Abstract
SUMMARY: Marginal sinus fistulas (MSFs) are uncommon vascular anomalies. Occasionally, the dominant venous drainage is forced retrograde up the inferior petrosal sinus and into the cavernous sinus, causing chemosis, proptosis, and ocular hypertension, mimicking a carotid cavernous fistula. This atypical clinical presentation may lead to misdiagnosis and inappropriate hazardous treatment of an MSF. Identifying the site of the fistula and understanding the anatomy of the venous drainage are critical in providing appropriate, safe, and efficacious endovascular treatment.
The marginal sinus surrounds the foramen magnum, communicating anteriorly with the venous plexus of the clivus and posteriorly with the occipital sinus (if present). Correspondingly, dural fistulas of the marginal sinus typically drain via the sigmoid sinus–jugular bulb.1 However, in some cases, the jugular venous outflow becomes stenotic or thrombosed, forcing the venous drainage retrograde through the inferior petrosal sinus (IPS) and into the cavernous sinus (CS). Patients with this anatomy may present with clinical findings identical to those of a carotid cavernous fistula (CCF).2
The clinical or angiographic misdiagnosis of a marginal sinus fistula (MSF) may lead to inappropriate or dangerous treatment. We review the clinical presentation, imaging findings, and endovascular treatment of 2 patients with MSFs, which presented clinically as CCFs.
Case Reports
Case 1
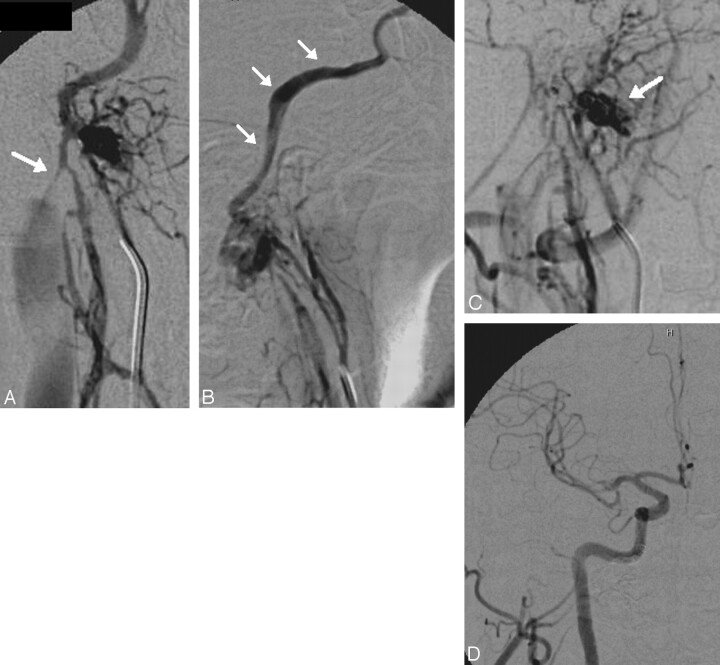
This 49-year-old man presented with an 18-month history of a pulsatile thrill in the right ear with subsequent decreased hearing and a 6-month history of progressive chemosis and proptosis without associated pain or oculoparesis. MR imaging revealed a dilated and arterialized IPS and superior opthalmic vein (SOV). External carotid angiography (Fig 1) revealed a coalescence of abnormal arteries in the region of a marginal sinus recipient venous pouch with early venous filling and reflux into the IPS and CS. Selective catheterization of the ascending pharyngeal artery more clearly demonstrated an MSF (grade 2). One microcatheter was placed in the ascending pharyngeal artery for the purposes of mapping, and a second microcatheter was manipulated into the recipient pouch. Due to instability of the microcatheter, the pouch could only be partially embolized with coils. After partial occlusion of the MSF pouch and the IPS, selective ascending pharyngeal injections demonstrated persistent flow through the fistula. This remaining fistula pouch was then embolized from a transarterial approach by using ethylene vinyl alcohol (Onyx; ev3, Irvine, Calif). A transarterial-to-venous Onyx injection was achieved, producing complete angiographic occlusion of the fistula. The patient had resolution of the chemosis and proptosis within 24 hours.
Fig 1.
