Abstract
BACKGROUND AND PURPOSE: The aim of this retrospective study was to report the incidence, clinical presentation, and midterm clinical and imaging results of endovascular treatment of 10 aneurysms of the vertebrobasilar junction.
MATERIALS AND METHODS: Between January 1995 and January 2007, 2112 aneurysms were treated in our institution. Ten aneurysms in 10 patients were located on the vertebrobasilar junction and 7 aneurysms (70%) were associated with proximal basilar fenestration. There were 5 men and 5 women, ranging from 29 to 75 years of age. Nine aneurysms presented with subarachnoid hemorrhage, and one was a giant partially thrombosed aneurysm with mass effect on the brain stem.
RESULTS: Nine ruptured aneurysms were treated by primary coil occlusion. One giant unruptured aneurysm was initially treated with bilateral vertebral artery occlusion, 2 months later followed by selective coil occlusion of the remaining aneurysm lumen via the posterior communicating artery. At imaging follow-up of 6–30 months in 7 patients, all aneurysms were adequately occluded. In 2 patients, the vertebrobasilar junction and distal vertebral arteries (including the aneurysm) thrombosed completely on follow-up without clinical sequelae.
CONCLUSION: Vertebrobasilar junction aneurysms are rare, with an incidence of 0.5% of treated aneurysms at our institution. Vertebrobasilar junction aneurysms are frequently associated with proximal basilar fenestration. Most patients present with subarachnoid hemorrhage. Endovascular treatment is effective and safe in excluding the aneurysms from the circulation.
Aneurysms located at the vertebrobasilar junction are uncommon lesions and are often associated with fenestration of the proximal basilar artery.1, 2 Surgical access to the vertebrobasilar junction is difficult, and local anatomy is complex due to the presence of perforators to the brain stem and lower cranial nerves.2–8 Successful endovascular treatment of vertebrobasilar junction aneurysms with detachable coils has been reported in several case reports and small case studies.9–21
In this study, we report the incidence, clinical presentation, and outcome of endovascular treatment in 10 patients with vertebrobasilar junction aneurysms treated in a 12-year period in a single center.
Patients and Methods
Patients
Between January 1995 and January 2007, 2112 aneurysms were treated in our institution. Surgery was performed in 970 aneurysms, and endovascular treatment in 1142 aneurysms. Of 2112 treated aneurysms, 10 aneurysms in 10 patients were located on the vertebrobasilar junction, resulting in an incidence of 0.5% of treated intracranial aneurysms. All 10 aneurysms were treated by endovascular techniques. Characteristics of patients and aneurysms are summarized in the Table. There were 5 men and 5 women with a mean age of 49.6 years (range, 29–75 years). Of 10 aneurysms, 9 (90%) had ruptured and one (10%) presented with symptoms of mass effect on the brain stem. Of 9 patients with a ruptured vertebrobasilar junction aneurysm, clinical conditions at the time of treatment were Hunt and Hess (HH) I-II in 3, HH III in 3, and HH IV-V in 3. Seven of 10 aneurysms (70%) were associated with proximal basilar fenestration, and 3 aneurysms were dumbbell-shaped.
