Abstract
BACKGROUND AND PURPOSE: Spinal arteriovenous shunts usually require digital subtraction angiography (DSA) for evaluation. We report a unique time-resolved spinal MR angiographic (TRSMRA) technique with a temporal resolution of 3–6 seconds and spatial resolution of approximately 1 mm3 that has the potential to noninvasively detect, localize, and follow-up these cases.
MATERIALS AND METHODS: Eleven patients with clinical presentation and/or MR findings suspicious for a spinal arteriovenous shunt were referred for TRSMRA. Patients subsequently underwent spinal DSA to confirm the presence or absence of a shunt or were followed clinically until an alternative diagnosis was found. TRSMRA was also used to predict the level of the shunt in the positive cases. In addition, 2 of these patients as well as a 12th patient referred to us posttreatment received a follow-up TRSMRA to assess treatment outcome.
RESULTS: Early venous shunting was identified by using TRSMRA in 6 cases. All 6 were confirmed to have an AV shunt on subsequent spinal DSA. The shunt level predicted by TRSMRA consistently correlated with DSA to within 1 vertebral level. In the 5 patients with a negative screening TRSMRA, DSA or clinical outcome confirmed the absence of an arteriovenous shunt in all of the cases. Posttreatment TRSMRA in 3 patients accurately assessed the success or failure of treatment.
CONCLUSION: Combining acceleration techniques to achieve high frame rate TRSMRA provides sufficient temporal and spatial resolution to identify, localize, and follow patients suspected of having a spinal arteriovenous shunt. Further study in a larger population is warranted to assess the accuracy of this technique.
Spinal vascular malformations represent a heterogeneous group of vascular anomalies. They are usually categorized into 4 types: spinal dural arteriovenous fistulas (DAVFs), intramedullary glomus malformations, extensive juvenile malformations, and perimedullary spinal cord arteriovenous fistulas, respectively.1 Spinal DAVF is the most common among them and is typically located along a nerve root sleeve within a neural foramen.2 The arterial supply is primarily from a dural branch of the radicular artery. The fistula drains intradurally via retrograde flow through a single medullary vein to the anterior or posterior median vein, resulting in engorgement of the coronal venous plexus.3 The lesions are more common in men, presenting at 40–60 years of age with symptoms of progressing myelopathy. The pathophysiology involves arteriovenous shunting of blood, causing venous hypertension and passive venous congestion, reduced intramedullary blood flow, and finally ischemia and cord dysfunction.4 Fortunately, these lesions are a treatable cause of myelopathy, and, therefore, early diagnosis is valuable.
Spinal vascular disease has been a diagnostic challenge in neuroimaging. Spinal arteriovenous shunt is a lesion that can mimic neoplasm, demyelination, or infection on physical examination and imaging.5 The most consistent MR signs for the presence of a shunt are seen on T2-weighted images as increased signal intensity in the central cord and enlarged subarachnoid flow voids; however, the cord signal intensity is seen over a mean of 6–7 vertebral levels and is not predictive of the shunt level.6 MR angiography techniques, such as phase contrast, are most sensitive to higher velocity lesions and are not capable of detecting low velocity lesions. Time-of-flight techniques provide static anatomic detail, but with acquisition times in the order of minutes, they lack sufficient temporal resolution to elucidate the hemodynamic properties of this lesion. Vessels that appear to fill on sequential images on digital subtraction angiography (DSA) are seen in only a single image on 2D acquisition or single volume in 3D acquisition. Because of this limitation, once an arteriovenous shunt is suspected clinically and with conventional MR imaging, intra-arterial DSA is often required to confirm the diagnosis and localize the site.
The purpose of this case series is to describe a new, noninvasive time-resolved MR angiographic (MRA) technique, which combines a rapid multiphase dynamic MRA acquisition with parallel imaging to provide a temporal resolution of 3–6 seconds and spatial resolution of approximately 1 mm isotropic.7–11 We present our preliminary experience with this technique in the diagnosis, pretreatment localization, and posttreatment follow-up in 12 patients with a suspected arteriovenous shunt.