A 45-year-old man with a right marginal sinus DAVF. Posteroanterior (PA) (A) and lateral projections (B) from a right ascending pharyngeal angiogram demonstrate an arteriovenous fistula supplied by numerous branches of the neuromeningeal trunk with dominant outflow retrograde (B, white arrows) into the IPS, CS, and ultimately the SOV, accounting for a clinical presentation indistinguishable from a CCF. Antegrade outflow via the ipsilateral jugular vein is limited by a high-grade flow-related stenosis at the skull base (A, white arrow). Access into the recipient pouch was achieved via a transfemoral transjugular approach. However, during the process of coil embolization, the microcatheter was kicked out of the recipient pouch and could not be navigated back in, to complete the occlusion of the fistula. C, Superselective angiogram from a catheter positioned within a neuromeningeal branch of the ascending pharyngeal artery demonstrates persistent filling of the recipient pouch (arrow) following partial coil embolization. For this reason, the embolization was completed with a transarterial-to-venous infusion of Onyx-18. D, Control angiography in the PA projection demonstrates complete ablation of the fistula with no residual arteriovenous shunt surgery.
Case 2
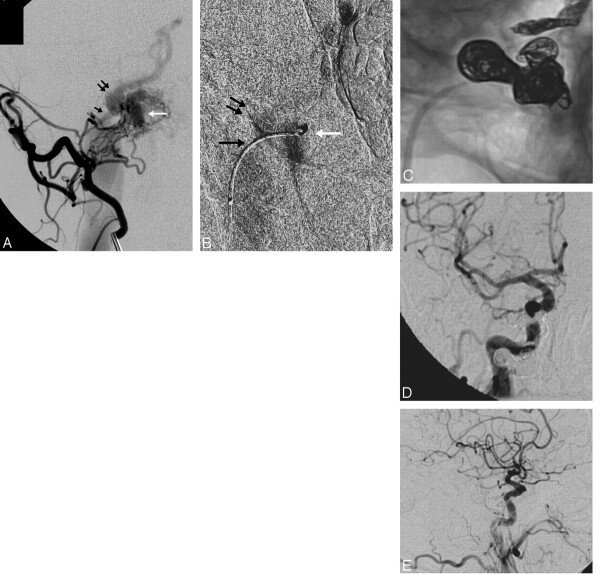
The patient was a 67-year-old man with a gradual onset of right-eye proptosis and chemosis for several months, worsening headaches, and a subjective bruit that was exacerbated with exercise. MR imaging revealed right-eye proptosis, prominent SOV, and arterialization of the CS and IPS. Selective conventional angiography revealed the site of the fistula to be medial to the origin of the IPS at the level of the foramen magnum (grade 2), with feeders coming from the right occipital artery, right ascending pharyngeal artery, right posterior auricular artery, and right internal carotid artery (Fig 2). The fistula was accessed and successfully coiled via a transjugular approach. The patient's bruit resolved postprocedurally, and visual symptoms resolved in several days.
Fig 2.
A 67-year-old man with progressive proptosis, chemosis, headache, and pulse synchronous bruit. A, Oblique view from a selective injection of the right occipital artery demonstrates arteriovenous shunt surgery into a marginal sinus recipient pouch (white arrow). Antegrade drainage into the ipsilateral jugular vein is limited by a high-grade stenosis at the junction of the pouch and the IJ vein (black arrow). The dominant venous drainage is via a patulous connection with the IPS (double arrow), which is proximally dilated and flows out into the CS and SOV. A microcatheter was successfully navigated into the fistulous pouch. B, Selective venography performed from a microcatheter positioned within the recipient venous pouch (white arrow) confirms the anatomy of the pouch and its venous outflow through the stenotic channel into the IJ vein (black arrow) and retrograde up the IPS (double arrows). The introduction of coils into the pouch resulted in instability of the microcatheter, which was repeatedly pushed back into the proximal IPS. For this reason, the IPS was coil-occluded to stabilize the microcatheter within the pouch. After this was performed, additional coils were introduced into the recipient pouch, resulting in complete occlusion of the fistula. C, Native oblique view demonstrates coils distributed within the right IPS and marginal sinus recipient pouch. Common carotid angiography in the PA (D) and lateral (E) projections demonstrate complete occlusion of the fistula, with no residual arteriovenous shunt surgery. Following the procedure, the patient's symptoms completely resolved.
Discussion/Results
MSFs are uncommon vascular anomalies. McDougall et al2 reported on 290 fistulas, of which 14 were MSFs (4.8%). Most lesions were relatively benign, presenting with pulse synchronous bruit in 11 of 14 cases. However, 3 patients presented with hemorrhage (subarachnoid and intracerebellar) and/or neurologic symptoms (ataxia/tremor).