Patient and aneurysm characteristics of 10 patients with vertebrobasilar junction aneurysms
| Patient No./Sex/ Age | Clinical Presentation | Aneurysm | Timing | Treatment | Initial/Final Result (%) | Outcome (months) | Angiographic Follow-Up | Remarks |
|---|---|---|---|---|---|---|---|---|
| 1/F/75 | SAH + IVH, HH V | 10-mm, dumbbell, fenestration | 2 days | Coil occlusion | 100 | GOS 3 (43) | Refused | Dependent in nursing home |
| 2/F/46 | SAH, HH III | 12 mm, fenestration | 2 days | Coil occlusion | 90–90 | GOS 1 (30) | 30 months | Additional AcomA aneurysm coiled |
| 3/M/31 | SAH, HH III | 7 mm | 10 days | Coil occlusion | 100–100 | GOS 1 (18) | 6 months | – |
| 4/F/52 | SAH, HH II | 5 mm, fenestration | 2 days | Coil occlusion | 100–100 | GOS 1 (24) | 24 months | – |
| 5/F/44 | SAH + IVH, HH IV | 6 mm, fenestration | 3 days | Coil occlusion, EVD | 100 | GOS 5 (0.2) | – | Died of vasospasm |
| 6/M/64 | SAH + IVH, HH I | 10 mm, dumbbell fenestration | 2 days | Coil occlusion, EVD | 100–100 | GOS 1 (27) | 12 months | Died 27 months later of cardiac disease |
| 7/M/29 | SAH + IVH, HH III | 12-mm, dumbbell | 2 days | Coil occlusion | 90–100 | GOS 1 (6) | 6 months | Progressive thrombosis, aneurysm + V4 segments bilaterally |
| 8/M/55 | SAH + IVH, HH II | 5 mm, fenestration | 6 days | Coil occlusion | 100–90 | GOS 1 (27) | 27 months | |
| 9/F/70 | SAH + IVH, HH V | 17 mm, fenestration | 1 day | Coil occlusion EVD | 100 | GOS (0.1) | – | Died of SAH |
| 10/M/30 | Mass effect: neck pain, right sided muscle, weakness, and swallowing difficulty | 47 mm, partially thrombosed | – | Bilateral vertebral artery occlusion, V3 right, V4 left, later coiling via PcomA | 0–100 | GOS 1 (24) | 24 months | Progressive complete thrombosis and partial involution aneurysm + V4 segments bilaterally; mass effect cured |
Note:—IVH indicates intraventricular hemorrhage; GOS, Glasgow Outcome Scale; –, not applicable; EVD, extraventricular drainage; PcomA, posterior communicating artery; AcomA, anterior communicating artery; SAH, subarachnoid hemorrhage; HH, Hunt and Hess scale.
Endovascular Procedure
Coiling of aneurysms was performed on a biplane angiographic unit (Integris BN 3000 Neuro; Philips Medical Systems, Best, the Netherlands) with the patient under general anesthesia and systemic heparinization. Heparin was continued intravenously or subcutaneously for 48 hours after the procedure, followed by low-dose aspirin for 3 months orally. After location of the aneurysm on bilateral vertebral biplane angiography, rotational 3D angiography was performed in 7 of 10 patients (in 3 earlier patients, 3D angiography was not yet available). Coiling was performed with Guglielmi detachable coils (GDC, Boston Scientific, Fremont, Calif) or Trufill DCS coils (Cordis, Miami Lakes, Fla). The aim of coiling was to pack the aneurysm as densely as possible, until not a single additional coil could be placed. Complications of coiling were recorded. One patient was initially treated with bilateral vertebral occlusion, 2 months later followed by additional coiling via the posterior communicating artery.
Initial angiographic results of coiling were classified as complete occlusion (100%), near complete occlusion (90–100%), and incomplete occlusion (<90%).
Clinical and Angiographic Follow-Up
Patients who survived the hospital admission period were scheduled for a follow-up visit in the outpatient clinic 6 weeks after treatment and for follow-up angiography after 6 months. On both occasions, neurologic status was evaluated. Results of follow-up angiography were classified in the same way as for initial angiographic results.
