Abstract
BACKGROUND AND PURPOSE: Intratumoral hemorrhage occurs frequently in pituitary macroadenoma and manifests as pituitary apoplexy and recent or old silent hemorrhage. T2*-weighted gradient-echo (GE) MR imaging is the most sensitive sequence for the detection of acute and old intracranial hemorrhage. T2*-weighted GE MR imaging was used to investigate intratumoral hemorrhage in pituitary macroadenomas.
MATERIALS AND METHODS: Twenty-five consecutive patients who underwent total or subtotal resection of pituitary macroadenoma with heights from 17 to 53 mm, including 1 patient with classic pituitary apoplexy, underwent MR imaging before surgery, including T2*-weighted GE MR imaging. For histologic assessment of the hemorrhage in whole surgical specimens, we used hematoxylin-eosin staining.
RESULTS: T2*-weighted GE MR imaging detected various types of dark lesions, such as “rim,” “mass,” “spot,” and “diffuse” and combinations, indicating clinical and subclinical intratumoral hemorrhage in 12 of the 25 patients. The presence of intratumoral dark lesions on T2*-weighted GE MR imaging correlated significantly with the hemorrhagic findings on T1- and T2-weighted MR imaging (P < .02 and <.01, respectively), and the surgical and histologic hemorrhagic findings (P < .001 and <.001, respectively).
CONCLUSION: T2*-weighted GE MR imaging could detect intratumoral hemorrhage in pituitary adenomas as various dark appearances. Therefore, this technique might be useful for the assessment of recent and old intratumoral hemorrhagic events in patients with pituitary macroadenomas.
Intratumoral hemorrhage occurs most frequently in pituitary adenoma among all types of brain tumors.1 Pituitary apoplexy (or classic pituitary apoplexy) is characterized by acute clinical syndrome (sudden onset of headache, vomiting, visual disturbance, ophthalmoplegia, and altered consciousness) and has been detected in 2% to 14% of patients who underwent surgery for pituitary macroadenomas.2–6 Also, clinically silent pituitary hemorrhage (or subclinical pituitary apoplexy) has been frequently detected in many clinical series, so the real incidence of intratumoral hemorrhage in pituitary adenoma may be higher.7 Subclinical intratumoral ischemia or hemorrhagic infarction may be common in pituitary adenoma and may result in cyst formation or hematoma, or both.2,6–10 Conventional MR imaging and surgical findings suggest that approximately 50% of pituitary adenomas contain cysts or hematomas, or both.10 Such intratumoral vasogenic events may be important in the progression of pituitary macroadenomas. Exact evaluation of intratumoral hemorrhage may also be important in surgical indication and planning.2,5,6,8,9
The most sensitive neuroimaging technique for the detection of blood is T2*-weighted gradient-echo (GE) MR imaging, which is widely applied for the evaluation of intracerebral hemorrhage in both the acute and chronic stages.11–16 T2*-weighted GE MR imaging shows a hemorrhage as marked low signal intensity, because the differences in the magnetic susceptibility resulting from the presence of blood or paramagnetic blood products create local magnetic field inhomogeneities.11,12
T2*-weighted GE MR imaging can sensitively detect the paramagnetic effects of deoxyhemoglobin and methemoglobin in hyperacute intracerebral hemorrhage.11,12,14–17 Postmortem analysis of patients with intracerebral hemorrhage has proved that focal low signal intensity detected by T2*-weighted GE MR imaging is indicative of past microbleeding, and that areas of low signal intensity indicate hemosiderin deposits.13 T2*-weighted GE MR imaging is also widely used for the detection of diffuse axonal injury after severe head trauma with diffusion-weighted images.17 T2*-weighted GE MR imaging has not been used for the evaluation of hemorrhagic events in patients with pituitary adenoma. Our study evaluated T2*-weighted GE MR imaging for the detection of intratumoral hemorrhage in patients with pituitary macroadenomas.
