Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to analyze angiographic and clinical results before and after additional endovascular therapy in patients with previously coiled but reopened cerebral aneurysms and to identify possible risk factors for retreatment of an aneurysm.
MATERIALS AND METHODS: Follow-up with selective digital subtraction angiography was performed in 323/596 (54.2%) patients harboring 342 aneurysms with a mean follow-up time of 28.6 months. The patients were divided into 3 groups: group A, who remained stable after initial treatment; group B, who showed minor morphologic changes; and group C, who underwent repeat treatment. Univariate and multivariate regression analyses were performed to determine possible risk factors for aneurysmal retreatment.
RESULTS: Single or multiple retreatment was performed in 33 of 323 (10.2%) patients. Retreatment of small aneurysms (≤10 mm) with small necks (≤4 mm) was performed in 6 of 214 aneurysms. When summarizing all other aneurysms as a “risk group” (n = 128), the odds ratio (OR) for retreatment in the “risk group” was 3.11 (95% CI: 1.43–6.75; P = .004). In patients with residual aneurysm after the first treatment, OR for retreatment was 3.96 (95% CI: 1.48–10.65; P = .006), whereas a neck remnant, clinical presentation, and aneurysmal localization were not predictive. We observed no resulting morbidity and mortality from the 33 retreatment procedures.
CONCLUSION: In our series, the retreatment of aneurysmal recurrences was a safe procedure. The best single predictors of aneurysmal recurrence were aneurysmal anatomy (neck width >4 mm and diameter >10 mm) and the presence of a residual aneurysm after initial treatment. A limitation in our study was the significant number of patients lost to follow-up (22.7%).
Endovascular coil embolization of cerebral aneurysms is associated with low morbidity and mortality rates and has become widely used in patients with ruptured and unruptured intracranial aneurysms.1–8
Recent data on the incidence of rebleeding after endovascular aneurysm therapy indicate that the rate is as low as 0.11% to 0.32% p.a.5, 9, 10
A major concern of endovascular treatment is the possibility of reopening of a coiled aneurysm and the necessity of a retreatment with its inherent risks and costs.11–13 Nevertheless, since the estimates for aneurysm recurrence can be expected in a range from 6.1% to 33.6%14–16 after endovascular treatment, the issue of a retreatment must be discussed individually with each patient. In symptomatic patients or patients with a major aneurysmal reopening, the decision to treat is made more easily. In minor aneurysmal reopening, careful decision-making for either conservative observation or retreatment must balance the procedure-related risk of retreatment against the risk of a bleeding or rebleeding.
New bioactive coil materials were developed to prevent recurrence and retreatment.17 The first clinical observations of these devices, however, reveal variable results.18–23
We present the data of our institutional review on both the stability of endovascular treatment in patients with aneurysm and the rate, morbidity, and mortality of the retreatment procedures. Previous papers have focused on the rate of aneurysmal recurrences.14–16 In this study, we compare patients who had aneurysmal recurrences that required further treatment and those with recurrences who were subject to further follow-up without intervention. On the basis of these data, we sought to identify the parameters to predict the clinically relevant (since re-treated) aneurysmal recurrences. These characteristics might allow the identification of patients who are most likely to benefit primarily from advanced treatment (eg, bioactive coils), who might become the target group for innovative methods of treatment to reduce sample size for clinical trials.
Patients and Techniques
Between November 1992 and December 2005, endovascular aneurysmal embolization with Guglielmi detachable coils (GDC; Boston Scientific, Natick, Mass) was performed in 596 consecutive patients harboring 627 aneurysms at our institution. A total of 323 patients harboring 342 aneurysms with available angiographic follow-up evaluation at 5 months or more (range, 5–132 months; mean, 28.6 months) were included in this study.
Selective digital subtraction angiography (DSA) was performed as follow-up investigation in all included patients. MR angiography (MRA) as a noninvasive adjunct to DSA was performed in 241 (70.5%) aneurysms. When results of the DSA and MRA were comparable at follow-up, a changeover to MRA as a single follow-up technique has been carried out in long-time stable cases (n = 182, 53.2%).
For comparative purposes, the patients were divided into 3 groups:
Group A: These patients remained stable after initial treatment and showed no morphologic changes compared with the results after treatment (252 patients, 270 aneurysms).
Group B: Patients in this group showed minor morphologic changes compared with initial treatment results at any further checkup. The changes were categorized as minor in a minimal coil compaction at the aneurysmal neck. Therefore, they were not referred for re-treatment. On additional control angiograms, the morphologic situation in these patients remained stable (38 patients, 39 aneurysms).