Materials and Methods
From April 2004 to February 2006, 11 consecutive patients (6 men and 5 women; age range, 29–77 years; mean age, 51.5 years) with initial MR and clinical findings suggestive of the presence of a spinal arteriovenous shunt were referred for time-resolved spinal MR angiographic (TRSMRA).
Two neuroradiologists experienced in spinal DSA and TRSMRA techniques performed a consensus interpretation to identify patients at high suspicion (early venous shunting) or low suspicion (no evidence of early venous shunting). The level of the arteriovenous shunting was identified by cine review of anterograde flow characteristics, morphologic transition from feeding arteries to arterialized veins, and correlation with unsubtracted source data. Based on the results, patients with a high suspicion of an arteriovenous (AV) shunt underwent a conventional intra-arterial spinal DSA to confirm the diagnosis, plan, and execute treatment. The subsequent DSA was planned to include the suspicious vertebral level plus or minus no less than 2 adjacent levels. Patients who were deemed to have a lower likelihood of a shunt on TRSMRA (no evidence of early venous drainage) either underwent a spinal DSA or were followed clinically until an alternative diagnosis was made. One patient with low-suspicion TRSMRA underwent spinal DSA at the request of the clinical service. The TRSMRA results were compared with the DSA results. In subjects where DSA was not performed, clinical follow-up was used as a surrogate for the absence of a shunt.
Two patients received posttreatment TRSMRA to assess for partial or complete obliteration of the lesion. A 12th patient (70-year-old man), who had been diagnosed previously and treated for DAVF, was referred to us for posttreatment follow-up TRSMRA and was included in the analysis.
Image Acquisition
Imaging of patients with a suspected spinal arteriovenous shunt was performed on a 1.5T whole-body MR scanner (Avanto; Siemens Medical Solutions, Erlangen, Germany) with an abdominal array. The spinal neuroaxis was evaluated with a sagittal acquisition including the entire spinal canal from lateral foramen to lateral foramen. The entire spinal axis was covered in 2 overlapping acquisitions with varying cranio-caudal coverage. A 3D gradient-echo multiphase pulse sequence (TR/TE: 2.8 ms/1.2 ms; flip angle 20°; RF and gradient spoiling; bandwidth 500–600 Hz/pixel; 512 readout; 40–44 sections; 1.2 × 0.7 × 1.3 mm voxels; section thickness 1.6–2.5 mm depending on the temporal resolution; 75% partial Fourier in all 3 dimensions; generalized autocalibrating partially parallel acquisition 2 times acceleration per 24 reference lines; and 3 time-resolved imaging of contrast kinetics [TRICKS] regions) was used for imaging. The frame rate of this sequence ranged from 2.32 to 6.75 seconds (depending on body habitus, FOV, etc). An injection of 15 mL of a gadolinium-based contrast agent (Magnevist; Berlex, Princeton, NJ) was administered in an antecubital vein using a power injector at a rate of 5.0 mL/s at the initiation of the image acquisition (average image acquisition time approximately 30–45 seconds based on the number of sections) and concurrent with the breath hold. The gadolinium bolus was followed with an equal volume saline chase. Datasets were subtracted in-line, and maximum intensity projection (MIP) processing produced a time series of images throughout the passage of the bolus of contrast agent through the aorta, spinal and radicular arteries, and radicular, intervertebral, vertebral, ascending lumbar, and azygous veins.
The TRICKS7 acquisition involves segmenting the ky–kz plane of k-space into regions, where the readout is along kx and phase-encoding is along ky and kz. In this protocol, the 3 regions consisted of an equal number of lines at low, middle, or high spatial frequencies. For every frame, the low frequency region was always acquired, but only one of the middle and high frequency regions was acquired in an alternating fashion. The unacquired region was copied from the subsequent frame to fill in the missing data. The frame rate is effectively increased by a factor of 1.5.