Materials and Methods
Patients
This prospective study included only patients with surgically proved presence of macroadenoma. Several preliminary studies found that characterization of micro- and intrasellar adenoma by T2*-weighted GE MR imaging was inconclusive, and frequently impossible because of skull base artifacts (data not shown). A total of 36 patients with pituitary macroadenoma were treated surgically in our institution between October 2002 and June 2006, and preoperative MR examinations were performed in all patients.
The trans-sphenoidal approach is the most common surgical technique even for large or giant adenomas; however, adenomas with extensive suprasellar or lateral extensions cannot be totally removed by a single trans-sphenoidal approach.18 Gross total or subtotal removal was achieved in 27 of the 36 cases of macroadenoma based on postoperative MR imaging.19 Partial removal was achieved in 9 cases, which were excluded because of the large discrepancy in the area of evaluation of the tumor between the imaging and surgical or histologic studies. Furthermore, we excluded 2 cases of recurrent pituitary adenoma to eliminate the artificial change caused by previous surgery.
Therefore, 25 consecutive patients (10 men and 15 women) initially treated for macroadenoma were included in this study. The age range of the patients was 29 to 75 years (mean ± SD, 52.2 ± 12.3 years). The heights of the macroadenoma ranged from 17 to 53 mm (mean ± SD, 27.3 ± 8.7 mm) as measured by preoperative MR imaging, including T2*-weighted GE imaging.
Histologic and immunohistochemical examination showed that 21 patients had primary nonfunctioning pituitary adenoma (absence of hormonal hypersecretion), including 1 with clinical (classic) pituitary apoplexy, 2 with growth hormone–producing adenoma, 2 with prolactin-producing adenoma, and 1 with adrenocorticotropic hormone-producing adenoma. Thirteen patients had headache associated with pituitary adenoma, including 6 with head heaviness, 4 with past episodes of severe headache (including the patient with clinical pituitary apoplexy), 2 with continuous dull pain, and 1 patient with intermittent dull pain. For analysis, we divided the patients into those with or without various headaches.20,21 There were 22 patients who had visual disturbance. No patients had associated metabolic disease including hemochromatosis or manganese excess.
MR Imaging
With a 1.5T system, we performed MR examination of the pituitary adenomas in all patients. Coronal T1-weighted fast spin-echo (FSE) MR images (TR, 400–500 ms; TE, 8–20 ms; section thickness, 3–4 mm; intersection gap, 0–1 mm; matrix, 256 × 160–320; and FOV, 16–20 × 16–20 cm) and coronal T2-weighted FSE images (TR, 3500–4400 ms; TE, 97.2–108 ms; section thickness, 3–4 mm; intersection gap, 0–1 mm; matrix, 160–384 × 192–448; and FOV, 16–20 × 16–20 cm) were obtained in all patients. The exception was 1 patient with apoplexy, who was admitted emergently to another hospital, where sagittal T1- and T2-weighted FSE imaging was performed (Fig 1) before transfer to our hospital for emergent surgery. MR imaging with contrast material was also performed in all patients, except for 1 patient, who had an allergy to contrast material. Coronal T2*-weighted GE MR imaging of the pituitary adenoma was obtained in all patients (TR, 500-1000 ms; TE, 15–26 ms; flip angle, 20°; section thickness, 3–5 mm; intersection gap, 0–2 mm; matrix, 193–256 × 160–320; and FOV, 16–22 × 16–22 cm). We also performed 3D time-of-flight MR angiograms in all patients to evaluate vascular abnormality of the internal or anterior cerebral arteries.
Fig 1.