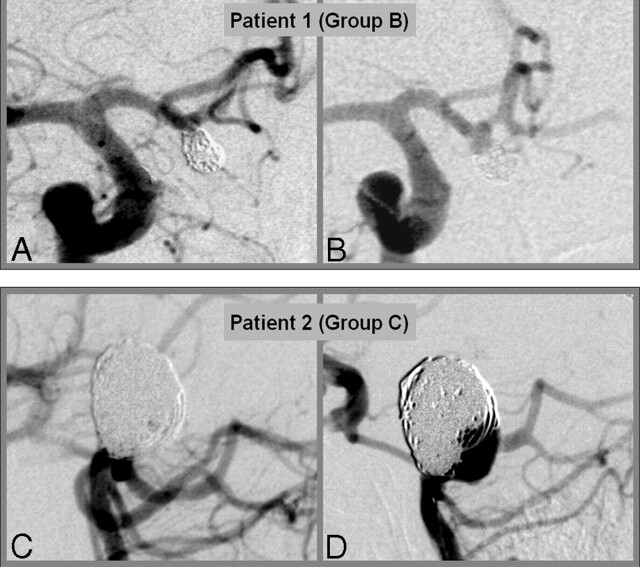
Group C: These patients showed major morphologic changes compared with initial treatment results at any further checkup. The changes were categorized as major as a result of a growth of an aneurysmal part not covered by coils in terms of significant coil loosening, coil compaction, or coil mass extrusion beyond the initial aneurysmal boundary, and contrast filling within the aneurysmal sac. This group received either surgical or endovascular repeat treatment (33 patients, 33 aneurysms). Examples from patients categorized in groups B and C are displayed in Fig 1.
Fig 1.
Examples for aneurysms categorized into group B (A,B) and group C (C,D).
A,C, Initial treatment result.
B,D, Situation at 6 months with control angiogram.
All treatments in our series as reported were done with bare platinum coils (Boston Scientific and Micrus Endovascular, San Jose, Calif). All stents used in our patients were Neuroform (Boston Scientific). Because of the relatively low total number of patients treated with remodelling techniques, a subgroup analysis was not conducted. There were 7 stenting procedures in group A, none in group B, and 1 in group C and 3 ballooning procedures in group A, 1 in group B, and none in group C.
Definition of Angiographic Results
After GDC embolization, multiple angiographic projections were obtained to assess the result according to the classification as proposed by Roy et al.24 Embolization was considered to be complete (c/o) if there was no contrast filling of the dome, body, or neck of the aneurysm. A neck remnant (n/r) was defined as residual filling of the neck or part of the neck of an aneurysm; a residual aneurysm (r/a) was indicated by contrast agent in the body or dome, or both, of an aneurysm. A recurrence was defined as any increase in aneurysmal filling at follow-up. A decreased filling in terms of a progressive thrombosis was categorized as a stable result. Aneurysmal size was calculated on the basis of a calibration method with an affixed coin of known dimensions.
Follow-Up Strategy and Selection Criteria for Retreatment
Follow-up cerebral angiograms were scheduled at 6 and 12 months after embolization. In stable cases, we requested MRA every 12 months to evaluate aneurysmal reopening. If follow-up angiograms demonstrated evidence of minor aneurysmal recanalization in terms of minimal coil compaction at the aneurysmal neck (Fig 1), another 6-month follow-up was recommended.
Additional treatment (endovascular coil embolization or direct surgical clipping) was suggested if the recurrence was categorized as morphologically significant (Fig 1). Recurrences were categorized to be significant if 1 or more of the following morphologic modifications were given: coil loosening with increasing patency between loops, resulting in progressive contrast filling within the aneurysmal sac; coil compaction with unchanged aneurysmal boundaries, but exposition of an aneurysmal part not covered by coils; and aneurysmal regrowth in increasing boundaries compared with initial aneurysmal size. The major criterion for the decision for retreatment was the exposure of the aneurysmal sac uncovered by coils that resulted in a recurrence of more than 2 mm to provide an appropriate cavity for an additional placement of coils (Fig 1). On the basis of multiple projection images (DSA), the most senior-level neuroradiologist available in the angiography laboratory (U.G., H.Z., J.F.) made the decision whether an aneurysm had to be treated again.
Demographic and Clinical Data
The male-to-female ratio was 1:2.8 (group A), 1:3.3 (group B), and 1:1.5 (group C). The median age of the patients was 50 years (range, 12–80 years) in group A, 51 years (range, 22–76 years) in group B, and 51 years (range, 18–77 years) in group C. Patients with subarachnoid hemorrhage (SAH) were categorized according to the scale of Hunt and Hess (HH). Table 1 displays the clinical presentation before initial treatment. To classify patient outcome, we used the Glasgow Outcome Scale (GOS) with the following categories: 1) dead, 2) vegetative state, 3) severe disability, 4) moderate disability but able to live independently, and 5) good recovery, able to return to work.