Results
Twelve patients underwent 16 TRSMRA examinations yielding 7 true-positives (6 initial diagnoses and 1 follow-up), 9 true-negatives, 0 false-positives, and 0 false-negatives (Tables 1 and 2). TRSMRA showed early venous shunting in 6 of the 11 patients with a suspected arteriovenous shunt, all of which were confirmed with spinal DSA. In these 6 true-positive cases, the location of the shunt predicted by TRSMRA corresponded with the conventional angiography findings to within 1 vertebral level in all 6 cases (Table 3). In the remaining 5 cases, initial MR findings and/or clinical presentation were moderately suspicious for the presence of an arteriovenous shunt, but TRSMRA did not visualize early venous shunting. One of these patients received a subsequent spinal DSA, which confirmed the absence of a shunt. In the remaining 4 cases, spinal DSA was not performed for multiple reasons, including history, presentation, and clinical course. Two of these patients were treated for transverse myelitis as part of Sjogren and Devic syndromes and showed dramatic improvement with a course of intravenous corticosteroids. Similarly, the remaining 2 patients were followed for more than 1 year and demonstrated a benign clinical course and/or improvement in MR imaging findings. Although confirmatory DSA would have been preferred in these patients, we took their clinical outcome as evidence for the absence of a spinal arteriovenous shunt and characterized them as true-negatives.
Table 1:
TRSMRA for diagnosis
| Case No. | Age, y/Gender | TRSMRA | DSA | Diagnosis* | Result |
|---|---|---|---|---|---|
| 1 | 36/F | Positive | Positive | Perimedullary fistula | TP |
| 2 | 43/F | Negative | Not done | Neurovascular Sjogren | TN |
| 3 | 61/F | Negative | Negative | Spinal SAH from indeterminate etiology | TN |
| 4 | 73/M | Positive | Positive | DAVF | TP |
| 5 | 35/M | Negative | Not done | Devic syndrome | TN |
| 6 | 50/M | Negative | Not done | Symptoms from dystrophic calcification and vascular ectasia | TN |
| 7 | 68/M | Positive | Positive | DAVF | TP |
| 8 | 29/F | Positive | Positive | AVM | TP |
| 9 | 33/M | Positive | Positive | DAVF | TP |
| 10 | 77/F | Positive | Positive | AV shunt in nerve sheath tumor | TP |
| 11 | 61/M | Negative | Not done | Multiple sclerosis | TN |
Note:—F indicates female; M, male; DAVF, spinal dural arteriovenous fistula; AVM, arteriovenous malformation; AV, arteriovenous; SAH, subarachnoid hemorrhage; TP, true positive; TN, true negative.
Ultimate diagnosis.
Table 2:
TRSMRA for follow-up
| Case No. | Age (Gender) | TRSMRA | DSA | Follow-Up Assessment | |
|---|---|---|---|---|---|
| 4 (a) | 73/M | Positive | Positive | Residual fistula | TP |
| 4 (b) | Negative | Not done | No residual shunting | TN | |
| 10 (a) | 77/F | Negative | Negative | No residual shunting | TN |
| 12 (a) | 70/M | Negative | Negative | No residual shunting | TN |
| 12 (b) | Negative | Negative | No residual shunting | TN |
Note:—F indicates female; M, male; TP, true positive; TN, true negative.
Table 3:
Predicted and actual levels of fistulous connection
| Case No. | TRSMRA Localization | DSA Localization |
|---|---|---|
| 1 | Conus | Conus |
| 4 | Left S3 | Left S4 |
| 7 | Left T10 or T11 | Left T10 |
| 8 | Cervicothoracic junction | C6 to T2 |
| 9 | Right T5 or T6 | Right T6 |
| 10 | Right L5 | Right L5 |
Three of the patients received follow-up TRSMRA (Table 2). In case 4, TRSMRA identified a persistently patent fistula on follow-up (case 4a) despite attempted endovascular embolization. Subsequent DSA confirmed this finding. Surgical obliteration was performed, and a postoperative TRSMRA (case 4b) showed complete obliteration of the shunt. Clinically, the patient demonstrated a resolution of his condition. In case 10, endovascular embolization was performed to treat the DAVF. Posttreatment TRSMRA (case 10a) showed absence of AV shunting; subsequent spinal DSA confirmed this finding. Case 12 had been diagnosed previously and treated for DAVF and was referred to us for follow-up imaging (Table 2). TRSMRA demonstrated no AV shunting (case 12a), and subsequent spinal DSA confirmed this finding. Follow-up TRSMRA 4 months later (case 12b) was also negative. Spinal DSA again confirmed this finding.