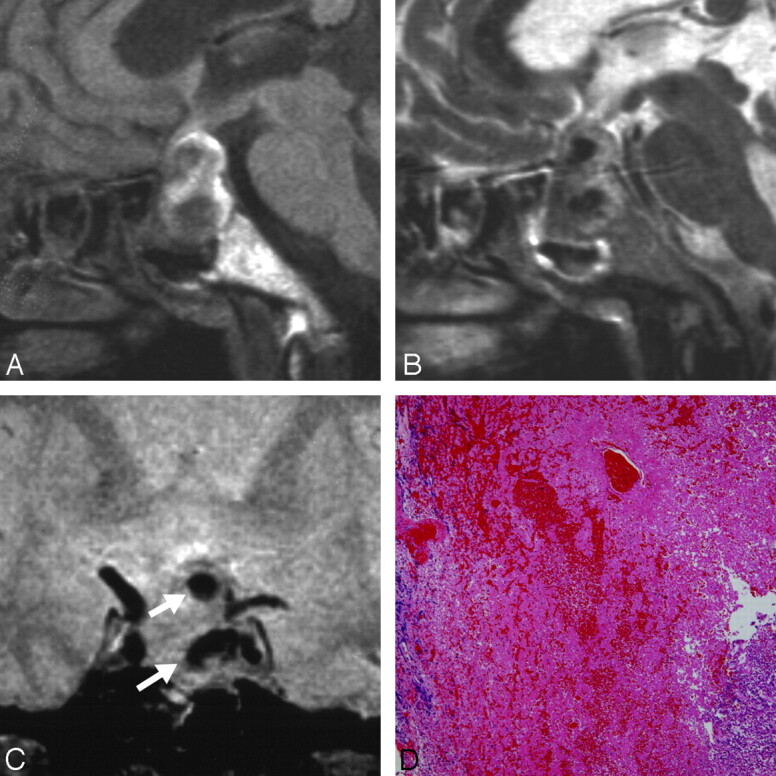
Patient 1. A 65-year-old woman presented with classic pituitary apoplexy manifesting as a sudden onset of severe headache, nausea, vomiting, and visual disturbance. Also, she became drowsy because of severe dehydration and hyponatremia. A, Sagittal T1-weighted MR image showing low- to isointense masses in the nonenhanced pituitary lesion with diffuse high signal intensity. B, Sagittal T2-weighted MR image showing dark masses in the mixed high signal intensity area. C, Coronal T2*-weighted gradient-echo image showing dark masses indicating hematoma (white arrows). D, Photomicrograph showing hemorrhage and a small amount of chromophobic adenoma (H&E, original magnification ×40).
Radiologic Assessments
Three independent observers reviewed the conventional T1- and T2-weighted FSE images and T2*-weighted GE images, and assessed the presence of various patterns of intensity suggestive of intratumoral hemorrhage.
The nonenhanced cystic regions were regarded as an intratumoral cyst or hematoma, and the solid regions were identified on gadolinium-enhanced images. Signal intensity on the T2-weighted FSE and T2*-weighted GE images was defined as low if equal to or lower than the signal intensity of white matter, isointense if higher than that of white matter and lower than that of gray matter, and high if equal to or higher than that of gray matter. Low signal intensity was also described as “dark” on these sequences. Signal intensity on the T1-weighted FSE images was defined as low if equal to or lower than the signal intensity of gray matter, isointense if higher than that of gray matter and lower than that of white matter, and high if equal to or higher than that of white matter.
Regional or special findings of low signal intensity on T2-weighted FSE and T2*-weighted GE images were classified as a “dark mass” with a diameter of more than 5 mm and a “dark spot” with a diameter of less than 5 mm, as illustrated in Figs 1B and C (the 5-mm boundary was based on the criteria for microbleeds in the brain on T2*-weighted GE images22). Intratumoral low signal intensity surrounding intratumoral cysts or hematomas on T2-weighted FSE and T2*-weighted GE images was classified as “dark rim” as illustrated in Figs 2B, 3B, and 2C and 3C, respectively. Diffusely observed (scattered) intratumoral dark spots on T2*-weighted GE images were classified as “diffuse dark” as illustrated in Fig 4C. Each neuroradiologist or neurosurgeon made initial evaluations independently, and consensus among them resolved any disagreements regarding final conclusions. Intratumoral hemorrhagic lesions were identified when there were high signal intensity intratumoral lesions on T1-weighted FSE images, and low or dark signal intensity lesions on T2-weighted FSE images and/or on T2*-weighted GE images (dark mass, rim, spots, or diffuse dark).4,9,23
Fig 2.