Results
Initial Angiographic Results and Results at Follow-Up
Nine patients were treated with coil occlusion of the aneurysm. Initial aneurysm occlusion was complete in 7 and near-complete in 2 aneurysms. Two patients died shortly after treatment of sequelae of subarachnoid hemorrhage (SAH). One patient became dependent as a result of SAH and refused follow-up angiography. Thus, of 9 patients treated with coil occlusion, 6 had angiographic follow-up at a mean of 17.5 months (range, 6–30 months). Aneurysm occlusion at follow-up was complete in 4 and near-complete in 2 aneurysms. In patient 7, a 29-year-old man with an initial nearly completely occluded aneurysm, at angiographic follow-up, both distal vertebral arteries appeared to be thrombosed spontaneously, including the remnant of the aneurysm without clinical symptoms. In patient 10, a 30-year-old man with a giant partially thrombosed aneurysm initially treated with bilateral vertebral artery occlusion followed by coil occlusion of the aneurysmal lumen via the posterior communicating artery 2 months later, angiographic follow-up 6 months thereafter showed spontaneous thrombosis of both distal vertebral arteries, including the aneurysm. MR imaging 24 months after presentation revealed remarkable shrinkage of the aneurysm with decreased mass effect on the brain stem.
Representative Cases
Case 1.
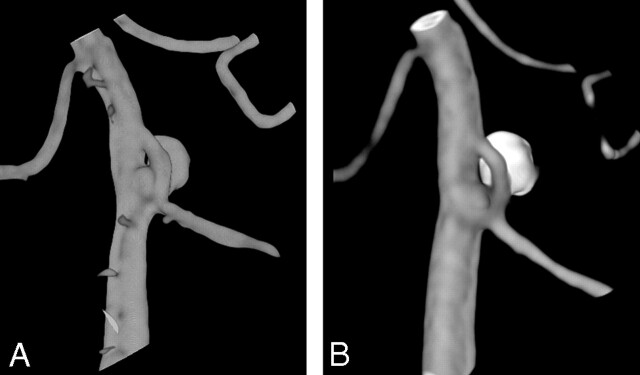
A 52-year-old woman (patient 4) was admitted with SAH HH II. 3D angiography showed a small vertebrobasilar junction aneurysm on a proximal basilar fenestration (Fig 1A), and the aneurysm was entirely occluded with coils without complications. The patient recovered completely, and follow-up angiography 6 months later demonstrated stable complete occlusion of the aneurysm (Fig 1B).
Fig 1.
52-year-old woman with good-grade SAH. A and B, 3D angiogram (A) shows a small vertebrobasilar junction aneurysm on the proximal part of a basilar fenestration and 6 months (B) later demonstrates stable complete occlusion of the aneurysm.
Case 2.
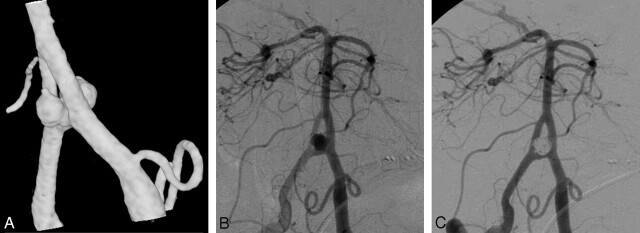
A 64-year-old man (patient 6) was admitted with an SAH and intraventricular hemorrhage in good clinical grade (Fig 2). On angiography, the origin of the right subclavian artery was occluded with steal from the left-to-right vertebral artery. A dumbbell-shaped aneurysm was present on the vertebrobasilar junction with a fenestration of the proximal basilar artery, and this aneurysm was completely occluded with coils. A follow-up angiogram 12 months later demonstrated stable complete occlusion. The patient died 27 months after coiling of cardiac infarction.
Fig 2.
A 64-year-old man with ruptured vertebrobasilar junction aneurysm. A and B, 3D (A) and 2D (B) angiograms demonstrate a dumbbell-shaped vertebrobasilar junction aneurysm on the bridging artery of a basilar fenestration. C, After coiling is performed, complete occlusion is seen.
Case 3.