Table 1:
Absolute numbers and relative parts of aneurysms in groups A, B, and C*
| Characteristics | Group A Aneurysms (n = 270) (%) | Group B Aneurysms (n = 39) (%) | Group C Aneurysms (n = 33) (%) |
|---|---|---|---|
| Hunt and Hess grade | |||
| No SAH | 101 (37.4) | 17 (43.6) | 10 (30.3) |
| I | 56 (20.7) | 11 (28.2) | 8 (24.2) |
| II | 32 (11.9) | 6 (15.4) | 4 (12.1) |
| III | 45 (16.7) | 3 (7.7) | 5 (15.2) |
| IV | 17 (6.3) | 0 (0.0) | 1 (3.0) |
| V | 19 (7.0) | 2 (5.1) | 5 (15.2) |
| Initial treatment result | |||
| Complete occlusion (c/o) | 197 (73.0) | 25 (64.1) | 16 (48.5) |
| Neck remnant (n/r) | 49 (18.1) | 10 (25.6) | 8 (24.2) |
| Residual aneurysm (r/a) | 24 (8.9) | 4 (10.3) | 9 (27.3) |
| Aneurysm size | |||
| Small (≤10 mm) and small neck (≤4 mm) | 181 (67.0) | 27 (69.2) | 6 (18.2) |
| Small (≤10 mm) and wide neck (>5 mm) | 41 (15.2) | 1 (2.6) | 11 (33.3) |
| Large (>10 mm) and small neck (≤4 mm) | 10 (3.7) | 0 (0.0) | 4 (12.1) |
| Large (>10 mm) and wide neck (>4 mm) | 38 (14.1) | 11 (28.2) | 12 (36.4) |
| Aneurysm location | |||
| ICA | 59 (21.9) | 10 (25.6) | 6 (18.2) |
| ACA/AcomA | 67 (24.8) | 7 (17.9) | 7 (21.2) |
| MCA | 23 (8.5) | 1 (2.6) | 3 (9.1) |
| PCA/PcomA | 34 (12.6) | 5 (12.8) | 3 (9.1) |
| BA | 65 (24.1) | 15 (38.5) | 14 (42.4) |
| VA | 22 (8.1) | 1 (2.6) | 0 (0.0) |
Note:—ICA indicates internal carotid artery; ACA, anterior cerebral artery; AcomA, anterior communicating artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; PcomA, posterior communicating artery; BA, basilar artery; VA, vertebral artery; SAH, subarachnoid hemorrhage.
Complete treatment (group A), recurrence without retreatment (group B), and recurrence with retreatment (group C).
Data Analysis
We performed statistical analysis with SPSS version 13 (SPSS, Chicago, Ill). We calculated risk for anatomic localizations, aneurysmal anatomy, clinical presentation (HH), and initial treatment result. We performed univariate binary logistic regression analysis to identify predictors of aneurysmal retreatment. Parameters with a P value of <0.1 in the univariate analysis were entered into a multivariate logistic regression analysis. The odds ratio (OR) with 95% confidence intervals (95% CI) was calculated for these groups. The positive predictive value (PPV) and negative predictive value (NPV) were calculated if the risk was significantly increased. Significance level was predefined as P < .05.
Results
Follow-up angiography was performed in 323 of 596 (54.2%) patients harboring 342 aneurysms. Mean angiographic follow-up for these patients was 28.6 months (range, 6–132 months). Reasons for absence of angiographic follow-up included angiograms performed elsewhere (12/596, 2.0%), patients refusing follow-up (14/596, 2.4%), patients in poor clinical condition (37/596, 6.2%), patients dying after primary SAH (75/596, 12.6%), and patients lost to follow-up (135/596, 22.7%).
Aneurysmal recurrences were found in 72 of 342 (21.1%) treated aneurysms with available follow-up data. Aneurysmal reopening was detected at a mean of 17.9 months (range, 0–77 months) with first retreatment sessions performed at a mean of 16.6 months. Second and third retreatment sessions were conducted on an average of 27.8 months (range, 5–63 months) after the previous procedure.
Retreatment was done in 33 of 342 (9.6%) aneurysms. Single treatment of an aneurysmal recurrence was performed in 26 of 342 (7.6%) patients. A total of 4 patients (1.2%) underwent repeat treatment twice and 3 patients (0.9%), 3 times. Three patients received surgical clipping as first retreatment. In one of these patients, a second retreatment procedure was performed with GDC embolization. Hemorrhage recurred in 5 of 323 (1.6%) patients with follow-up angiograms during the entire period of observation.
Regression Analysis
In the univariate regression analysis, the category of “unfavorable anatomy” (aneurysmal size >10 mm and/or neck width >4 mm), and the initial treatment result were found to be significantly associated with retreatment (Table 2). Aneurysmal size and aneurysmal neck width were associated with each other (Pearson χ2 123.35, P < .001). For the multivariate logistic regression analysis, all aneurysms with a neck width >4 mm and/or aneurysmal size >10 mm were combined in “unfavorable anatomy,” and the remaining aneurysms were defined as “favorable anatomy.” “Unfavorable anatomy” was significantly associated with retreatment (Table 2).