Patient 2. A 30-year-old man presented with subacute hematoma manifesting as right temporal hemianopia and progressive right visual disturbance. He occasionally had a mild headache but no sudden and eventful headaches. His serum prolactin level was 533.2 ng/mL. A, Coronal T1-weighted MR image showing a large pituitary lesion containing a large area of high signal intensity. B, Coronal T2-weighted MR image showing a large area of low signal intensity with a marked low signal intensity rim. C, Coronal T2*-weighted GE image showing low intensity hematomas with a clear dark rim (white arrow), and a dark mass in the solid portion of the tumor (white arrowhead). D, Photomicrograph showing hemorrhage in chromophobic adenoma (H&E, original magnification ×40).
Fig 3.
Patient 3. A 43-year-old man had typical cysts in a nonfunctioning adenoma. A, Coronal T1-weighted MR image showing a large pituitary lesion. B, Coronal T2-weighted MR image showing the equivocal hypointense rim of a cyst (white arrowheads). C, Coronal T2*-weighted GE image showing the faint hypointense rim of a cyst (white arrows). D, Photomicrograph showing hemorrhage in chromophobic adenoma (H&E, original magnification ×40).
Fig 4.
Patient 4. A 39-year-old woman had a solid nonfunctioning pituitary adenoma without cyst or hematoma. She had no past or present headache. A, Coronal T1-weighted MR image showing the isointense tumor. B, Coronal T2-weighted MR image showing the mixed high signal intensity pituitary lesion. C, Coronal T2*-weighted GE image showing diffuse dark appearance of the adenoma. D, Photomicrograph showing hemorrhage in chromophobic adenoma (H&E stain, original magnification ×40).
Skull base artifacts should be eliminated carefully on T2*-weighted GE images.12 Skull base artifacts were frequently seen in the most anterior section during coronal sections of the adenoma, and these sections were frequently excluded from assessments. Inferior small portions near the sella of the adenomas were frequently impossible to assess because of skull base artifacts (Fig 4). All MR angiograms were assessed to eliminate vascular abnormalities surrounding and inside of macroadenomas as potential causes of dark elements (flow voids) on T2*-weighted GE MR imaging.12
Surgical and Histologic Assessments
All patients underwent trans-sphenoidal resection of the tumors, which was performed by 1 neurosurgeon. All adenomas that were removed in the series were soft, friable, and removable through suction and were resected piece by piece with use of a curette, as in the common procedure for removal of pituitary adenomas. Intraoperative findings relating to intratumoral hemorrhage were classified as cysts including xanthochromic fluid, liquefied hematoma (reddish or brownish fluid), and coagulation (coagulated hematoma, or tumor tissue marbled small coagulated hematomas).9 Whole resected materials were submitted for histologic assessments.
One neuropathologist specializing in interpretation of brain and pituitary tumors performed the histologic assessment of the hemorrhage in the whole surgical specimens, independent of the other participants and completely unaware of the clinical and neuroradiologic information. Intratumoral hemorrhage, calcification, necrosis, melanins, and iron deposits as potential dark elements on T2*-weighted GE images were assessed systematically with hematoxylin-eosin (H&E) staining.12 Intratumoral hemorrhage was evaluated with H&E and was classified as follows: ++, significantly present; +, clearly present; ±, slightly observed; and -, not observed. A positive hemorrhagic finding was determined as “++” or “+,” and a negative hemorrhagic finding was determined as “±” or “−.” Intratumoral calcifications and melanins were assessed systematically.12 Histologic deposition of iron was assessed with use of a Prussian blue stain in all adenomatous specimens and was classified as “present (positive)” or “not observed (negative)” for statistical analysis.
Statistical Analysis
We used the Fisher exact probability test to determine whether the presence of intratumoral dark lesions on T2*-weighted GE MR images correlated with the clinical, surgical, or histologic findings, and the findings of other MR images related to intratumoral hemorrhage. A value of P < .05 was considered statistically significant.
