Abstract
BACKGROUND AND PURPOSE: This study was undertaken to analyze the outcomes and treatment-related complications of the polyglycolic/polylactic acid (PGLA)-coated Matrix platinum coils in the treatment of intracranial aneurysms and compare these results with those derived from the same single-institutional experience with use of uncoated, bare platinum coils.
MATERIALS AND METHODS: In this study, we compared 2 groups of patients in a retrospective fashion. The first group consisted of 70 consecutive patients who underwent 82 aneurysm treatments with Matrix coils during the 14-month period of study, from January 2003 to February 2004. We compared this cohort with 70 consecutive patients who underwent a total of 80 aneurysm treatments with bare platinum coils in the 12 months immediately preceding the use of PGLA-coated coils, from January through December 2002. We then recorded the treatment characteristics, angiographic outcomes, and any complications.
RESULTS: There were similar baseline demographic characteristics between the 2 study groups except in age, anatomic location, and length of follow-up. The overall recurrence rate of aneurysms was 41% among the Matrix-treated group and 32% among the patients treated with bare platinum. Among the 42 patients treated with 100% Matrix, the rate of recurrence was 31%. Of the recurrences, 21% of the Matrix group, 19% of the 100% Matrix group, and 9% of the bare platinum group required retreatment. The overall rate of complications was 10% in the Matrix-treated group and 7% in the bare platinum group. There was not a statistically significant difference in the rate of recurrence of aneurysms or complications between the 2 groups.
CONCLUSIONS: On the basis of our single-center experience, there is insufficient evidence to support the use of Matrix coils over bare platinum coils, given their disadvantages.
The endovascular treatment of aneurysms became widespread in the United States after Food and Drug Administration (FDA) approval of the Guglielmi detachable coil (GDC) in 1995. Because techniques and technologies have advanced, coiling has become an accepted alternative to surgical clipping of most cerebral aneurysms. This platinum coil, which rapidly became the standard for endovascular treatment, functions by partially filling the aneurysm. The coil mass within the aneurysm slows the blood flow within it, which causes the development and organization of the clot, leading to fibrosis.1 Results with GDC have indicated that it is effective in preventing rebleeding when the aneurysm is completely occluded and is also effective when incompletely occluded.2 However, compaction of the coils and recurrence of aneurysms occur at a rate of between 14% and 54%, depending on the series.3–7 Rates of aneurysm recurrence are higher in giant aneurysms, those that are partially thrombosed, and in cases where only partial coil occlusion is possible.4, 5
To reduce the rate of recurrence, several groups have performed experimental modifications of the platinum coils in animals.8–11 These modifications have included coating the coils with growth factors and cells. In 2002, the FDA approved the GDC coil coated with a copolymer consisting of polyglycolic/polylactic acid (PGLA), the Matrix coil (Boston Scientific, Natick, Mass). These coils would theoretically decrease the rate of recanalization by accelerating neointimal formation and fibrosis on the coils; there is experimental evidence to support this mechanism.12 However, longer-term data on the clinical effectiveness of the coated coils is lacking.
Published studies on GDC outcomes have reported a range of aneurysm recurrence rates and are usually derived from a single institution's experience.4, 5, 7 During the development and initial evaluation of new techniques of aneurysmal obliteration, these GDC series are often used as a comparison. However, this manner of evaluation does not control for device selection bias, differences in physician experience and techniques, or institutional differences in outcomes. We sought to determine the results and complications associated with use of the PGLA-coated (Matrix) coils and compare them with use of uncoated platinum coils at our own institution.
Materials and Methods
Patients and Methods
The Institutional Review Board reviewed and approved all components of the study design. During a period of 14 months (between January 2003 and March 2004), 82 aneurysms in 70 patients were treated with endovascular occlusion with use of PGLA-coated platinum coils. Baseline patient demographic characteristics are summarized in Table 1. This group consisted of 19 male and 51 female patients (mean age, 57 years; age range, 26–86 years; SD, 14 years). Of this group, 33% had suffered subarachnoid hemorrhage; 65 (79%) aneurysms were in the anterior circulation, and 17 (21%) were in the posterior circulation. A total of 24 (30%) aneurysms were classified as “wide necked” (≥4 mm). Balloon remodeling or adjunctive stent placement was performed in 25 (30%) cases. A total of 10 patients had multiple aneurysms treated: 8 with 2 aneurysms, and 2 with 3 aneurysms. PGLA-coated coils were used alone or in combination with other platinum coils at the treating physicians’ discretion. Forty-two of the 82 aneurysms were treated with 100% PGLA-coated coils and were also analyzed as a separate subgroup, “100% Matrix.” Aneurysms treated with only bare platinum coils during this later period (January to March 2004) were not included in this analysis.