To further illustrate the utility of the technique, we describe 1 case in detail. Case 4 was a 73-year-old man who presented with progressive lower extremity weakness. Initial MR showed enlarged spinal veins from foramen magnum to sacrum and increased T2 signal intensity from level T8 through L4, findings highly suspicious for a spinal DAVF (Fig 1A, -B). TRSMRA localized the fistula in the left pelvis at approximately S3 (Fig 1C–H). Spinal DSA confirmed a sacral DAVF at the S4 level, and embolization was attempted (Fig 1I). At the end of the procedure, the dural fistula was seemingly obliterated. Five days later, a TRSMRA was performed that showed persistent arteriovenous shunting. Spinal DSA confirmed the residual fistula, and repeat embolization was unsuccessful. After surgical excision, postoperative TRSMRA showed no evidence of a persistent AV shunt.
Fig 1.
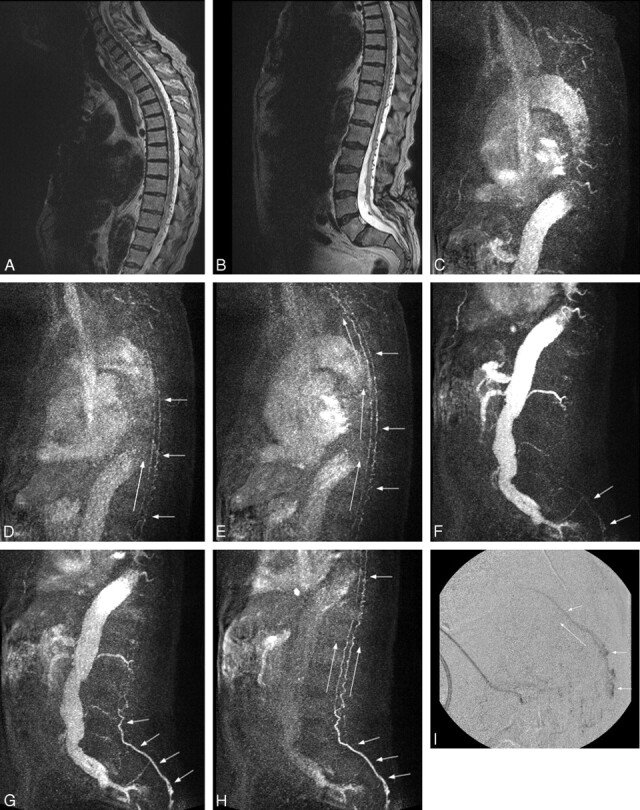
A and B, Sagittal T2-weighted sequence in case 4 demonstrates serpiginous flow voids along the ventral and dorsal spinal cord from the cervical region to the sacrum. Increased T2-signal intensity is seen within the distal cord/conus. These findings are highly suspicious for a fistula, the level of which cannot be determined on these conventional images. The patient has incidental low-lying conus. C–E, Three MIP partitions from a sagittal TRSMRA (temporal resolution = 5.07 seconds) centered in the cervical and upper thoracic spine show early filling of venous structures on the ventral and dorsal surface of the cord (short arrows). Filling proceeds from caudal to cephalad (long arrows). F–H, Three MIP partitions from a sagittal TRSMRA (temporal resolution = 5.07 seconds) centered in the thoracic, lumbar, and upper sacral spine demonstrate early venous filling (short arrows) beginning within the pelvis and proceeding cephalad (long arrows). Unsubtracted images (not shown) showed the earliest venous drainage within the sacrum. A coronal oblique acquisition further localized the fistula to approximately the S3 level. I, Lateral projection from a superselective catheter spinal DSA shows early venous filling within the sacral canal proceeding cephalad compatible with a spinal DAVF. This correlates with the appearance and physiologic dynamics demonstrated in F–H.