Results
Fifteen of the 25 (60%) patients had a pituitary macroadenoma containing nonenhanced intratumoral portions (cyst or hematoma) with a diameter of more than 5 mm, and 10 patients had a solid tumor. Intratumoral high signal intensity on T1-weighted FSE images, indicating hemorrhage, was found in 8 (32%) patients.23 An intratumoral dark mass or cyst with a dark rim on T2-weighted FSE images, which indicated hematoma or hemorrhagic cyst, respectively, was found in 9 (36%) patients. T2*-weighted GE MR imaging showed the solid portion of pituitary adenoma as mainly isointense and the cystic portion as mainly high signal intensity. T2*-weighted GE MR imaging detected various types of dark lesions, which indicated new or old intratumoral bleeding, in 12 (48%) patients. The dark lesions were classified as a rim in 9 patients, a mass in 3 patients, a spot in 5 patients, diffuse in 2 patients, and a combination of a rim and others in 5 patients. No vascular abnormality surrounding and inside the tumor was observed on MR angiograms in all patients.24
Intraoperative findings relating to intratumoral hemorrhage were observed in 10 of the 25 patients: cysts including xanthochromic fluid in 3, liquefied hematoma (reddish or brownish fluid) in 4, and coagulation (coagulated hematoma, or tumor tissue marbled small coagulated hematomas) in 3 patients. Histologic evidence of intratumoral hemorrhage was clearly observed in 16 patients: 2 patients with significantly present (++) and 14 with clearly present (+) hemorrhage. Intratumoral calcifications and melanins were not observed in all specimens. Intratumoral necrosis was observed only in the case of a patient with clinical pituitary apoplexy. Intratumoral iron deposits were observed in 12 of the 25 adenomas.
The presence of intratumoral regional dark lesions (mass, rim, or spots, or all; or diffuse) on T2*-weighted GE MR imaging did not correlate with a preoperative headache and intratumoral areas unenhanced by gadolinium, but it correlated significantly with the presence of positive hemorrhagic findings on T1- and T2-weighted FSE MR imaging (P < .02 and <.01, respectively) and the presence of various intraoperative and histologic hemorrhagic findings (P < .001 and <.001, respectively) (Table). The presence of positive iron findings did not correlate with positive findings on T2*-weighted GE MR imaging or T2-weighted FSE sequence.
Intratumoral dark lesions on T2*-weighted GE MR imaging and other hemorrhagic findings
| Signs Relating to Intratumoral Hemorrhage | Intratumoral Dark Lesions on T2*-Weighted GE MR Imaging |
P Value | |
|---|---|---|---|
| Yes | No | ||
| Intratumoral high intensity on T1-weighted FSE MR imaging | <.02* | ||
| Yes | 7 | 1 | |
| No | 5 | 12 | |
| Intratumoral dark lesions on T2-weighted FSE MR imaging | <.01* | ||
| Yes | 8 | 1 | |
| No | 4 | 12 | |
| Intraoperative findings of intratumoral hemorrhage | <.001* | ||
| Yes† | 10 | 0 | |
| No | 2 | 13 | |
| Histologic findings of intratumoral hemorrhage | <.001* | ||
| Yes‡ | 12 | 4 | |
| No | 0 | 9 | |
Note:—GE indicates gradient-echo; FSE, fast spin-echo.
Statistically significant.
Cysts including xanthochromic fluid, liquefied hematoma, and coagulation.
Positive histologic hemorrhagic finding (++ or +).
Discussion
Our study detected intratumoral hemorrhage in pituitary macroadenomas by T2*-weighted GE MR imaging as various types of intratumoral dark lesions (rim, mass, spots, diffuse, and combinations), which indicated new or old intratumoral bleeding in 12 of the 25 patients. The presence of intratumoral dark lesions on T2*-weighted GE MR imaging correlated significantly with the presence of hemorrhagic findings on T1- and T2-weighted MR imaging, and intraoperative and histologic examinations.
GE MR imaging can depict acute and chronic hemorrhage not seen with conventional SE techniques at low and midfield strengths and is clearly the most sensitive currently available technique for the detection of intracerebral hemorrhage.11,12,16 We could detect intratumoral dark lesions indicating clinical and clinically silent hemorrhage in pituitary adenoma more frequently on T2*-weighted GE MR images than on conventional T1- and T2-weighted FSE MR images. However, GE MR imaging for the clinical detection of hemorrhage has several essential limitations.