Table 1:
Baseline demographic characteristics (all procedures)
| Characteristics | PGLA-Coated Coils | Bare Platinum Coils | P Value* |
|---|---|---|---|
| Age (mean +/− SD) (yrs) | 57 +/− 14 | 52 +/− 13 | .04 |
| SAH (%) | 33 | 45 | .11 |
| Sex ratio (%) (M:F) | 27:73 | 24:76 | .70 |
| Circulation (%) (Ant:Post) | 79:21 | 60:40 | .008 |
| Average size (mm) (SD) | 8.5 (6.1) | 8.1 (5.4) | .63 |
| Size category (mm) (%) | .50 | ||
| 0–10 mm | 63 (77) | 67 (84) | |
| 11–24 mm | 17 (21) | 11 (14) | |
| >25 mm | 2 (2) | 2 (2) | |
| >4 mm neck | 24 (29) | 19 (24) | .80 |
| <4 mm neck | 58 (71) | 61 (76) | .80 |
| Follow-up period (mos) mean (SD) | 9.4 (5.2) | 12.1 (7.0) | .009 |
Note:—SAH indicates subarachnoid hemorrhage; PGLA, polyglycolic/polylactic acid.
ns = P > .05.
A second group consisted of 72 consecutive patients with a saccular aneurysm who were treated in the preceding 12 months by the same treating physicians at the same institution, before the introduction of the bioactive coils. We used these patients as a comparison cohort to determine our own institutional outcome rates. Two patients in this group treated with parent vessel sacrifice for dissecting or fusiform aneurysms were excluded. From January through December 2002, 80 aneurysms in the remaining 70 patients were treated with standard (ie, noncoated or bare) platinum coils from a variety of manufacturers; 79% were treated entirely with GDC (Boston Scientific) bare platinum coils. The remainder was treated with DCS Orbit coils (Cordis Neurovascular, Miami Lakes, Fla), bare platinum coils (Micrus Endovascular, San Jose, Calif), or a combination of these 3 types at the discretion of 3 staff interventionalists.
The second group consisted of 17 male and 53 female patients (mean age, 52 years; range, 14–76 years; SD, 13 years). There were 45% of treated aneurysms that were ruptured, and 5 patients had suffered a subarachnoid hemorrhage previously from a different aneurysm. A total of 48 (60%) aneurysms were in the anterior circulation, and 32 (40%) were in the posterior circulation. There were 19 (24%) aneurysms classified as “wide-necked” (≥4 mm). Balloon remodeling or adjunctive stent placement was performed in 19 (24%) cases. A total of 10 patients had 2 aneurysms treated. We determined the aneurysmal dome and neck sizes by the largest dimension as measured from initial pretreatment arteriograms, or by CT and MR imaging measurements in partially thrombosed aneurysms. We divided the aneurysmal sizes into 3 categories: 0 to 10 mm, 11 to 24 mm, and 25 mm and greater. The anatomic locations (anterior or posterior circulation) of the aneurysms treated for both groups are summarized in Table 2.
Table 2:
Aneurysm locations
| Locations | PGLA-Coated Coils | Bare Platinum Coils |
|---|---|---|
| Anterior circulation total | 65 | 47 |
| Proximal ICA | 10 | 6 |
| Ophthalmic | 8 | 15 |
| SupHypo | 7 | 5 |
| PComA | 14 | 6 |
| AntChor | 1 | 2 |
| ICA terminus | 7 | 2 |
| AComA | 16 | 9 |
| ACA | 0 | 2 |
| MCA | 2 | 0 |
| Posterior circulation total | 17 | 33 |
| PICA | 2 | 5 |
| VBJ | 1 | 1 |
| SCA | 2 | 4 |
| TOB | 12 | 22 |
| PCA | 0 | 1 |
| Total | 82 | 80 |
Note:—PGLA indicates polyglycolic/polylactic acid; proximal ICA, proximal internal carotid artery; Ophthalmic, ophthalmic artery; SupHypo, superior hypophyseal artery; PComA, posterior communicating artery; AntChor, anterior choroidal artery; ACA, anterior cerebral artery; MCA, middle cerebral artery; PICA, posterior inferior cerebellar artery; VBJ, vertebrobasilar junction; SCA, superior cerebral artery; TOB, top of basilar artery; PCA, posterior cerebral artery.