Table 2:
Univariate and multivariate regression analyses*
| Predictor Variables | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Aneurysm location | 1.06 (0.86–1.31) | .56 | n.a. | n.a. |
| Aneurysm size | 3.97 (1.90–8.32) | ≤.001 | n.a. | n.a. |
| Aneurysm neck | 2.47 (1.19–5.11) | .015 | n.a. | n.a. |
| Unfavorable anatomy† | 3.45 (1.65–7.22) | ≤.001 | 3.11 (1.43–6.75) | .004 |
| Initial treatment result | 2.16 (1.35–3.47) | ≤.001 | 1.86 (1.13–3.07) | .014 |
| Complete occlusion vs neck remnant | 1.71 (0.70–4.17) | .240 | n.a. | n.a. |
| Complete occlusion vs residual aneurysm | 5.05 (1.94–13.11) | ≤.001 | 3.96 (1.48–10.65) | .006 |
| Hunt and Hess | 1.13 (0.91–1.39) | .283 | n.a. | n.a. |
| SAH vs non-SAH | 1.38 (0.64–3.01) | .412 | n.a. | n.a. |
Note—n.a. indicates not applied; SAH, subarachnoid hemorrhage.
Retreatment is defined as “response variable.” Variables identified as predictors for retreatment by univariate analysis (P<0.1) were entered into the multivariate analysis.
Pearson χ2 test showed dependency of aneurysmal size and neck. Therefore, the multivariate analysis was performed for the combined variable “favorable anatomy” (aneurysmal size, ≤10 mm; neck width, ≤4 mm).
Furthermore, the multivariate logistic regression analysis confirmed the initial treatment result (complete occlusion vs neck remnant and residual aneurysm) to be a significant predictor. After categorization into subgroups, only “residual aneurysm” was a significant predictor, whereas “neck remnant” was not (Table 2).
Reasons for Retreatment
Asymptomatic aneurysmal recanalization owing to coil compaction without rupture accounted for most of the first retreatment sessions (22/33, 66.7%). Other reasons for first retreatment included SAH (4/33, 12.1%), asymptomatic aneurysmal regrowth (5/33, 15.2%), and migration of coils in thrombus material within the aneurysmal sac (1/33, 3.0%).
In 1 patient who was treated initially with the stent remodeling technique, no contrast filling of the aneurysm was observed after deployment of the stent; therefore, coiling was set aside. Because the first control angiogram of this patient showed aneurysmal filling with contrast media, occlusion with a single coil was again achieved (1/33, 3.0%).
A total of 4 patients underwent retreatment twice (1 after surgical clipping as first retreatment technique), and 3 had a retreatment session 3 times (all endovascular). Within this group of patients, the reason for second and third retreatment included aneurysmal regrowth with coil loosening (n = 6), coil compacting (n = 2), adjacent enlarging aneurysm (n = 1), and recurrence of hemorrhage as a result of aneurysmal regrowth (n = 1).
Outcome after Retreatment and Rebleeding
Good outcome (GOS 5) after first retreatment (including surgical clipping in 3/33, 9.1%) was achieved in 87.8% (29/33) of patients. In 6.1% (2 /33) of patients, outcome was unchanged compared with the preoperative status. In 6.1% (2/33), outcome was rated GOS 1 after rebleeding. Early rebleeding occurred in 0.6% (2/323) of patients (initial clinical presentation in both patients was HH V) within 24 hours after incomplete coil embolization. Both patients underwent repeat treatment immediately after rebleeding. One patient died, and one patient showed a good recovery score (GOS 5).
Late rebleeding occurred in another 0.9% (3/323) of patients. One patient (initial clinical presentation HH II) missed the 6-month control angiogram and presented 11 months after initial complete aneurysmal occlusion with an HH grade V hemorrhage. Retreatment was performed, but the patient died of multiple cerebral infarcts resulting from cerebral vasospasm. The second patient (initial clinical presentation HH IV) presented 56 months after a first retreatment (GOS 5) with an HH grade III hemorrhage. In this patient, a control angiogram was also missed since the first retreatment. After the second retreatment, a third session 4 years later was necessary. Outcome for this patient was GOS 4. The third patient presented with rebleeding (HH 1) on the evening after the regular control angiogram. This patient was treated surgically; a second treatment session (endovascular) 5 months later was necessary, with a final GOS of 5. All other patients with multiple retreatment sessions showed no clinical changes compared with their particular preoperative status.
Dependent on initial HH grade, the rate of retreatment ranged from 1 of 18 (5.6%) patients with an HH of IV to 5 of 26 (19.2%) patients with an HH of V. Clinical presentation was not identified as a predictor for retreatment by univariate analysis (P > .1) and therefore was not entered into the multivariate analysis (Table 2).
