Versus-Noncontrast MRA
Few studies have directly compared contrast-enhanced MRA with traditional 3D TOF-MRA for the evaluation of coiled aneurysms. Cottier et al46 compared contrast-enhanced MRA with unenhanced MRA and found that contrast enhancement was only beneficial in the evaluation of giant aneurysms. However, other studies comparing the 2 techniques demonstrated improvement in lesion detection with intravenous contrast. Leclerc et al36 compared TOF and gadolinium-enhanced MRA and found that the contrast-enhanced technique had a higher sensitivity for the detection of neck remnants in a series of 20 patients (only 5 of whom had aneurysm recurrences). Farb et al38 similarly observed greater sensitivity of CE-MRA in comparison with TOF-MRA in a series of 28 patients. Pierot et al37 also found that CE-MRA provided better visualization of residual and recurrent aneurysms than TOF-MRA with less artifact. However, they did not find that this improved visualization was manifest in terms of a greater sensitivity for the detection of recurrent aneurysms and concluded that 3D TOF-MRA was also a valid and useful technique for surveillance of coiled aneurysms. In our experience, TOF-MRA without contrast is generally accurate and closely correlates with the findings of contrast-enhanced techniques. However in several cases, contrast enhancement aided the visualization of small remnants and uncovered a larger neck remnant or filling of the coil pack that was not anticipated on the noncontrast MRA technique. For this reason, we continue to use CE-MRA along with TOF-MRA in the evaluation of coiled aneurysms.
MRA Techniques for the Evaluation of Coiled Aneurysms: Recommended Follow-Up Protocol
At our institutions, MRA has supplanted many of the conventional angiographies that would have otherwise been performed for the follow-up of treated aneurysms. The decision to perform follow-up conventional angiography is most often made on a patient-by-patient basis. In those patients who present specific technical challenges or who are at higher risk of adverse events from conventional angiography (eg, renal insufficiency, contrast allergy, or severe diffuse atheromatous disease), MRA is typically performed as the only follow-up examination.
The initial MR imaging study is performed in close temporal proximity to the initial treatment (24–48 hours). This study provides a direct correlate with the immediate post-treatment DSA and provides a baseline examination for subsequent comparison with serial MRA studies. Any residual filling of the aneurysm identified at the conclusion of the initial procedure on DSA can be directly correlated with these immediate posttreatment MRA data and targeted for follow-up. A side-by-side comparison of the immediate posttreatment MRA with subsequent follow-up MRAs is easier and more straightforward than the comparison of the immediate posttreatment angiogram with a subsequent follow-up MRA, particularly for the purpose of accurately measuring and assessing small increases in the size of aneurysm residuals. In addition, the immediate posttreatment MRA indicates whether MR imaging can be used effectively as a follow-up technique in the future. This is particularly important in those patients who undergo embolization supported by an adjunctive intravascular stent. In some vascular locations, the stent results in significant signal-intensity loss within and immediately adjacent to the stented segment, likely largely related to stent-induced radio-frequency shielding.47,48 It is difficult to predict which stent-coil constructs will elicit enough artifact to interfere with posttreatment imaging. In some cases, stent artifact obscures the aneurysm to the degree that MRA is ineffective for use in follow-up. If no baseline imaging is performed, signal-intensity loss within the stent on a follow-up MRA may be misinterpreted as the development of in-stent stenosis. Similarly, stent-induced signal-intensity loss within the adjacent coil mass may be erroneously interpreted as complete occlusion of the treated aneurysm. Correlation of the posttreatment angiogram with an immediate posttreatment MR image can avoid these errors and will immediately identify those patients who are in need of conventional angiography for subsequent follow-up.
Our evolving schedule for aneurysm follow-up currently consists of an immediate posttreatment MRA. In those patients in whom the MRA-DSA data correlate adequately, further follow-up consists of MRA at 3–6 months, 12–15 months, and 24–36 months. We had initially been performing follow-up conventional angiography concurrently with either the 3- to 6- month or 12- to 15-month follow-up MRA to ensure continued concordance between the techniques, but as we have gained more experience and confidence in the MRA data, this has become less frequent.