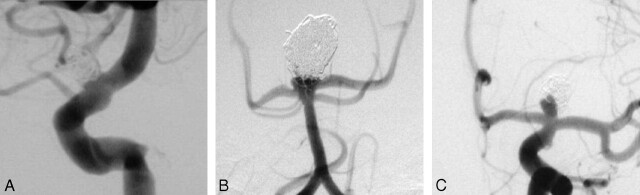
Aneurysms were assigned to 1 of 3 grades at the end of treatment and subsequently on follow-up angiograms (Fig 1). We modified the aneurysmal grading scale used by Raymond et al.7, 13 We combined the categories “Dog Ear” and “Residual Neck” into 1 category, “Neck Remnant” (see below). Aneurysms that were completely occluded with no contrast filling within the interstices of the coil, within the original aneurysm, or within an aneurysmal neck were considered “Obliterated” (OBL). If there was contrast filling at the base of the aneurysm within the neck or within an area subjacent to the main coil mass but not at the actual neck, this was classified as a “Neck Remnant” (Neck). In general, we followed a conservative approach such that any contrast filling in the region of the neck was considered a neck remnant. If there was contrast filling within the interstices of the coil, or more contrast filling than simply at the neck, because of expansion of the aneurysm or compaction of the coil, this was classified as a “Residual” or “Recurrent Aneurysm,” depending on whether it was present immediately after the treatment (Residual) or at follow-up (Recurrent). At last follow-up, we classified these 2 categories as being together (Residual + Recurrence) in Table 6. We determined all follow-up aneurysm grading from conventional angiograms that we performed at a minimum of 6 months after the procedure. We determined pretreatment aneurysmal dimensions, neck size, and angiographic outcome grading from digitally subtracted angiographic images using ≥4 projections. For purposes of statistical comparisons, patients with only MR angiography or CT angiography follow-up were not graded or included.
Fig 1.
Aneurysm outcome grading scheme. A, Grade 1 “Obliterated” aneurysms had no contrast filling within the interstices of the coils or at the neck and were considered completely obliterated. B, Grade 2 “Neck Remnant” was used to classify cases with contrast filling at the base of the aneurysm within the neck or within an area subjacent to the main coil mass, but not at the actual neck. C, Grade 3 “Residual” or “Recurrent” was used if there was contrast filling within the interstices of the coil, or more contrast filling than simply at the neck, because of expansion of the aneurysm or compaction of the coil, depending on whether it was present immediately after the treatment (“Residual”) or at follow-up (“Recurrent”).
Table 6:
Initial and final results of treatment (no. of aneurysms/%) during follow-up interval
| Category of Aneurysm | Matrix Coils |
Bare Platinum Coils |
||
|---|---|---|---|---|
| Initial Result | Final Result | Initial Result | Final Result | |
| OBL | 23/28% | 29/43% | 32/40% | 28/53% |
| Neck | 30/37% | 9/13% | 30/38% | 8/15% |
| Residual + recurrence | 29/35% | 30/44% | 18/22% | 17/32% |
Note:—OBL indicates obliterated.
We automatically considered any aneurysms that had enough of a recurrence or residuum to undergo retreatment with additional coils to be recurrent/residual. We used this classification because we felt that any amount of filling large enough to allow placement of more coils would not be best described as simply a “neck remnant.” However, for a variety of reasons, not all aneurysms graded as residual or recurrent underwent recoiling.
The amount of PGLA-coated coils used, as a percentage of the total length of coils inserted, was calculated, and statistical comparison was made between the bare platinum-treated cohort and the entire PGLA-treated cohort. We also performed a comparison between the bare platinum-treated cohort and only those aneurysms treated with 100% PGLA-coated coils that had follow-up available (see Results).
Treatments
Intravenous heparin to an activated clotting time (ACT) of approximately 250 seconds was used for all patients who had suffered a subarachnoid hemorrhage. Aspirin (325 mg) was usually administered the evening following the procedure and then daily thereafter. The patients whose aneurysm had not ruptured were all orally premedicated for at least 3 days with aspirin (325 mg) and clopidogrel bisulfate (75 mg, Bristol-Myers Squibb, New York, NY), which was maintained for the subsequent 30 days. Clopidogrel was discontinued after 30 days, and the aspirin continued indefinitely. During the endovascular procedure, the patients were anticoagulated to an ACT of approximately 300 seconds.
The heparin was allowed to dissipate after the procedure and was only maintained in cases in which intraluminal thrombus of the parent vessel occurred. If clotting occurred, intra-arterial (IA) lytics, intravenous heparin, or antiplatelet agents such as the glycoprotein IIb/IIIa inhibitor abciximab (Eli Lilly, Indianapolis, Ind) were administered. The guide catheters and microcatheters were continuously flushed with heparinized saline (3000 U/100 mL) under arterial pressure at a rate of 3 mL/h. All endovascular treatments of aneurysms were performed with the patient under general anesthesia.