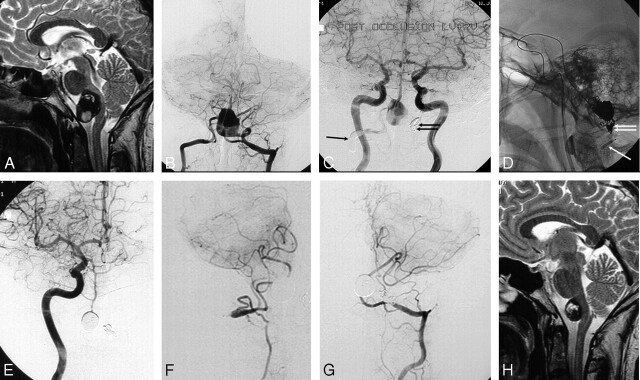
A 30-year-old man (patient 10) had sudden neck pain during skiing, followed by right-sided muscle weakness and difficulty in swallowing (Fig 3). MR imaging revealed a giant partially thrombosed vertebrobasilar junction aneurysm compressing the brain stem. Because good-caliber posterior communicating arteries were present on both sides, it was decided to treat this aneurysm with bilateral vertebral artery occlusion, distal to the posterior inferior cerebellar artery (PICA) on the left side and proximal to the PICA on the right side. The aim of this treatment was to diminish and reverse the flow in the basilar artery, hopefully followed by thrombosis of the aneurysm lumen.22 However, at angiography 2 months later, the aneurysm lumen was still open. Subsequently, the remaining lumen was occluded with coils via the left posterior communicating artery. On follow-up angiography 6 months later, both distal vertebral arteries appeared to be thrombosed, including the aneurysm. The distal right vertebral artery that initially was occluded proximal to the PICA had been recanalized via the thyrocervical trunk with adequate flow to the right PICA. MR imaging demonstrated remarkable shrinkage of the aneurysm, with decreased mass effect on the brain stem. The patient's symptoms had resolved completely.
Fig 3.
A 29-year-old man with sudden neck pain followed by right-sided muscle weakness and difficulty in swallowing. A and B, MR image (A) and frontal bilateral vertebral angiogram (B) show a giant partially thrombosed vertebrobasilar junction aneurysm compressing the brain stem. C, Bilateral frontal carotid angiogram after occluding the right vertebral artery proximal to the PICA with a balloon (arrow) and the left vertebral artery distal to the PICA with coils (double arrow). Flow to the basilar artery is reversed with outflow to the right PICA, yet the aneurysm lumen still fills. D, Lateral radiograph during coiling of the aneurysm lumen via the posterior communicating artery 2 months later. The arrow indicates deflated balloon remnant in the right vertebral artery. The double arrow indicates coils in the left vertebral artery. E–G, Six months later, a frontal view of right carotid angiogram (E) demonstrates filling of the basilar artery via the right posterior communicating artery. Frontal view of the right thyrocervical trunk (F) shows recanalization of the distal right vertebral artery with filling of the PICA territory. Frontal view of the left vertebral angiogram (G) shows filling of the left PICA territory. The aneurysm is completely occluded. H, MR imaging 2 years after presentation shows remarkable shrinkage of the aneurysm. The patient was free of symptoms.