Hypointensity on T2*-weighted GE MR imaging is not specific for hemorrhage and may be caused by calcification, or melanin or iron deposits.11,12 In our study, we systematically excluded the presence of calcification and melanin as potential dark elements on T2*-weighted GE images with histologic assessment of whole surgical materials. Melanin pigmentation of pituitary adenomas was not observed in our series and, to the best of our knowledge, has never been reported.
The incidence of calcifications within pituitary adenomas is about 1.72% on radiologic evaluation and 6.75% on histologic evaluation. Prolactin-producing adenoma, for which the surgical indications are limited, is the most common type in patients with calcified pituitary adenoma.25,26 Systematic examination revealed no histologic calcification in our series. Although very rare, intratumoral calcification may not be excluded on the basis of preoperative findings on T2*-weighted GE MR images. This limitation should be compensated by CT, especially in patients with high levels of serum prolactin.
Hypointensity on T2*-weighted GE images may also be caused by intratumoral iron deposits. No significant correlation was found between the presence of histologic iron deposits and positive findings on T2*-weighted GE images. The iron deposits in adenomas were also not related to hypointensity on T2-weighted SE images.27 Iron deposits may not be a major cause of hypointensity on T2*-weighted GE images. Intrasellar flow voids as a result of aneurysms or vascular malformation are another possible cause of dark elements on T2*-weighted GE images but were excluded by 3D time-of-flight MR angiograms in our study. Intrasellar vascular abnormalities associated with pituitary macroadenoma may be extremely rare.24
Intraparenchymal large hematomas appear as several different patterns regardless of the age of the hematoma. The most common appearance of a “dark rim,” “low signal intensity rim,” or “peripheral signal intensity loss” can be explained as the “boundary effect,” based on differences in magnetic susceptibility at the border of tissues and by the deoxygenation of blood occurring in the interdigitation of blood and tissue at the periphery of the lesion.16 The presence of perilesional hemosiderin in chronic hematoma causes the “rim” appearance on T2*-weighted GE MR images. Our study found that a “dark rim” was the most common appearance of intratumoral hemorrhage in pituitary macroadenoma.
Pituitary adenomas frequently (about 50%) include cystic or hematoma formation, or both.10 In our study, 16 of the 25 macroadenomas had cysts or hematomas, or both. Nonenhanced areas appearing as high signal intensity on T1-weighted FSE imaging may indicate subacute or early chronic phase intratumoral hemorrhage.9,23 In our study, all cases with a high signal intensity cavity on T1-weighted FSE imaging certainly also had a “dark rim (or mass)” on T2*-weighted GE MR imaging (Fig 2). However, cases with a nonenhanced cystic cavity appearing as low signal intensity to isointensity on T1-weighted FSE imaging sometimes had a “dark rim” on T2*-weighted GE MR imaging (Fig 3). A cystic cavity also with these patterns of intensity may indicate an old or weakly hemorrhagic cystic cavity. Even with GE MR imaging, hemosiderin, especially small and scattered deposits, may become progressively less visible with time.28 Intratumoral cysts with a “dark rim” on T2*-weighted GE MR imaging might indicate an acute or old hemorrhagic cyst, but even without a dark rim on T2*-weighted GE MR imaging, the cyst cannot be conclusively identified as nonhemorrhagic.28
Our study identified the “diffuse dark” characteristic of pituitary adenoma on T2*-weighted GE MR imaging, which was not detected by conventional T1- and T2-weighted FSE imaging. The solid portion of the tumor contained an extensive and diffuse dark area on T2*-weighted GE MR imaging. There were 2 of 25 pituitary adenomas that had a diffuse, dark macroadenoma on T2*-weighted GE MR imaging. The diffuse, dark part of the adenoma was dark and radish, and it mixed with small coagulated hematomas on intraoperative findings. Histologic examination found positive hemorrhagic findings in these 2 macroadenomas (Fig 4). These patients had a nonfunctioning pituitary adenoma without a history of headache or recurrent headache. This type of highly and diffusely hemorrhagic adenoma may represent a category of silent pituitary apoplexy, which can only be detected on T2*-weighted GE MR imaging.