The Matrix coils were prepared as per the instructions for use (ie, immersed in heparinized saline for 10 to 20 seconds before introduction into the microcatheter). The Matrix soft and UltraSoft (Boston Scientific) stretch-resistant coils became available in May 2003 and were used in selective cases at the discretion of the treating physician thereafter.
Statistical Analysis
We analyzed the relationships between categoric data by calculating contingency tables. We tested patterns for statistical significance with χ2 tests. When continuous data were available for groups, analysis depended on what was the independent variable. When the independent variable was categorical and the dependent variable was continuous, we calculated means and SD and tested differences for statistical significance with t tests. When the independent variable was continuous and the dependent variable was categoric, we performed logistic regression. To evaluate multiple independent variables simultaneously, a multivariable nominal logistic regression analysis was performed. We carried out all analyses using JMP 5.0 (SAS Institute, Cary, NC).
Results
Comparison of baseline demographic properties of the treatment groups is presented in Table 1. There were 80 aneurysms in 70 patients treated with bare platinum coils and 82 aneurysms in 70 patients treated with Matrix coils. The breakdown of anatomic location of the treated aneurysms is summarized in Table 2.
There were statistically significant differences between the groups in 3 variables: age, anatomic location, and length of follow-up. Patients in the Matrix group tended to be older than the GDC group, which would tend to reduce the apparent success of the Matrix coils, because success of the outcome tended to decline with the age of the patient (logistic regression, P = .005). Patients in the Matrix group had a greater proportion of aneurysms in the anterior circulation than the GDC group, which would tend to increase the apparent success of the Matrix coils, because success of the outcome tended to be greater with the anterior than with posterior circulation (χ2, P = .04). As expected, patients in the GDC group had a statistically significant longer interval of follow-up than those in the Matrix group. There was follow-up information for 35 of 42 (83%) patients in the Matrix group, and for 53 of 80 (66%) patients in the GDC group. This difference was also statistically significant (χ2, P = .046) and is addressed below (see Discussion).
Table 3 summarizes several specific attributes of treatment in each cohort. Because of aneurysmal neck size, overall configuration, or other anatomic factors, we treated a subset of both treatment groups by using adjunctive devices. In the earlier GDC group, this technique was performed in almost all (18/19) cases with balloon remodeling14 because only coronary stents were available at that time. The Matrix group was treated after the Neuroform stent (Boston Scientific) became available, which was used as an adjunct to treatment in most cases (18/25). There was no statistically significant difference in the numbers of patients in both groups treated with either balloon remodeling or stent assistance (P = .92 for all Matrix cases vs bare platinum; P = .32 for 100% Matrix vs bare platinum). Two patients (2%) in the Matrix group, 1 (2%) in the 100% Matrix group, and 4 (5%) in the bare platinum group had intentional subtotal coil embolization to preserve a branch vessel originating from, or immediately adjacent to, the aneurysm.
Table 3:
Treatment characteristics
| No. and Type of Aneurysms (%) | PGLA-Coated Coils (All) | 100% PGLA-Coated Coils | Bare Platinum Coils |
|---|---|---|---|
| Stent-assisted | 18/82 (22%) | 8/42 (19%) | 1/80 (1%) |
| Balloon remodeling | 7/82 (9%) | 1/42 (2%) | 18/80 (23%) |
| Total treated with adjunctive device | 25/82 (30%) | 9/42 (21%) | 19/80 (24%) |
| Intentional subtotal embolization to preserve parent vessel | 2/82 (2%) | 1/42 (2%) | 4/80 (5%) |
Note:—PGLA indicates polyglycolic/polylactic acid.