Location and Morphologic Features of Aneurysms
The rate of patients who underwent repeat treatment was significantly associated with the size of the aneurysm. The mean size in group A was 7.9 mm (range, 2–29 mm); in group B, 8.6 mm (range, 2–23 mm); and in group C, 13.2 (range, 3–44 mm).
Retreatment in small aneurysms with small necks was performed in 6 of 214 (2.8%) of patients. In all other aneurysmal configurations (small aneurysms with wide neck and large aneurysms), the rate of retreatment ranged from 19.7% to 28.6%. When summarizing all these types of aneurysms as a “risk group” (128/342, 37.4%), the OR for retreatment was considerably increased to 3.11 (95% CI: 1.43–6.75; P = .004). The PPV was 0.21 (95% CI: 0.14–0.29). The NPV was 0.97 (95% CI: 0.94–0.99). The sensitivity was 0.81 (95% CI: 0.65–0.93). The specificity was 0.67 (95% CI: 0.62–0.73).
Retreatment was performed in 6 of 75 (8.0%) aneurysms of the internal carotid artery (ICA), in 7 of 83 (8.4%) aneurysms of the anterior (communicating) cerebral artery (ACA/AcomA), in 3 of 27 (11.1%) aneurysms of the middle cerebral artery (MCA), in 3 of 42 (7.1%) aneurysms of the posterior (communicating) cerebral artery (PCA/PcomA), and in 14 of 94 (14.9%) aneurysms of the basilar artery (BA). No retreatment was performed in the 23 aneurysms of the vertebral artery (VA).
Morphologic Features at Initial Session of Embolization
In line with others, we found basilar tip aneurysms to have the highest relative propensity for a recurrence (Table 1). The rate of retreatment dependent on location ranged from 0 of 23 in the VA aneurysms to 14 of 94 (14.9%) in the basilar tip aneurysms. Aneurysm location was not identified as a predictor for retreatment by univariate analysis (P > .1) and therefore was not entered into the multivariate analysis (Table 2).
Angiographic Results before and after Retreatment
In accordance with the classification of Roy et al,24 the immediate angiographic results in group A were considered to be complete obliteration (c/o) in 73.0% (n = 197), neck remnants (n/r) in 18.1% (n = 49), and residual aneurysm (r/a) in 8.9% (n = 24) of cases. In group B, the angiographic results were c/o in 64.1% (n = 25), n/r in 25.6% (n = 10), and r/a in 10.3% (n = 4) of cases. In group C, angiographic results were c/o in 48.5% (n = 16), n/r in 24.2% (n = 8), and r/a in 27.3% (n = 9) of cases (Table 1).
Surgical clipping as a first retreatment was performed in 9.0% (n = 3) of patients. Angiographic results after first endovascular repeat treatment were c/o in 45.5% (n = 15) and n/r in 45.5% (n = 15). Seven patients underwent a second retreatment session (preceding results were c/o in 4 patients, n/r in 2 patients; and aneurysm regrowth after surgical clipping in 1 patient); c/o was achieved in 3, n/r in 2, and r/a in 2 patients. Three patients underwent a third retreatment session (preceding results: c/o in 1 patient, n/r in 1, and r/a in 1 patient) in which complete occlusion of the aneurysms was achieved.
The rate of retreatment dependent on the initial treatment result was 16 of 238 (6.7%) in aneurysms with initial complete occlusion, 8 of 67 (11.9%) in aneurysms with neck remnants, and 9 of 37 (24.3%) in aneurysms with a residual aneurysm as a result of the initial treatment.
When residual aneurysms was defined as a “risk group,” the OR for retreatment was significantly increased to 3.96 (95% CI: 1.48–10.65; P = .006). The PPV was 0.24 (95% CI: 0.12–0.41). The NPV was 0.92 (95% CI: 0.88–0.95). The sensitivity was 0.27 (95% CI: 0.13–0.45). The specificity was 0.91 (95% CI: 0.87–0.94).
Discussion
How Often Is An Aneurysmal Recurrence Observed and How Often Is It Treated?