The anatomy of the venous outflow is the critical factor in patient presentation and treatment planning.3–16 In grade 1 lesions, the venous drainage is unrestricted and typically through the adjacent ipsilateral jugular vein. These patients present with a pulse synchronous bruit. Any change in the bruit, such as decreased intensity or resolution, may indicate an upgrading of the fistula. Grade 2 fistulas have a restriction or obstruction of venous drainage via the jugular bulb (Fig 2B). In these cases, venous outflow is shunted retrograde through the IPS and into the CS. These patients present with orbital venous hypertension and a clinical syndrome more typical for a CCF. If the IPS drainage becomes stenotic or occludes, the fistula can be further upgraded. Grade 3 lesions demonstrate retrograde cortical venous drainage, and these, correspondingly, have the highest propensity to hemorrhage. Although patients with grade 1 and asymptomatic grade 2 may be observed in some cases, patients with grade 2 with ocular hypertension and all grade 3 lesions warrant intervention.2 Treatment strategies previously reported initially centered on surgical ligation, with reports of endovascular strategies being ineffective.6 However, endovascular management of these and other dural arteriovenous fistulas (DAVFs) is both curative and safe.3,17–25
In the present series, the diagnosis of MSF was complicated by a clinical presentation similar to that of a CCF. Misdiagnosis on the basis of clinical presentation and/or noninvasive imaging may lead to the inappropriate dismissal of an MSF as a benign indirect CCF. Furthermore, conventional angiographic investigation may not be performed, and the high-risk features (not infrequently associated with the MSF) may go undiagnosed. If the neurointerventionist misinterprets an MSF and embolizes the superior ophthalmic vein, CS, IPS, or jugular vein without ablating the recipient venous pouch, the fistula may upgrade with the elimination of these lower risk venous outflow pathways, potentially forcing the drainage into higher risk cortical venous pathways. In addition, these embolizations could block future endovascular access to the fistula site and thereby preclude definitive endovascular treatment targeted at occluding the marginal sinus recipient pouch.
As with all DAVFs, the accurate identification and subsequent endovascular embolization or surgical ligation of the proximal recipient vein (in this case the marginal sinus pouch) is required for safe and efficacious therapy. This is best achieved by selective transvenous catheterization of the fistula pouch with subsequent coil embolization (case 2). As with other DAVFs, this is best facilitated by performing roadmaps or by using an “overlay” fluoro-fade technique in an imaging projection that best separates the marginal sinus pouch to be catheterized from the normal anatomic venous drainage. A more selective roadmap of the pouch is usually best achieved with a 4F or 5F diagnostic catheter positioned within a branch of the external carotid artery, which provides a dominant supply to the fistula. This more selective roadmap reduces the degree of obscuration of the fistula site, often produced with a more proximal external or common carotid injection. Transvenous access to the fistula pouch can usually be achieved either directly from the ipsilateral internal jugular (IJ) through the contralateral IJ-contralateral IPS-circular sinus-ipsilateral IPS approach2 or retrograde through an SOV-CS-IPS approach. Liquid embolic agents injected via transarterial access or retrograde through transvenous access26 can be useful when total transvenous coil occlusion of the fistula has not been achieved and access into the fistula has been lost or becomes tenuous. If a transvenous Onyx injection is performed, this must be done with the microcatheter positioned within the fistulous pouch and is most effective when the outflow has been slowed to some extent by partial coil embolization.26
Conclusion
MSFs are uncommon vascular anomalies whose symptoms can mimic CCFs. Correctly identifying the location and anatomy of the recipient pouch is critical in providing appropriate, safe, and efficacious treatment. This diagnosis should be considered whenever fistulas in the region of the jugular vein are identified. Selective external carotid branch angiography with oblique views can be useful in separating the jugular bulb laterally from the marginal sinus and can aid in the identification, catheterization, and embolization of MSFs. As with all dural fistulas, cure is best achieved by a treatment strategy targeted to achieve complete occlusion of the recipient pouch.