In the procedures involving the use of Matrix coils, the percentage of Matrix coils used typically varied between 30% and 100%, with a slight majority of procedures (42/82, 51%) using 100% Matrix coils. A preliminary analysis showed a weak, but statistically significant (logistic regression, P = .046), tendency for outcome to improve as the percentage of Matrix coils used increased. Therefore, we compared the bare platinum group with both the entire group of Matrix cases as well as only the procedures that used 100% Matrix coils. Results from both are presented in Table 4. The performance of Matrix and GDC coils was virtually identical, and in all cases P > .50, which suggested that the minor differences were more likely because of sampling error than any real difference in coil performance. There was also no statistically significant difference in angiographic outcome between the cohort that was treated with 100% Matrix coils and the GDC-treated group. These angiographic outcomes are also consistent with the rates of recanalization reported from previous bare-platinum series.4, 5, 15
Table 4:
Treatment results
| PGLA-Coated Coils |
Bare Platinum Coils |
P Value (χ2) | |||||
|---|---|---|---|---|---|---|---|
| OBL | Neck | Resid + Recurr | OBL | Neck | Resid + Recurr | ||
| All sizes | 43% (29/68) | 16% (11/68) | 41% (28/68) | 53% (28/53) | 15% (8/53) | 32% (17/53) | .51 |
| 0–10 mm | 52% (26/50) | 14% (7/50) | 34% (17/50) | 61% (25/41) | 17% (7/41) | 22% (9/41) | .44 |
| 11–24 mm | 20% (3/15) | 20% (3/15) | 60% (9/15) | 20% (2/10) | 10% (1/10) | 70% (7/10) | .83 |
| ≥25 mm | 0 | 33% (1/3) | 67% (2/3) | 50% (1/2) | 0 | 50% (1/2) | – |
| 100% PGLA-Coated Coils | Bare Platinum Coils | ||||||
| All sizes | 46% (16/35) | 23% (8/35) | 31% (11/35) | 53% (28/53) | 15% (8/53) | 32% (17/53) | .63 |
| 0–10 mm | 54% (15/28) | 21% (6/28) | 25% (7/28) | 61% (25/41) | 17% (7/41) | 22% (9/41) | .82 |
| 11–24 mm | 17% (1/6) | 17% (1/6) | 67% (4/6) | 20% (2/10) | 10% (1/10) | 70% (7/10) | .92 |
| ≥25 mm | 0 | 100% (1/1) | 0 | 50% (1/2) | 0 | 50% (1/2) | – |
Note:—PGLA indicates polyglycolic/polylactic acid; OBL, obliterated; Resid + Recurr, residual and recurrence.
Because the differences in the demographic variables had the potential to affect the apparent relative successes of the coil types, we performed a multivariable logistic regression analysis to consider the potential confounding variables simultaneously and evaluate their relative importance. Coil type, patient age, aneurysm size, and aneurysm location (anterior vs posterior circulation) were the independent variables, and outcome was the dependent variable. Patient age, and aneurysm size and location were statistically significant (Wald χ2, P = .005, P = .02, P = .04, respectively), and coil type was not (Wald χ2, P =.31). Because this analysis will tend to adjust all factors in relationship to each other, it confirms the previous evidence that coil type is not a factor in the outcome of the procedure.
The need for retreatment of an aneurysm was also recorded and statistically compared as another metric of treatment efficacy, independent of the angiographic outcome on the 3-point scale. Table 5 summarizes retreatments. Overall, 21% of the PGLA-treated group and 9% of the bare platinum-treated group required retreatment. This result approached but did not achieve a statistically significant difference (P = .07). The difference did not approach statistical significance when only the aneurysms treated with 100% Matrix (19% retreatment) were compared (P = .24). It is important to note that there was no statistically significant relationship between the need for retreatment and the use of stent or balloon remodeling (P = .75), though the numbers were small.
Table 5:
Retreatments
| No. of Aneurysms (%) | PGLA-Coated Coils | 100% PGLA-Coated Coils | Bare Platinum Coils |
|---|---|---|---|
| Overall | 17/82 (21%) | 8/42 (19%) | 7/80 (9%) |
| Originally treated with Neuroform stent or balloon remodeling | 4/17 | 1/8 | 1/7 |
| Size (mm) | |||
| 0–10 | 10/59 (17%)* | 6/34 (18%) | 3/57 (5%)* |
| 11–24 | 6/20 (30%)* | 2/7 (29%) | 2/19 (11%)* |
| ≥25 | 1/3 (33%)* | 0 | 2/4 (50%)* |
| >4 Neck | 4 | 1 | 1 |
| <4 Neck | 14 | 0 | 6 |
Note:—PGLA indicates polyglycolic/polylactic acid.
Percentages represent the % of aneurysms within a particular size category.
The evolution of angiographic outcome between the initial posttreatment results and the final angiographic outcome is also important. This is summarized in Table 7. There was no statistically significant difference in the initial angiographic outcome between the Matrix and GDC coils. A similar evolution in angiographic status during the follow-up interval occurred in both groups: several aneurysms (37% for Matrix and 38% for GDC coils) graded as having “Neck” remnants went on to be graded as either “Recurrences” or “Obliterated.” In those aneurysms graded as “Obliterated” initially, 3 (13%) of the Matrix-treated cases went on to have a recurrence, whereas none of the GDC-treated cases had a recurrence. As stated above, for a variety of reasons, not all those cases graded as “Recurrences” were re-treated. In those aneurysms graded as “Residual” initially, most in both groups were graded similarly at last follow-up (“Residual/Recurrence”). A small number of cases (5 [17%] in the Matrix group and 2 [11%] in the GDC group) with “Residual” initially went on to have occlusions and were graded as “Obliterated.”