Discussion
In this study, we found that aneurysms on the vertebrobasilar junction are rare, with an incidence of 0.5% of all treated aneurysms at our institution. Most patients presented with SAH, and 7 of 10 aneurysms were associated with proximal basilar fenestration. The relation of vertebrobasilar junction aneurysms with basilar fenestration is well established and explained by intrinsic defects in the medial vessel wall of fenestrated arteries. In the fetus, the basilar artery is formed by fusion of bilateral longitudinal neural arteries during the fifth gestational week. During this fusion process, temporary bridging arteries connecting the longitudinal neural arteries regress as fusion is completed. If these bridging arteries persist, they result in fenestration of the basilar artery.23, 24 The lateral walls of the fenestrated artery have a normal intrinsic architecture. The medial walls, however, have focal defects at both ends of the fenestration that may lead to aneurysm formation similar to cerebral artery bifurcations.25, 26
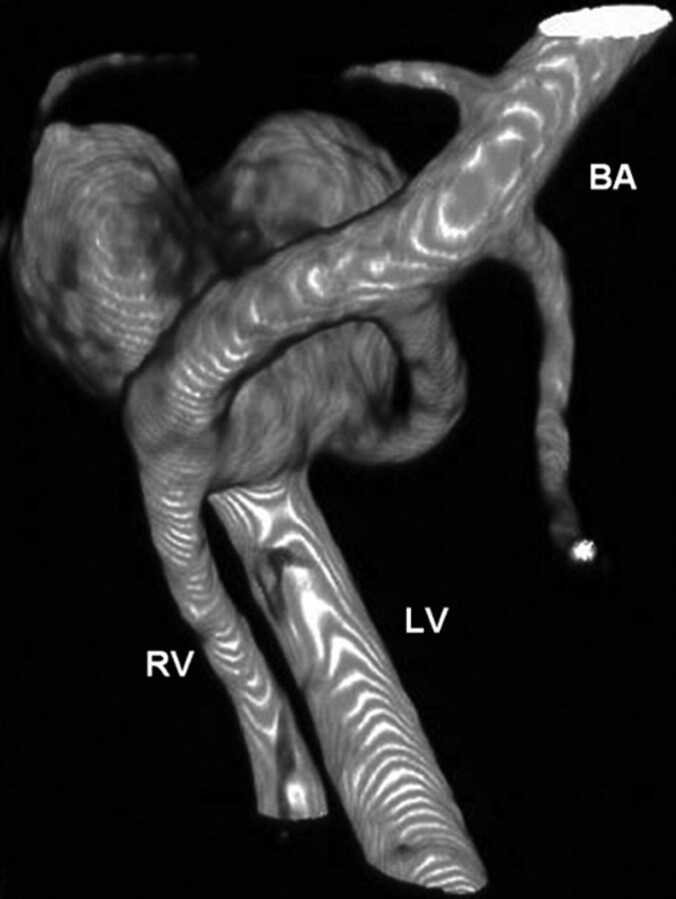
Basilar artery fenestration is reported in 0.6% of angiograms27 and in about 5% of autopsy series.28 Basilar artery fenestrations are most commonly located in the proximal basilar trunk close to the vertebrobasilar junction. In the presence of a basilar fenestration, the incidence of an aneurysm is reported to be 7%.29 On the other hand, the incidence of fenestration in the presence of a vertebrobasilar junction aneurysm is reported to be 35%.2 In our study, the incidence of fenestration with vertebrobasilar junction aneurysms was 70%, twice as high. This higher incidence may be the result of better imaging capabilities with 3D angiography; on 2D angiographic images, a small fenestration may be easily overlooked. We found 3D angiography very helpful in evaluating the anatomy of the aneurysms and finding a working projection for coiling. All aneurysms associated with fenestrations were located on the proximal part of the fenestration on the bridging artery. From 3D images, it was readily apparent whether the parent bridging artery could be sacrificed or should be spared in the process of coil occlusion of the aneurysm. In some aneurysms, inadvertent occlusion of the parent bridging artery while occluding the neck would have had no consequences for flow to the basilar artery, but in others, sparing the parent bridging artery would be sensible in preserving flow to the basilar artery (Fig 4).
Fig 4.
3D angiogram shows a vertebrobasilar junction aneurysm on the bridging artery of a basilar fenestration. With this anatomy, sparing the parent bridging artery would be sensible in preserving flow to the basilar artery. RV indicates right vertebral artery; LV, left vertebral artery; BA, basilar artery. (Compare with Fig 2.)