Discussion
With normal artery-to-vein transit time of approximately 10–20 seconds in the spinal cord,12 visualization of spinal arteriovenous shunts demands a high frame rate image acquisition technique to capture the arterial and venous phases. Accordingly, conventional spinal DSA, with temporal resolution of up to 10 frames per second and the ability to selectively inject contrast at individual arterial levels, is considered the “gold standard” for diagnosing AV fistula and other AV malformations. DSA can define the precise location of the shunt, as well as the feeding and draining vessels.
Despite the advantages, spinal DSA is invasive and associated with several risks. It is a costly and tedious procedure, requiring as many as 40 injections into multiple bilateral thoracic intercostals, lumbar, and sacral arteries.13 Concomitantly the patient is exposed to long fluoroscopy times and a large volume of iodinated contrast agents. Preangiographic localization by noninvasive imaging can allow subsequent targeting of DSA to a few spinal levels and cut down on the risks of a complete examination. Although the rate of complications from spinal angiography is low,14 one can anticipate a further reduction in morbidity and mortality if the procedure time can be shortened. In addition, if the TRSMRA technique is shown to be adequately sensitive and specific in a large sample size, reliance on DSA for diagnosis, treatment planning, and follow-up could be decreased.
For these purposes, several groups have attempted to develop MRA techniques to diagnose and localize arteriovenous shunts.15–19 Initially, 2D and 3D phase-contrast techniques were developed.20,21 In a study by Bowen et al15 in 1995, a contrast-enhanced (CE) 3D time-of-flight sequence with an 11-minute acquisition time was applied in 8 patients with spinal DAVFs. Abnormal intradural vessels were identified in all 8, and in 6 of these patients the medullary vein draining the fistula was demonstrated, indicating the level of the fistula.15 Using a 3D CE MRA sequence in 21 patients, Saraf-Lavi et al16 reported a 50% accuracy of predicting the correct fistula level and a 73% accuracy with the correct level ±1 level. Binkert et al17 used 3D CE MRA sequence in spinal vascular disease patients and detected arteriovenous shunting in 2 of 3 cases of DAVF. Later, in 2002, Farb et al18 used an auto-triggered elliptic centric-ordered 3D gadolinium-enhanced sequence in 9 patients and after multiple imaging sessions, detected and localized AV shunting in 8 of these patients.
More recently, Luetmer et al19 used elliptic centric CE MRA with a 49-second acquisition time to correctly diagnose DAVF in 20 of 22 patients and determine the level of fistula to within 1 level in 13 of these cases. Assessing the effect of MRA on subsequent conventional angiography, this study showed a 50% reduction in the fluoroscopy time and contrast volume if preangiographic localization is performed. In our experience, a preprocedure spinal TRSMRA study similarly reduced the number of arterial injections, the amount of iodinated contrast used, the amount of radiation exposure, and the procedure time.
CT angiography is also being explored as a tool for diagnosing spinal arteriovenous malformations. Lai et al22,23 recently reported a multidetector CT angiography technique to detect and localize spinal DAVFs. Their results with 8 patients show promise in a technique other than MRA for noninvasive diagnosis of AV shunts; however, the use of radiation and iodinated contrast are disadvantages of CTA in comparison with MRA techniques. In addition, despite improvement in temporal resolution with 64-section CT, the acquisition technique does not allow for cine review of the entire spine, and fistula localization method is similar to that of “unresolved” spinal MRA techniques.