Table 7:
Evolution of final angiographic outcome based on initial results (no. of aneurysms)
| Matrix Coils | Bare Platinum Coils | ||
|---|---|---|---|
| OBL (23 aneurysms) | OBL (32 aneurysms) | ||
| OBL | 14 | OBL | 19 |
| Neck | 1 | Neck | 2 |
| Recurrence | 3 | Recurrence | 0 |
| No. retreated | 2 | No. retreated | 0 |
| Neck (30 aneurysms) | Neck (30 aneurysms) | ||
| OBL | 10 | OBL | 7 |
| Neck | 4 | Neck | 5 |
| Recurrence | 9 | Recurrence | 9 |
| No. retreated | 6 | No. retreated | 6 |
| Residual + Recurrence (29 aneurysms) | Residual + Recurrence (18 aneurysms) | ||
| OBL | 5 | OBL | 2 |
| Neck | 4 | Neck | 1 |
| Recurrence | 18 | Recurrence | 8 |
| No. retreated | 9 | No. retreated | 1 |
Note:—OBL indicates obliterated.
Number of aneurysms with final result is less than initial number as a result of patients lost to follow-up.
Complications
Complications are summarized in Table 8. In the bare platinum-treated group, there were 7 treatment-related complications in 5 (7%) patients. There were 2 retroperitoneal hemorrhages. One of these patients received a blood transfusion. During attempted treatment in another patient, a coil was stretched, which led to termination of the procedure. There was one case of IA formation of a thrombus distal to the location of the aneurysm that was treated with mechanical lysis and prolonged heparinization without evidence of neurologic deficit. One patient suffered a postoperative cerebral infarction from delayed thrombosis (4 days) of the posterior inferior cerebellar artery after coiling of a ruptured aneurysm arising at the origin of this vessel. One death resulted from perforation of the aneurysm and hemorrhage during coiling of a ruptured aneurysm of the basilar apex.
Table 8:
Complications
| Complications | PGLA-Coated Coils (7 patients) | Bare Platinum Coils (5 patients) |
|---|---|---|
| Retroperitoneal hemorrhage | 1 | 2 |
| Stretched coil | 4 | 1 |
| Parent vessel thrombus | 2 | 1 |
| CVA | 2 | 1 |
| Aneurysmal perforation | 0 | 1 |
| Death | 1 | 1 |
Note:—PGLA indicates polyglycolic/polylactic acid; CVA, cerebrovascular accident.
In the PGLA-treated group, there were 10 treatment-related complications in 7 (10%) patients. There were 4 instances of a stretched coil, one of which was associated with IA thrombus and required thrombolysis. The patient sustained no adverse neurologic consequences. One patient had a mild stroke presumed secondary to thromboembolic complications after an elective coiling. No device-related events occurred in this patient. There was 1 retroperitoneal hemorrhage that did not require a blood transfusion. One patient experienced thromboembolic complications from partial distal migration of a coil during treatment. Attempts to remove the coil were unsuccessful, and despite thrombolytic therapy, the patient sustained an infarct of the anterior and middle cerebral arteries and died.
In our study, there were no cases of chemical meningitis or excessive inflammatory response, as has been reported in a previous study.16 None of the aneurysms were treated with a combination of Matrix and other bioactive coils.
Discussion
There were statistically significant differences between the groups in 3 baseline demographic variables: age, pattern of circulation, and length of follow-up. We believe the small shift toward older patients and a higher percentage in the anterior circulation in the more recently treated cohort (Matrix coils) may reflect the expanding indications for endovascular versus open treatment that have taken place. The degree to which follow-up angiograms were available deserves some comment. There was follow-up information for 83% of patients in the Matrix group, and for 66% of patients in the GDC group. Overall, these rates are consistent with historical outcome studies with the GDC coil and are equal or greater than several recently published studies on bioactive coils.17, 18 There was a statistically significant longer interval of follow-up in the bare platinum group. This interval was expected because the bare platinum group was drawn from the year preceding the Matrix group. Also, this interval would not affect the outcomes because there was no association between length of follow-up and outcome (logistic regression, P =.94). There was also a statistically significant difference among the percentage of cases with 6-month follow-up between the groups. This difference was not enough to preclude meaningful statistical comparison.
A variety of angiographic outcome scales have been used in the past to assess the efficacy of coils. There are also a range of opinions as to what constitutes a “treatment failure,” and all of these may or may not impact the risk of aneurysmal rupture. We have used a common angiographic outcome scale, which did not demonstrate superiority of one coil type. Assessing the need for retreatment is also valuable and, in this instance, yielded evidence that bare platinum coils may be superior to Matrix bioactive coils. Fiorella et al19 also recently reported a similar rate of retreatment (13.8%) in a series of aneurysms treated with Matrix coils.