Surgical access to the vertebrobasilar junction is hampered by the petrous bone and the direct proximity of the aneurysm to the brain stem, with perforating arteries and lower cranial nerves. Several lateral approaches directed through parts of the petrous bone may be used for direct access to the vertebrobasilar junction.5–7 Although surgical approach to the vertebrobasilar junction is difficult, endovascular access is easy; therefore, coil occlusion is the treatment of choice with good initial and midterm results.9, 13, 20, 21 In our series, all aneurysms were adequately occluded at initial treatment and follow-up. There were no complications of treatment and no recurrent hemorrhage during follow-up. A remarkable, and as yet unknown, phenomenon occurred at angiographic follow-up in 2 patients with initially incompletely occluded vertebrobasilar junction aneurysms: In both patients, the vertebrobasilar junction and distal vertebral arteries thrombosed completely some time after coiling, including the aneurysm. In both patients, flow to the basilar artery and PICAs was preserved via posterior communicating arteries and vertebral arteries, respectively. Both patients were symptom-free. Perhaps, alterations of flow were responsible for this phenomenon. In selected cases with insufficient caliber of the posterior communicating arteries, unilateral or bilateral stent placement over the vertebrobasilar junction can be considered to preserve antegrade flow to the basilar artery.
Conclusion
Vertebrobasilar junction aneurysms are rare, with an incidence of 0.5% of treated aneurysms. Most vertebrobasilar junction aneurysms are associated with proximal basilar fenestration. Most patients present with SAH. Endovascular treatment is effective and safe in excluding the aneurysms from the circulation.
References
- 1.Miyazaki S, Kamata K, Yamaura A. Multiple aneurysms of the vertebrobasilar system associated with fenestration of the vertebral artery. Surg Neurol 1981;15:192–95 [DOI] [PubMed] [Google Scholar]
- 2.Campos J, Fox AJ, Vinuela F, et al. Saccular aneurysms in basilar artery fenestration. AJNR Am J Neuroradiol 1987;8:233–36 [PMC free article] [PubMed] [Google Scholar]
- 3.Grand W, Budny JL, Gibbons KJ, et al. Microvascular surgical anatomy of the vertebrobasilar junction. Neurosurgery 1997;40:1219–23 [DOI] [PubMed] [Google Scholar]
- 4.Kawase T, Bertalanffy H, Otani M, et al. Surgical approaches for vertebro-basilar trunk aneurysms located in the midline. Acta Neurochir (Wien) 1996;138:402–10 [DOI] [PubMed] [Google Scholar]
- 5.Seifert V, Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg 1996;85:373–79 [DOI] [PubMed] [Google Scholar]
- 6.Seifert V. Direct surgery of basilar trunk and vertebrobasilar junction aneurysms via the combined transpetrosal approach. Neurol Med Chir (Tokyo) 1998;38(suppl):86–92 [DOI] [PubMed] [Google Scholar]
- 7.Seifert V, Raabe A, Stolke D. Management-related morbidity and mortality in unselected aneurysms of the basilar trunk and vertebrobasilar junction. Acta Neurochir (Wien) 2001;143:343–49 [DOI] [PubMed] [Google Scholar]
- 8.Sugita K, Kobayashi S, Takemae T, et al. Aneurysms of the basilar artery trunk. J Neurosurg 1987;66:500–05 [DOI] [PubMed] [Google Scholar]
- 9.Bavinzski G, Killer M, Gruber A, et al. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg 1999;90:843–52 [DOI] [PubMed] [Google Scholar]
- 10.Collice M, Arena O, D'Aliberti G, et al. Aneurysms of the vertebro-basilar junction area: preliminary experience in endovascular and surgical management. Acta Neurochir (Wien) 1997;139:124–33 [DOI] [PubMed] [Google Scholar]