Investigators have suggested the development of parallel imaging techniques to further refine spinal MRA and decrease imaging times without sacrificing spatial resolution.19 Our technique for TRSMRA combines TRICKS with parallel imaging, partial Fourier, and very short time of repetition.11 It has evolved, with temporal resolution of 5–6 seconds in the initial patients, improving to 3 seconds in the more recent patients. There are, however, disadvantages to this technique. As temporal resolution is increased, spatial resolution and/or signal-to-noise-ratio (SNR) may be compromised. With our technique, we maintained a spatial resolution of approximately 1-mm isotropic and traded off SNR. The increased noise that resulted was perceived to be minimal and did not render the images nondiagnostic. Therefore, the tradeoff was favorable. Although limited to a small series of patients, our MRA technique was able to identify venous shunting and correctly diagnose all 6 of the positive cases. We were able to localize the shunt site to within 1 vertebral level in the 6 positive cases. Despite the lack of spinal DSA examination in several of the negative TRSMRA cases, clinical outcome allowed us to characterize the remaining 6 of our cases as true-negatives. As such, we did not encounter any false-negative or false-positive cases.
Previous groups have used temporally “unresolved” spinal MRA techniques, such as elliptic centric-ordered 3D CE MRA,18,19 for diagnosing arteriovenous shunts and have been able to detect and localize these lesions in a most of their patients. High frame rate is, therefore, not an absolute requirement for localization of these lesions; however, the novelty of time-resolved MRA techniques is the ability to provide increased confidence in fistula localization, which primarily relates to the ability to allow evaluation of flow in an anterograde fashion rather than inferring it in retrograde fashion, as has been described by previous authors. The clinical use of time-resolved imaging may also be appreciated by taking an example of a treated fistula: enlarged veins can be seen on MRA performed both before and after treatment; however, by using a technique with a good temporal resolution, one can distinguish enlarged veins that fill early (pretreatment) versus those that fill later (posttreatment). Unresolved MRA techniques with good spatial resolution may allow visualization of the intradural veins; however, they cannot make the distinction of early versus normal venous filling or demonstrate anterograde filling of the lesion like DSA. Other advantages of the TRSMRA technique include a shorter image acquisition time and the lack of requiring contrast agent bolus timing.
Limitations of this study include the small sample size, lack of the gold-standard spinal DSA for all cases, and the nonstandardized technique. First, though there were no false-positives, false-negatives, or inaccurate localization in our series of patients, true sensitivity, specificity, and predictive value of the technique can only be determined by larger studies. Second, DSA was not performed in most of our negative cases and would have been desirable as evidence for the absence of an arteriovenous shunt versus clinical evidence alone. However, the procedure is not a minor undertaking, and patients with a lower suspicion for an arteriovenous shunt warranted a less rigorous evaluation. Third, the technique that we used for TRSMRA used standard imaging parameters but was nonstandard in relation to the temporal resolution, varying from patient to patient from 3 to 6 seconds. Fortunately, we were able to accurately visualize the presence and location of the shunting across that range of temporal resolutions. Finally, it should be realized that because of the principal difference in the amount and length of contrast injections between MRA and DSA, our MRA technique, like all other noninvasive techniques, cannot simulate direct arterial injections; it can, therefore, be better equated with performing DSA with a single aortic injection rather than injections at selective arterial levels.
Conclusion
Our technique presents improved temporal and spatial resolution compared with the previously reported spinal MRA techniques for the evaluation of spinal vascular malformations and has shown promising results. Time-resolved imaging allows for anterograde evaluation of normal and abnormal blood flow from arterial to venous phases and has added value compared with unresolved MRA techniques. We have used this technique to diagnose patients with a high suspicion of spinal AV shunts, exclude the diagnosis in a subset with lower suspicion, localize the level in positive cases, and follow the effectiveness of treatment. If established to be sensitive and specific, TRSMRA may be used adjunctively to reduce the costs, time, morbidity, and mortality associated with conventional spinal DSA.