Given the bioactivity of Matrix coils, one might hypothesize interval occlusion and obliteration of any amount of aneurysmal filling that is present initially after treatment. The interval development of fibrosis at the neck of the aneurysm during follow-up has been described.20 However, the accelerated fibrosis and neointimal formation that have been seen in an animal experiment12 did not translate into an observable difference in angiographic outcomes in our patients. These data also demonstrate the finding that, again, one of the most significant predictors of ultimate angiographic outcome is the initial treatment result.
The inclusion of both narrow and wide-necked aneurysms as well as cases treated with the use of adjunctive devices such as stents or balloon remodeling was intentional, given the accrual of consecutive cases. This inclusion represents an accurate cross-section and a significant percentage of treated cases in our practice. Although not identical, we believe that the techniques are comparable, and the number of cases treated with either balloon remodeling or stent placement was similar, as was the number of wide-necked (≥4 mm) aneurysms in each group. We believe our institutional comparison is valuable also because some historical bare platinum outcome series were before the routine use of adjunctive measures (either balloon remodeling or stent-assisted coiling).
One weakness of our study was its research design. Assigning patients treated in different periods to different coil types resulted in differences in the demographics of the patients in the different coil groups. We performed multivariable analysis in an effort to compensate for these differences. The ideal design for a study of this type would involve random assignment to coil type, to remove any association with time, patient, or physician. Such a design would likely involve a multicenter, prospective setting and, unfortunately, has not been feasible up to this point. A prospective, randomized, multicenter, industry-sponsored Matrix and Platinum Science (MAPS) Trial is planned and may address these weaknesses in design.
Another criticism might be to question if the power of the study was sufficient to demonstrate a difference, given these rates of recurrence. The relatively small sample size is a weakness in a negative study, but it is a minor one. Statistical power is most important in negative studies in which there are clear trends that do not achieve statistical significance. The outcomes for the 2 coil types in our study were nearly identical; in fact, the trivial differences tended to favor bare platinum coils. Furthermore, multivariable analysis found 3 statistically significant variables (patient age, aneurysm size, and circulation pattern), so the failure of coil type to attain a statistically discernable result suggests that it can have, at most, very limited influence on the outcome. These shortcomings of our study aside, our analysis provided no evidence to support the hypothesis that Matrix coils provide superior angiographic outcomes compared with bare platinum coils.
Recently reported studies have demonstrated that treatment with bioactive coils is feasible and can be performed safely,17, 18, 21 that a greater packing attenuation can be achieved with coated coils,22 and comparison of single-center results are comparable to historical results with bare platinum coils.19 However, there has not been a report showing superior angiographic outcomes to bare platinum coils obtained with any of the available bioactive coils. Multiple studies have demonstrated only similar outcomes to historical results with bare platinum coils,23, 24 and 1 group has reported a worse rate of recanalization.25 In fact, Taschner et al26 recently reported a 20% rate of thromboembolic complications in their series of patients treated with a combination of bare platinum and Matrix coils. We did not find a statistical difference in the overall rates of complications between the bare platinum and Matrix populations (P = .88). In addition, our rate of thromboembolic complications in both groups was consistent with that reported previously.27
It has been our experience, and as reported by others,26 that the PGLA-coated coils are slightly more technically demanding to use. The Matrix coils require an extra step of preparation and are slightly harder to visualize under fluoroscopic guidance because of the relatively smaller amount of platinum in the coil volume. In addition, they are stiffer and seem to have more friction while in the delivery catheter and between previously placed coils. They are also sold at a cost premium, possibly because of an increased cost of production. It is important to emphasize that the coils evaluated in our study were first-generation Matrix coils and that newer-generation Matrix2 coils exist and may have yielded different results. The primary modification made to the coils relates to a smoother surface to the PGLA biopolymer coating and reduced coil-to-coil friction (unpublished data from Boston Scientific). Although these coils warrant further evaluation and will be evaluated in the MAPS trial, no studies have demonstrated improved clinical outcomes with Matrix2 coils compared with the first-generation Matrix coils.
Conclusion
Endovascular treatment of intracranial aneurysms with Matrix PGLA-coated coils is feasible with similar rates of treatment complications with bare platinum coils. However, they are significantly more costly and technically demanding to use. On the basis of the current results as well as those reported by others,19, 22 there is insufficient evidence to justify these disadvantages.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy or the Department of Defense, of the US Government.