The observed rate of recanalization (21.1%) and rate of retreatment (9.6%) in our study population compares well with those published in the literature, in which recanalization rates ranged from 10% to 33.6%, and retreatment rates were from 4.7% to 12.3% (Table 3). Murayama et al6 reported recanalization rates of 26.1% in aneurysms treated during their first 5 years experience with endovascular treatment. The rate decreased to 17.2% in those aneurysms treated in their later 6 years of experience. These findings are in concordance with our experiences. Although 61.7% (387/627) of aneurysms at our institution were treated after December 1999, the major portion of the aneurysms that needed retreatment (66.6%, 22/33) was treated before this date. Similar experiences were reported by Raymond et al.14
Table 3:
Previous literature regarding recurrences and retreatment of aneurysms after initial coil embolization
| Author | Pts/Ayms with Angiographic F/U (%) | Mean Angiographic F/U (months) | Recurrences (%) | Retreated Aneurysms (%) | Rebleeding (p.a.) (%) | Set of Retreated Recurrences (%) | Combined M/M Recoiling (%) |
|---|---|---|---|---|---|---|---|
| Byrne 199928 | 250/317 pts (78.9) | 6–12 | 38/259 (14.7) | 13/250 (5.2) | 0.6–2.4 | 13/38 (34.2) | Not reported |
| Cognard 199936 | 169/203 ayms (83.3) | 3.4 | 26/169 (15.5) | 8/169 (4.7) | 0.0 | 8/26 (30.8) | Not reported |
| Thornton 200237 | 141/196 ayms (71.9) | 16.7 | 25/141 (17.7) | Not reported | 0.5 | Not reported | Not reported |
| Murayama 20036 | 489/916 ayms (53.4) | 11 | 102/489 (20.9) | Not reported | 0.5–1.6 | Not reported | Not reported |
| Raymond 200314 | 381/501 ayms (76.5) | Not specified* | 128/381 (33.6) | 39/381 (10.2) | 0.3 | 39/128 (30.5) | Not reported |
| Slob 200429 | 400/488 ayms (82) | 6 | Not reported | 48/400 (12) | 1.1 | Not reported | 0 |
| Henkes 200616 | 1680/2759 ayms (60.9) | Not specified† | Not reported | 350/1680 (20.8) | Not reported | Not reported | 3 |
| Gallas 20052 | 571/705 ayms (81) | 36 | 85/571 (14.9) | 33/571 (5.8) | 0.06 | 33/85 (38.8) | Not reported |
| CARAT 20069 | 299 pts with clinical F/U | Not reported | Not reported | 35/299 (11.7) | 0.11 | Not reported | 11 |
| Kang 200615 | 250/522 ayms (47.9) | 8 | 91/250 (36.4) | 32/250 (12.8) | 0.79 | 39/91 (42.8) | 0 |
| Own data | 342/627 ayms (54.5) | 28.6 | 72/342 (21.1) | 33/342 (9.6) | 0.6 | 33/72 (45.8) | 0 |
Note:—Pts indicates patients; ayms, aneurysms; F/U, follow-up; p.a., per annum; MM, morbidity and mortality.
Early F/U (3–12 months) in 353/501 aneurysms and late F/U (>12 months) in 277/501 aneurysms.
Standard recommendation was to have F/U angiography at 6–12 months. Mean interval between first treatment and first F/U examination was 21 months (median, 14 months).
Which Aneurysms Underwent Repeat Treatment?
The best single predictor for patients who needed retreatment was aneurysmal anatomy (Table 2). Our results imply that it can be expected that a small aneurysm with a narrow neck will need no retreatment in about 97% of cases. From a practical standpoint, these aneurysms are definitely treated with conventional coil techniques. In turn, to show the benefit of bioactive coils for the clinically relevant end point “rate of retreatment” in these patients (who represent almost two thirds of our group), a very large study would be needed.
The risk of retreatment in the remaining patients with a neck width of >4 mm or an aneurysm size of >10 mm is probably increased about threefold. In contrast, Raymond et al14 found recurrences not significantly increased when the neck size was >4 mm. One possible explanation is that recurrences in small aneurysms actually do occur rarely but are treated in an even smaller portion (Table 1). This observation might be explained, in part, by the fact that small aneurysms have usually small recurrences, which do not exceed our predefined threshold of ≥2 mm for a reintervention. However, this finding is still meaningful from a clinical standpoint because these small recurrences are probably less likely to bleed but, as previously suggested,25 are more dangerous to treat. However, the risk of bleeding in these small recurrences cannot be ruled out completely.26
Location of the aneurysm was no significant predictor of recurrent treatment. In line with previous observations, we found variations in the rate of recurrent treatments,14, 16 which did not reach significance levels.
It is not surprising that the initial treatment result was a significant predictor for the rate of retreatment. If the initial aneurysmal treatment was achieved only with a residual aneurysm, a further growth of the remnant with need for retreatment was about 4 times as often in completely occluded aneurysms or in aneurysms with neck remnants. This finding is in line with previous observations in which a residual aneurysm was a significant predictor for recurrence (OR 3.60, 95% CI: 1.60–8.09).14 However, aneurysms with neck remnants had no significantly increased risk. These data suggest that complete treatment does not necessarily need to be enforced.
We found no association between the initial clinical presentations (HH grade) and the rate of retreatment. It is noteworthy also that the patients with incidentally treated aneurysms (HH 0, Table 1), in whom interventions can be planned and prepared in advance, did not necessarily have lower rates of recurrence and retreatment. In contrast, Raymond et al14 found significantly lower rates of recurrence in incidental aneurysms. Differences in patient selection might explain these results.