In those patients in whom the initial baseline MRA-DSA correlation is thought to be unreliable (eg, artifact related to the presence of aneurysm clips from prior surgery, aneurysms with particularly complex anatomy, or after the placement of a complex treatment construct involving both stents and coils), follow-up is performed primarily by using conventional angiography. Small aneurysm recurrences identified by MRA in the course of serial follow-up are usually investigated by conventional angiography to verify the diagnosis and ensure an accurate estimation of the size. In patients not requiring retreatment, these small recurrences may be subsequently followed noninvasively. In patients with larger recurrences on MRA, consent is often prospectively obtained in the clinic for both confirmatory angiography and aneurysm recoiling. Follow-up angiography is then performed with the patient under general anesthesia with the anticipated retreatment performed at the same time. This sequence facilitates a much more efficient mechanism for patient management and the use of anesthesia services.
We use a short TE (<2.5 ms) MRA sequence with both unenhanced and contrast-enhanced techniques on a 1.5T scanner (Table). All postcoiling MRAs are evaluated along with the catheter-based angiography performed at the time of coil embolization as well as the baseline posttreatment MRA study. Serial studies are compared directly with the baseline MRA with reference to the treatment DSA made to facilitate an understanding of the anatomy of the aneurysm-parent vessel complex. All images are evaluated using 3D software either integrated with the PACS system or as part of an imaging workstation. All presentations of the data, including the axial source data as well as MPR, MIP, and volume-rendered presentations, are evaluated. Images are also reviewed in conjunction with the treating neurointerventionalist whenever possible.
MRA protocol
| Routine MRA | Coiled Aneurysm |
||
|---|---|---|---|
| Ultrashort TE TOF MRA | Contrast-Enhanced MRA | ||
| Technique | 6-Slab MOTSA | 3-Slab MOTSA | 3D SPGR single volume |
| Flow comp | ON | OFF | OFF |
| BW | 15 kHz | 32 kHz | 32 kHz |
| Minimal TE | 3.7 ms | ∼2 ms | ∼2 ms |
| TR | 33 ms | 33 ms | 14 ms |
| Flip angle | 20° | 20° | 30° |
| Section thickness | 1.4 mm | 1.4 mm | 1.4 mm |
| F × P | 320 × 192 | 384 × 244 | 512 × 256 |
Note:—MOTSA indicates multiple overlapping thin-slab acquisition; TR, repetition time; SPGR, spoiled gradient-recalled-echo; Flow comp, flow compensation; BW, bandwidth; F, frequency; P, phase.
Conclusions
MRA is emerging as an alternative to conventional catheter-based angiography for the assessment of aneurysms after endovascular treatment. Short TE and CE-MRA can be applied to optimize this technique for this purpose. Conventional angiography remains the gold standard for the evaluation of treated aneurysms and should be liberally used to resolve any cases of diagnostic uncertainty on the noninvasive imaging.