- 11.Ezaki Y, Kazekawa K, Tsutsumi K, et al. A vertebrobasilar junction aneurysm associated with fenestration treated by intra-aneurysmal embolization. Acta Neurochir (Wien) 2003;145:807–08 [DOI] [PubMed] [Google Scholar]
- 12.Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 1992;77:515–24 [DOI] [PubMed] [Google Scholar]
- 13.Graves VB, Strother CM, Weir B, et al. Vertebrobasilar junction aneurysms associated with fenestration: treatment with Guglielmi detachable coils. AJNR Am J Neuroradiol 1996;17:35–40 [PMC free article] [PubMed] [Google Scholar]
- 14.Komiyama M, Nakajima H, Nishikawa M, et al. Treatment of a saccular aneurysm at the fenestration of the intracranial vertebral artery with Guglielmi detachable coils. Acta Neurochir (Wien) 1999;141:1125–27 [DOI] [PubMed] [Google Scholar]
- 15.Nagashima H, Okudera H, Orz Y, et al. Endovascular treatment of basilar trunk aneurysm associated with fenestration of the basilar artery. Neurosurg Rev 1999;22:219–21 [DOI] [PubMed] [Google Scholar]
- 16.Nichols DA, Brown RD Jr, Thielen KR, et al. Endovascular treatment of ruptured posterior circulation aneurysms using electrolytically detachable coils. J Neurosurg 1997;87:374–80 [DOI] [PubMed] [Google Scholar]
- 17.Picard L, Roy D, Bracard S, et al. Aneurysm associated with a fenestrated basilar artery: report of two cases treated by endovascular detachable balloon embolization. AJNR Am J Neuroradiol 1993;14:591–94 [PMC free article] [PubMed] [Google Scholar]
- 18.Pierot L, Boulin A, Castaings L, et al. Selective occlusion of basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery 1996;38:948–53 [DOI] [PubMed] [Google Scholar]
- 19.Saatci I, Cekirge HS, Karcaaltincaba M, et al. Endovascular treatment of kissing aneurysms at the fenestrated basilar artery: case report with literature review. Surg Neurol 2002;58:54–58 [DOI] [PubMed] [Google Scholar]
- 20.Uda K, Murayama Y, Gobin YP, et al. Endovascular treatment of basilar artery trunk aneurysms with Guglielmi detachable coils: clinical experience with 41 aneurysms in 39 patients. J Neurosurg 2001;95:624–32 [DOI] [PubMed] [Google Scholar]
- 21.Yoon SM, Chun YI, Kwon Y, et al. Vertebrobasilar junction aneurysms associated with fenestration: experience of five cases treated with Guglielmi detachable coils. Surg Neurol 2004;61:248–54 [DOI] [PubMed] [Google Scholar]
- 22.Sluzewski M, Brilstra EH, van Rooij WJ, et al. Bilateral vertebral artery balloon occlusion for giant vertebrobasilar aneurysms. Neuroradiology 2001;43:336–41 [DOI] [PubMed] [Google Scholar]
- 23.Giuffre R, Sherkat S. The vertebral artery: developmental pathology. J Neurosurg Sci 1999;43:175–89 [PubMed] [Google Scholar]
- 24.Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol 1968;32:205–61 [Google Scholar]
- 25.Black SP, Ansbacher LE. Saccular aneurysm associated with segmental duplication of the basilar artery: a morphological study. J Neurosurg 1984;61:1005–08 [DOI] [PubMed] [Google Scholar]
- 26.Finlay HM, Canham PB. The layered fabric of cerebral artery fenestrations. Stroke 1994;25:1799–806 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Tamakawa Y, Kishikawa T, et al. Fenestration of the basilar artery. Radiology 1973;109:79–82 [PubMed] [Google Scholar]
- 28.Wollschlaeger G, Wollschlaeger PB, Lucas FV, et al. Experience and result with post-mortem cerebral angiography performed as routine procedure of the autopsy. AJR Am J Roentgenol 1967;101:68–87 [DOI] [PubMed] [Google Scholar]
- 29.Sanders WP, Sorek PA, Mehta BA. Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies. AJNR Am J Neuroradiol 1993;14:675–80 [PMC free article] [PubMed] [Google Scholar]