Is Retreatment Beneficial and Stable?
The major problem with the prediction of recurrent treatment was the fact that we do not know whether the retreatment was indicated in the treated patients because objective or generally accepted criteria do not exist for a retreatment of reopened cerebral aneurysms after endovascular GDC treatment. The ideal end point would be aneurysm (re-)rupture because the intention of angiographic follow-up and additional treatment must imply the reduction of the risk of hemorrhage or recurrent hemorrhage. However, the prediction of retreatment is at least a step closer to that end point.
The natural history of aneurysmal neck remnants after endovascular coil embolization is often benign,27 but bleeding from incompletely coiled aneurysms is a well-documented threat.28 Byrne et al28 reported that rebleeding occurred in 3 of 38 (7.9%) recurrent aneurysms and in 1 of 221 (0.4%) aneurysms that appeared stable on angiograms. Slob et al29 observed no rebleeding in patients with complete or near-complete occlusion after additional coiling, but 2 episodes of rebleeding occurred during the added time of observation of 66 person-years (3.0%) in patients with incompletely occluded aneurysms. Therefore, aneurysms that are incompletely treated, which are more unstable, may be considered for retreatment in particular, as was done in our series.
The overall rate of rebleeding in our patient group was 0.6% p.a., which compares well with the data from the literature (Table 3). In consideration of these low rates of rebleeding, an aggressive retreatment strategy does not necessarily seem to be indicated. Even if rebleeding from completely occluded aneurysms on angiograms has been reported,5 a further impetus for control angiograms is the detection of treatable aneurysmal recurrences, which are thought to be a risk factor for SAH, perhaps more often with noninvasive techniques such as MRA.30, 31
A single retreatment of an aneurysm recurrence was performed in 7.6%, and multiple retreatments in 2.0% of our study population. These data suggest that single retreatment is stable in most of the patients. In line with our data, Henkes et al16 reported a first retreatment in 12.7% and 2 or more retreatment sessions in 5.3% in their patients, which suggests that most patients undergo repeat treatment only once.
Is Retreatment Safe?
Complication rates during retreatment are reported to be lower if compared with initial treatment (Table 3). Despite a single aneurysmal rupture during treatment, we observed no resulting morbidity and mortality from the retreatment procedures. The delay between presenting SAH and retreatment is usually long enough to lower the risk of an intraprocedural rupture, which is higher in recently ruptured aneurysms.32–34 From our experience, the complication rate for patients who undergo repeat treatment can be expected to be at least comparable or lower than the complication rate in the patients with incidental aneurysms. However, Park et al35 found morbidity for retreatment of aneurysms as high as 10%.
Limitations of the Study
Our reported rebleeding rate was well within the range reported in the literature (Table 3). Because of the considerable number of patients lost to follow-up (135/596, 22.7%), the significance of our results in regard to the incidence of rebleeding has to be referred to as limited. Also, the total number of patients included in this study (323) was a small sample. Therefore, we do not claim our results to be considered definite.
Conclusion
In our series, the retreatment of aneurysmal recurrences was a safe procedure, which was performed in approximately 10% of the patients with predominantly stable results in later follow-up. The best predictors of aneurysmal retreatment were aneurysmal anatomy and the presence of a residual aneurysm after initial treatment. Our data suggest that complete treatment does not need to be enforced by all means because neck remnants were not predictive for retreatment. However, because a significant number of patients (22.7%) were lost to follow-up, the quality of our data was limited. A large multicenter data base is needed to gather enough patients with the rare event of rebleeding to identify those who ultimately need retreatment.