References
- 1.Adams WM, Laitt RD, Jackson A. The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol 2000;21:1618–28 [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas SW, Sheppard L, Goldberg HI, et al. Intracranial aneurysms: detection and characterization with MR angiography with use of an advanced postprocessing technique in a blinded-reader study. Radiology 1997;203:807–14 [DOI] [PubMed] [Google Scholar]
- 3.Horikoshi T, Fukamachi A, Nishi H, et al. Detection of intracranial aneurysms by three-dimensional time-of-flight magnetic resonance angiography. Neuroradiology 1994;36:203–07 [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Masaryk TJ, Modic MT, et al. Intracranial aneurysms: evaluation by MR angiography. AJNR Am J Neuroradiol 1990;11:449–55 [PMC free article] [PubMed] [Google Scholar]
- 5.Kang HS, Han MH, Kwon BJ, et al. Postoperative 3D angiography in intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:1463–69 [PMC free article] [PubMed] [Google Scholar]
- 6.Sugahara T, Korogi Y, Nakashima K, et al. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002;23:1545–52 [PMC free article] [PubMed] [Google Scholar]
- 7.Anzalone N, Righi C, Simionato F, et al. Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2000;21:746–52 [PMC free article] [PubMed] [Google Scholar]
- 8.Boulin A, Pierot L. Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital subtraction angiography. Radiology 2001;219:108–13 [DOI] [PubMed] [Google Scholar]
- 9.Brunereau L, Cottier JP, Sonier CB, et al. Prospective evaluation of time-of-flight MR angiography in the follow-up of intracranial saccular aneurysms treated with Guglielmi detachable coils. J Comput Assist Tomogr 1999;23:216–23 [DOI] [PubMed] [Google Scholar]
- 10.Derdeyn CP, Graves VB, Turski PA, et al. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. AJNR Am J Neuroradiol 1997;18:279–86 [PMC free article] [PubMed] [Google Scholar]
- 11.Kahara VJ, Seppanen SK, Ryymin PS, et al. MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow-up of intracranial aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:1470–75 [PMC free article] [PubMed] [Google Scholar]
- 12.Weber W, Yousry TA, Felber SR, et al. Noninvasive follow-up of GDC-treated saccular aneurysms by MR angiography. Eur Radiol 2001;11:1792–97 [DOI] [PubMed] [Google Scholar]
- 13.Molyneaux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trail (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 14.Molyneaux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 15.Manabe H, Fujita S, Hatayama T, et al. Re-rupture of coil-embolized aneurysm during long-term observation: case report. J Neurosurg 1998;88:1096–98 [DOI] [PubMed] [Google Scholar]
- 16.Byrne JV, Sohn MJ, Molyneaux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 17.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–56 [DOI] [PubMed] [Google Scholar]
- 18.Hodgson TJ, Carroll T, Jellinek DA. Subarachnoid hemorrhage due to late recurrence of a previously unruptured aneurysm after complete endovascular occlusion. AJNR Am J Neuroradiol 1998;19:1939–41 [PMC free article] [PubMed] [Google Scholar]
- 19.Hope JK, Byrne JV, Molyneux AJ. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. AJNR Am J Neuroradiol 1999;20:391–99 [PMC free article] [PubMed] [Google Scholar]
- 20.Malisch TW, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176–83 [DOI] [PubMed] [Google Scholar]
- 21.Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg 1989;70:556–60 [DOI] [PubMed] [Google Scholar]
- 22.Todd NC. Aneurysm rebleeding after treatments that leave the aneurysm sac patent. Br J Neurosurg 1990;4:373–79 [DOI] [PubMed] [Google Scholar]
- 23.McDougall CG, Halbach VV, Dowd CF, et al. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393–99 [DOI] [PubMed] [Google Scholar]
- 24.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 25.Vanninen R, Koivisto T, Saari T, et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology 1999;211:325–36 [DOI] [PubMed] [Google Scholar]
- 26.Kuether TA, Nesbit GS, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery 1998;43:1016–25 [DOI] [PubMed] [Google Scholar]
- 27.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401–07 [PMC free article] [PubMed] [Google Scholar]