References
- 1.Barrocas AM, Derdeyn CP, Cross DT III, et al. Histologic and hemodynamic effects of endosaccular platinum coils for intracranial aneurysms. J Long Term Eff Med Implants. 2004;14:225–42 [DOI] [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 3.Byrne JV, Sohn MJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcome and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 4.Friedman JA, Nichols DA, Meyer FB, et al. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol 2003;24:526–33 [PMC free article] [PubMed] [Google Scholar]
- 5.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 6.Ng P, Khangure MS, Phatouros CC, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke 2002;33:210–17 [DOI] [PubMed] [Google Scholar]
- 7.Raymond J, Roy D, Bojanowski M, et al. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg 1997;86:211–19 [DOI] [PubMed] [Google Scholar]
- 8.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand white rabbit model. AJNR Am J Neuroradiol 2003;24:591–96 [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon OK, Han MH, Oh CW, et al. Embolization with autologous fibroblast-attached platinum coils in canine carotid artery aneurysms: histopathological differences from plain coil embolization. Invest Radiol 2003;38:281–87 [DOI] [PubMed] [Google Scholar]
- 10.Murayama Y, Viñuela F, Suzuki Y, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II: an experimental study in a swine aneurysm model. AJNR Am J Neuroradiol 1999;20:1992–99 [PMC free article] [PubMed] [Google Scholar]
- 11.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke 1999;30:1657–64 [DOI] [PubMed] [Google Scholar]
- 12.Murayama Y, Tateshima S, Gonzalex NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 13.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004 [DOI] [PubMed] [Google Scholar]
- 14.Mericle RA, Wakhloo AK, Rodriguez R, et al. Temporary balloon protection as an adjunct to endosaccular coiling of wide-necked cerebral aneurysms: technical note. Neurosurgery 1997;41:975–78 [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 16.Meyers PM, Lavine SD, Fitzsimmons BF, et al. Chemical meningitis after cerebral aneurysm treatment using two second-generation coils: report of two cases. Neurosurgery 2004;55:E1222–E1227 [DOI] [PubMed] [Google Scholar]
- 17.Arthur AS, Wilson SA, Dixit S, et al. Hydrogel-coated coils for the treatment of cerebral aneurysms: preliminary results. Neurosurg Focus 2005;18:E1–9 [DOI] [PubMed] [Google Scholar]
- 18.Linfante I, Akkawi NM, Perlow A, et al. Polyglycolide/polylactide-coated platinum coils for patients with ruptured and unruptured cerebral aneurysms: a single-center experience. Stroke 2005;36:1948–53 [DOI] [PubMed] [Google Scholar]
- 19.Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with Matrix detachable coils. Neurosurgery 2006;58:51–59; discussion 51–9 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez NR, Patel AB, Murayama Y, et al. Angiographic evidence of aneurysm neck healing following endovascular treatment with bioactive coils. AJNR Am J Neuroradiol 2005;26:912–14 [PMC free article] [PubMed] [Google Scholar]
- 21.Bendszus M, Solymosi L. Cerecyte coils in the treatment of intracranial aneurysms: a preliminary clinical study. AJNR Am J Neuroradiol 2006;27:2053–57 [PMC free article] [PubMed] [Google Scholar]
- 22.Kang HS, Han MH, Kwon BJ, et al. Short-term outcome of intracranial aneurysms treated with polyglycolic acid/lactide copolymer-coated coils compared to historical controls treated with bare platinum coils: a single-center experience. AJNR Am J Neuroradiol 2005;26:1921–28 [PMC free article] [PubMed] [Google Scholar]
- 23.Mitra D, Herwadkar A, Soh C, et al. Follow-up of intracranial aneurysms treated with Matrix detachable coils: a single-center experience. AJNR Am J Neuroradiol 2007;28:362–67 [PMC free article] [PubMed] [Google Scholar]
- 24.Wong GK, Yu SC, Poon WS. Clinical and angiographic outcome of intracranial aneurysms treated with Matrix detachable coils in Chinese patients. Surg Neurol 2007;67:122–26; discussion 126 [DOI] [PubMed] [Google Scholar]
- 25.Niimi Y, Song J, Madrid M, et al. Endosaccular treatment of intracranial aneurysms using Matrix coils: early experience and midterm follow-up. Stroke 2006;37:1028–32 [DOI] [PubMed] [Google Scholar]
- 26.Taschner CA, Leclerc X, Rachdi H, et al. Matrix detachable coils for the endovascular treatment of intracranial aneurysms: analysis of early angiographic outcomes. Stroke 2005;36:2176–80 [DOI] [PubMed] [Google Scholar]
- 27.Derdeyn CP, Cross DT III, Moran CJ, et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg 2002;96:837–43 [DOI] [PubMed] [Google Scholar]