References
- 1.De Oliveira E, Rhoton AL, Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol 1985;24:293–352 [DOI] [PubMed] [Google Scholar]
- 2.McDougall CG, Halbach VV, Dowd CF, et al. Dural arteriovenous fistulas of the marginal sinus. AJNR Am J Neuroradiol 1997;18:1565–72 [PMC free article] [PubMed] [Google Scholar]
- 3.Awad IA, Little JR, Akrawi WP, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990;72:839–50 [DOI] [PubMed] [Google Scholar]
- 4.Brown RD, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg 1994;81:531–38 [DOI] [PubMed] [Google Scholar]
- 5.Djindjian R, Merland JJ. Super-Selective Arteriography of the External Carotid Artery. New York: Springer-Verlag;1978
- 6.Gaston A, Chiras J, Bourbotte G, et al. Meningeal arteriovenous fistulae draining into cortical veins: 31 cases [in English, French]. J Neuroradiol 1984;11:161–77 [PubMed] [Google Scholar]
- 7.Halbach VV, Hieshima GB, Higashida RT, et al. Carotid cavernous fistula: indications for urgent therapy. AJNR Am J Neuroradiol 1987;8:627–33 [DOI] [PubMed] [Google Scholar]
- 8.Hurst RH, Hackney DB, Goldberg HI, et al. Reversible arteriovenous malformations-induced venous hypertension as a cause of neurological deficits. Neurosurgery 1992;30:422–25 [DOI] [PubMed] [Google Scholar]
- 9.Lalwani AK, Dowd CH, Halbach VV. Grading venous restrictive disease to predict the clinical presentation and guide therapy in patients with dural arteriovenous fistulas of the transverse sigmoid sinus. J Neurosurg 1993;79:11–15 [DOI] [PubMed] [Google Scholar]
- 10.Lasjaunias P, Chiu M, TerBrugge K, et al. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 1986;64:724–30 [DOI] [PubMed] [Google Scholar]
- 11.Malik GM, Pearce JE, Ausmann JI. Dural arteriovenous malformations and intracranial hemorrhage. Neurosurgery 1984;15:332–39 [DOI] [PubMed] [Google Scholar]
- 12.Thompson BG, Doppman JL, Oldfield EH. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg 1994;80:617–23 [DOI] [PubMed] [Google Scholar]
- 13.Vinuela F, Fox AJ, Pelz DM, et al. Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg 1986;64:554–58 [DOI] [PubMed] [Google Scholar]
- 14.Willinsky R, TerBrugge K, Lasjaunias P, et al. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol 1990;34:118–23 [DOI] [PubMed] [Google Scholar]
- 15.Wrobel CJ, Oldfield EH, DiChiro G, et al. Myelopathy due to intracranial dural arteriovenous fistulas draining intrathecally into spinal medullary veins: report of three cases. J Neurosurg 1988;69:934–39 [DOI] [PubMed] [Google Scholar]
- 16.Awad IA. Tentorial incisura and brain stem dural arteriovenous malformations. In: Awad IA, Barrow DL, eds. Dural Arteriovenous Malformations. Park Ridge, Ill: American Association of Neurological Surgeons Publications Committee;1993;131–46
- 17.Barnwell SL, Halbach VV, Dowd CF, et al. Dural fistulas involving the inferior petrosal sinus. AJNR Am J Neuroradiol 1990;11:511–17 [PMC free article] [PubMed] [Google Scholar]
- 18.Barnwell SL, Halbach VV, Dowd CF, et al. A variant of arteriovenous fistulas within the wall of dural sinuses. J Neurosurg 1991;24:199–204 [DOI] [PubMed] [Google Scholar]
- 19.Barnwell SL, Halbach VV, Higashida RT, et al. Complex dural arteriovenous fistulas: results of a new combined neurosurgical and interventional radiology treatment in 16 patients. J Neurosurg 1989;71:352–58 [DOI] [PubMed] [Google Scholar]
- 20.Djindjian R, Cophignon J, Theron J. Embolization by superselective arteriography from the femoral route: review of 60 cases—technique, indications, complications. Neuroradiology 1973;6:20–26 [DOI] [PubMed] [Google Scholar]
- 21.Halbach VV, Higashida RT, Hieshima GB, et al. Treatment of dural arterial venous malformations involving the superior sagittal sinus. AJNR Am J Neuroradiol 1988;9:337–43 [PMC free article] [PubMed] [Google Scholar]
- 22.Halbach VV, Higashida RT, Hieshima GB, et al. Dural fistulas involving the transverse and sigmoid sinuses: results of treatment in 28 patients. Radiology 1987;163:443–47 [DOI] [PubMed] [Google Scholar]
- 23.Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the cavernous carotid. AJNR Am J Neuroradiol 1989;10:377–84 [PMC free article] [PubMed] [Google Scholar]
- 24.Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol 1989;10:385–92 [PMC free article] [PubMed] [Google Scholar]
- 25.Halbach VV, Higashida RT, Hieshima GB, et al. Dural fistulas involving the cavernous sinus: results of treatment of 30 patients. Radiology 1987;163:437–42 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Lee DW, Jahan R, et al. Transvenous treatment of spontaneous dural carotid-cavernous fistulas using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol 2006;27:1346–49 [PMC free article] [PubMed] [Google Scholar]