- 28.Leffers AM, Wagner A. Neurologic complications of cerebral angiography: a retrospective study of complication rate and patient risk factors. Acta Radiol 2000;41:204–10 [DOI] [PubMed] [Google Scholar]
- 29.Warnock NG, Gandhi MR, Bergvall U, et al. Complications of intraarterial digital subtraction angiography in patients investigated for cerebral vascular disease. Br J Radiol 1993;66:855–58 [DOI] [PubMed] [Google Scholar]
- 30.Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology 1992;182:243–46 [DOI] [PubMed] [Google Scholar]
- 31.Dion JE, Gates PC, Fox AJ, et al. Clinical events following neuroangiography: a prospective study. Stroke 1987;18:997–1004 [DOI] [PubMed] [Google Scholar]
- 32.CARAT Investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke 2006;37:1437–42 [DOI] [PubMed] [Google Scholar]
- 33.Masaryk AM, Frayne R, Unal O, et al. Utility of CT angiography and MR angiography for the follow-up of experimental aneurysms treated with stents or Guglielmi detachable coils. AJNR Am J Neuroradiol 2000;21:1523–31 [PMC free article] [PubMed] [Google Scholar]
- 34.Westerlaan HE, van der Vliet AM, Hew JM, et al. Time-of-flight magnetic resonance angiography in the follow-up of intracranial aneurysms treated with Guglielmi detachable coils. Neuroradiology 2005;47:622–29 [DOI] [PubMed] [Google Scholar]
- 35.Gaurvit JY, Leclerc X, Pernodet M, et al. Intracranial aneurysms treated with Guglielmi detachable coils: usefulness of 6-month imaging follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol 2005;26:515–21 [PMC free article] [PubMed] [Google Scholar]
- 36.Leclerc X, Navez JF, Gauvrit JY, et al. Aneurysms of the anterior communicating artery treated with Guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol 2002;23:1121–27 [PMC free article] [PubMed] [Google Scholar]
- 37.Pierot L, Delcourt C, Bouquigny F, et al. Follow-up of intracranial aneurysms selectively treated with coils: prospective evaluation of contrast-enhanced MR angiography. AJNR Am J Neuroradiol 2006;27:744–49 [PMC free article] [PubMed] [Google Scholar]
- 38.Farb RI, Nag S, Scott JN, et al. Surveillance of intracranial aneurysms treated with detachable coils: a comparison of MRA techniques. Neuroradiology 2005;47:507–15 [DOI] [PubMed] [Google Scholar]
- 39.Kuo PH, Kanal E, Abu-Alfa AK, et al. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007;242:647–49. Epub 2007 Jan 9 [DOI] [PubMed] [Google Scholar]
- 40.Hartmann J, Nguyen T, Larsen D, et al. MR artifacts, heat production, and ferromagnetism of Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:497–501 [PMC free article] [PubMed] [Google Scholar]
- 41.Schmalbrock P, Yaun C, Chakeres DW, et al. Volume MR angiography: methods to achieve very short echo times. Radiology 1990;175:861–65 [DOI] [PubMed] [Google Scholar]
- 42.Wehrli FW, Chao PW, Youssem DM. Parameter dependence of susceptibility-induced signal losses in gradient-echo imaging. Magn Reson Imaging 1989;7:139 [Google Scholar]
- 43.Gonner F, Heid O, Remonda L, et al. MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1324–28 [PMC free article] [PubMed] [Google Scholar]
- 44.Walker MT, Tsai J, Parish T, et al. MR angiographic evaluation of platinum coil packs at 1.5T and 3T: an in vitro assessment of artifact production— technical note. AJNR Am J Neuroradiol 2005;26:848–53 [PMC free article] [PubMed] [Google Scholar]
- 45.Majoie CB, Sprengers ME, van Rooij WJ, et al. MR angiography at 3T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 2005;26:1349–56 [PMC free article] [PubMed] [Google Scholar]
- 46.Cottier JP, Bleuzen-Couthon A, Gallas S, et al. Intracranial aneurysms treated with Guglielmi detachable coils: is contrast material necessary in the follow-up with 3D time-of-flight MR angiography? AJNR Am J Neuroradiol 2003;24:1797–803 [PMC free article] [PubMed] [Google Scholar]
- 47.van Holten J, Wielopolski P, Bruck E, et al. High flip angle imaging of metallic stents: implications for MR angiography and intraluminal signal interpretation. Magn Reson Med 2003;50:879–83 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Truong TN, Yen C, et al. Quantitative evaluation of susceptibility and shielding effects of nitinol, platinum, cobalt-alloy, and stainless steel stents. Magn Reson Med. 2003;49:972–76 [DOI] [PubMed] [Google Scholar]