References
- 1.Spetzler RF, Detwiler PW, Riina HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg 2002;96:145–56 [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson JR, Miller GM, Goldman MS, et al. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol 1995;16:2049–57 [PMC free article] [PubMed] [Google Scholar]
- 3.Kendall BE, Logue V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology 1977;13:181–89 [DOI] [PubMed] [Google Scholar]
- 4.Aminoff MJ, Barnard RO, Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci 1974;23:255–63 [DOI] [PubMed] [Google Scholar]
- 5.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg 1984;60:238–47 [DOI] [PubMed] [Google Scholar]
- 6.Koch C, Kucinski T, Eckert B, et al. Spinal dural arteriovenous fistula: clinical and radiological findings in 54 patients [in German]. Rofo 2003;175:1071–78 [DOI] [PubMed] [Google Scholar]
- 7.Korosec FR, Frayne R, Grist TM, et al. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med 1996;36:345–51 [DOI] [PubMed] [Google Scholar]
- 8.Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med 1997;38:591–603 [DOI] [PubMed] [Google Scholar]
- 9.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002;47:1202–10 [DOI] [PubMed] [Google Scholar]
- 10.Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952–62 [PubMed] [Google Scholar]
- 11.Cashen TA, Carr JC, Shin W, et al. Intracranial time-resolved contrast-enhanced MR angiography at 3T. AJNR Am J Neuroradiol 2006;27:822–29 [PMC free article] [PubMed] [Google Scholar]
- 12.Launay M, Chiras J, Bories J. Angiography of the spinal cord: venous phase. Normal features. Pathological application. J Neuroradiol 1979;6:287–315 [PubMed] [Google Scholar]
- 13.Willinsky R, Lasjaunias P, Terbrugge K, et al. Angiography in the investigation of spinal dural arteriovenous fistula. A protocol with application of the venous phase. Neuroradiology 1990;32:114–16 [DOI] [PubMed] [Google Scholar]
- 14.Forbes G, Nichols DA, Jack CR Jr, et al. Complications of spinal cord arteriography: prospective assessment of risk for diagnostic procedures. Radiology 1988;169:479–84 [DOI] [PubMed] [Google Scholar]
- 15.Bowen BC, Fraser K, Kochan JP, et al. Spinal dural arteriovenous fistulas: evaluation with MR angiography. AJNR Am J Neuroradiol 1995;16:2029–43 [PMC free article] [PubMed] [Google Scholar]
- 16.Saraf-Lavi E, Bowen BC, Quencer RM, et al. Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: sensitivity, specificity, and prediction of vertebral level. AJNR Am J Neuroradiol 2002;23:858–67 [PMC free article] [PubMed] [Google Scholar]
- 17.Binkert CA, Kollias SS, Valavanis A. Spinal cord vascular disease: characterization with fast three-dimensional contrast-enhanced MR angiography. AJNR Am J Neuroradiol 1999;20:1785–93 [PMC free article] [PubMed] [Google Scholar]
- 18.Farb RI, Kim JK, Willinsky RA, et al. Spinal dural arteriovenous fistula localization with a technique of first-pass gadolinium-enhanced MR angiography: initial experience. Radiology 2002;222:843–50 [DOI] [PubMed] [Google Scholar]
- 19.Luetmer PH, Lane JI, Gilbertson JR, et al. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol 2005;26:711–18 [PMC free article] [PubMed] [Google Scholar]
- 20.Gelbert F, Guichard JP, Mourier KL, et al. Phase-contrast MR angiography of vascular malformations of the spinal cord at 0.5 T. J Magn Reson Imaging 1992;2:631–36 [DOI] [PubMed] [Google Scholar]
- 21.Mascalchi M, Quilici N, Ferrito G, et al. Identification of the feeding arteries of spinal vascular lesions via phase-contrast MR angiography with three-dimensional acquisition and phase display. AJNR Am J Neuroradiol 1997;18:351–58 [PMC free article] [PubMed] [Google Scholar]
- 22.Lai PH, Pan HB, Yang CF, et al. Multi-detector row computed tomography angiography in diagnosing spinal dural arteriovenous fistula: initial experience. Stroke 2005;36:1562–64 [DOI] [PubMed] [Google Scholar]
- 23.Lai PH, Weng MJ, Lee KW, et al. Multidetector CT angiography in diagnosing type I and type IVA spinal vascular malformations. AJNR Am J Neuroradiol 2006;27:813–17 [PMC free article] [PubMed] [Google Scholar]