References
- 1.Friedman JA, Nichols DA, Meyer FB, et al. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol 2003;24:526–33 [PMC free article] [PubMed] [Google Scholar]
- 2.Gallas S, Pasco A, Cottier JP, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2005;26:1723–31 [PMC free article] [PubMed] [Google Scholar]
- 3.Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery 2004;54:268–80; discussion 280–85 [DOI] [PubMed] [Google Scholar]
- 4.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 5.Molyneux A, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 6.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 7.Solymosi L, Chapot R, Bendszus M. Stent, balloon, or clip? The problem of wide-necked aneurysms. Klinische Neuroradiologie. 2005;3:145–60 [Google Scholar]
- 8.van Rooij WJ, Sluzewski M. Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2006;27:1678–80 [PMC free article] [PubMed] [Google Scholar]
- 9.CARAT Investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006;37:1437–42 [DOI] [PubMed] [Google Scholar]
- 10.Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms: incidence, morbidity, and risk factors. AJNR Am J Neuroradiol 2005;26:1739–43 [PMC free article] [PubMed] [Google Scholar]
- 11.Boet R, Wong GK, Poon WS, et al. Aneurysm recurrence after treatment of paraclinoid/ophthalmic segment aneurysms–a treatment-modality assessment. Acta Neurochir (Wien)2005;147:611–16; discussion 616 [DOI] [PubMed] [Google Scholar]
- 12.Campi A, Summers P, Molyneux A, et al. Why aneurysms needed retreatment in ISAT. Neuroradiology. 2006;48 Suppl 2:57–135 [Google Scholar]
- 13.Sluzewski M, van Rooij WJ, Rinkel GJ, et al. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology. 2003;227:720–24 [DOI] [PubMed] [Google Scholar]
- 14.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 15.Kang HS, Han MH, Kwon BJ, et al. Repeat endovascular treatment in post-embolization recurrent intracranial aneurysms. Neurosurgery 2006;58:60–70; discussion 60–70 [DOI] [PubMed] [Google Scholar]
- 16.Henkes H, Fischer S, Liebig T, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms: safety and effectiveness aspects. Neurosurgery 2006;58:224–32; discussion 224–32 [DOI] [PubMed] [Google Scholar]
- 17.Murayama Y, Suzuki Y, Vinuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I: in vitro study. AJNR Am J Neuroradiol 1999;20:1986–91 [PMC free article] [PubMed] [Google Scholar]
- 18.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 19.Sluzewski M, van Rooij WJ. Questionable interpretation of results of ACTIVE study on Matrix coils by Boston Scientific. AJNR Am J Neuroradiol 2005;26:1882–83 [PMC free article] [PubMed] [Google Scholar]
- 20.Gaba RC, Ansari SA, Roy SS, et al. Embolization of intracranial aneurysms with hydrogel-coated coils versus inert platinum coils: effects on packing density, coil length and quantity, procedure performance, cost, length of hospital stay, and durability of therapy. Stroke 2006;37:1443–50 [DOI] [PubMed] [Google Scholar]
- 21.Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with Matrix detachable coils. Neurosurgery 2006;58:51–59; discussion 51–59 [DOI] [PubMed] [Google Scholar]
- 22.Berenstein A, Song JK, Niimi Y, et al. Treatment of cerebral aneurysms with hydrogel-coated platinum coils (HydroCoil): early single-center experience. AJNR Am J Neuroradiol 2006;27:1834–40 [PMC free article] [PubMed] [Google Scholar]
- 23.Katsaridis V, Papagiannaki C, Violaris C. Guglielmi detachable coils versus Matrix coils: a comparison of the immediate posttreatment results of the embolization of 364 cerebral aneurysms in 307 patients: a single-center, single-surgeon experience. AJNR Am J Neuroradiol 2006;27:1841–48 [PMC free article] [PubMed] [Google Scholar]
- 24.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004 [DOI] [PubMed] [Google Scholar]
- 25.Sluzewski M, van Rooij WJ. Small aneurysm size is a risk factor for perforation during coiling. AJNR Am J Neuroradiol 2003;24:2122 ; author reply 2122 [PMC free article] [PubMed] [Google Scholar]
- 26.Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg 1989;70:556–60 [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg 2000;93:561–68 [DOI] [PubMed] [Google Scholar]
- 28.Byrne JV, Sohn MJ, Molyneux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 29.Slob MJ, Sluzewski M, van Rooij WJ, et al. Additional coiling of previously coiled cerebral aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol 2004;25:1373–76 [PMC free article] [PubMed] [Google Scholar]
- 30.Mallouhi A, Felber S, Chemelli A, et al. Detection and characterization of intracranial aneurysms with MR angiography: comparison of volume-rendering and maximum-intensity-projection algorithms. AJR Am J Roentgenol 2003;180:55–64 [DOI] [PubMed] [Google Scholar]
- 31.Majoie CB, Sprengers ME, van Rooij WJ, et al. MR angiography at 3T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 2005;26:1349–56 [PMC free article] [PubMed] [Google Scholar]
- 32.Sluzewski M, Bosch JA, van Rooij WJ, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg 2001;94:238–40 [DOI] [PubMed] [Google Scholar]
- 33.Cloft HJ, Kallmes DF. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol 2002;23:1706–09 [PMC free article] [PubMed] [Google Scholar]
- 34.Ries T, et al. Intravenous administration of acetylsalicylic acid during endovascular treatment of cerebral aneurysms reduces the rate of thromboembolic events. Stroke 2006;37:1816–21 [DOI] [PubMed] [Google Scholar]
- 35.Park HK, Horowitz M, Jungreis C, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2005;26:506–14 [PMC free article] [PubMed] [Google Scholar]
- 36.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–56 [DOI] [PubMed] [Google Scholar]
- 37.Thornton J, Debrun GM, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 2002;50:239–49; discussion 249–50 [DOI] [PubMed] [Google Scholar]