“Diffuse hemorrhage throughout the adenoma” has already been proposed as a type of silent pituitary apoplexy, observed as diffuse increased signal intensity throughout the most solid part of the adenoma on T1-weighted SE images.29 Our adenomas with the “diffuse dark” appearance on T2*-weighted GE MR imaging had no diffuse increased signal intensity on T1-weighted FSE imaging and may indicate the past silent, “diffuse-type” pituitary apoplexy. These adenomas were soft but slightly resistant to suction (intermediate consistency) at surgery.30 Further study of larger series is required to elucidate the pathophysiology and clinical significance of a “diffuse dark” macroadenoma detected only on T2*-weighted GE MR imaging.
Classical pituitary apoplexy is a clinical syndrome characterized by sudden onset of headache, vomiting, visual impairment, diplopia, disturbance of consciousness, and autonomic or hormonal dysfunction.2,6 From a clinical standpoint, the diagnosis of pituitary apoplexy may be difficult to establish in the acute stage, so it is usually decided retrospectively, but strict medical management and surgical decompression for endocrinologic and neurologic problems are usually required.2,6 Pituitary apoplexy is caused by acute hemorrhagic or ischemic infarction of the pituitary gland in patients harboring pituitary adenomas.2,4,6 Early in the course of pituitary apoplexy, MR imaging depicts a mass lesion as heterogeneous signal intensity with predominant hyperintensity on T1-weighted MR images and predominant hypointensity on T2-weighted images.4,23 In our series, 1 patient presented with classic pituitary apoplexy (Fig 1), in which the T1- and T2-weighted MR images were similar to the previously reported rare image of the early phase of pituitary apoplexy.24 T2*-weighted GE MR imaging could clearly detect the intratumoral hemorrhage as a “dark lesion (mass),” which may reflect the distribution of paramagnetic deoxyhemoglobin in the acute phase of hemorrhage. This “dark mass” had very high contrast with the surrounding structures. The cases of subacute apoplexy had a clear dark rim (Fig 2).3 T2*-weighted GE MR imaging shows a high potential for the neuroimaging diagnosis of pituitary apoplexy.
Accurate assessment of intratumoral hemorrhage in pituitary macroadenoma has increased in importance. Patients with pituitary macroadenomas can sometimes be followed up by MR imaging because of a relatively benign prognosis.31,32 Recurrent hemorrhage could cause tumoral enlargement and aggravate the patient's symptoms, so such patients should be followed carefully.9 The presence of a hemorrhagic cavity often facilitates removal of a macroadenoma, and such information may be useful for surgical planning.2 This more sensitive method of neuroimaging detection of an intratumoral hemorrhage may influence the decision making in various clinical and intraoperative conditions of patients with pituitary macroadenoma.30,32
The interface between regions with different magnetic susceptibility is markedly hypointense on T2*-weighted GE images.11,12 Therefore, marked hypointensity near the sphenoid sinus air or pituitary adenomatous interface because of diamagnetic susceptibility gradients can obscure the inferior portions of the pituitary adenoma. Several preliminary studies found that characterization of micro- and intrasellar adenoma by T2*-weighted GE MR imaging was inconclusive and frequently impossible because of skull base artifacts. Therefore, this prospective study included only patients with pituitary macroadenoma, including pituitary apoplexy. Most cases of intratumoral cyst or hemorrhage are predominantly found in patients with macroadenoma with suprasellar extension. Although the T2*-weighted GE sequence has several limitations, most clinical requirements for MR observation of intratumoral hemorrhage in patients with pituitary macroadenomas can be satisfied.
T2*-weighted GE MR imaging is better than other conventional sequences for the detection of hemorrhage, but, in our study, we found only 12 (75%) of 16 histologically proved intratumoral hemorrhages using a 1.5T unit. Several variations of MR imaging techniques and MR units with higher field strengths than 1.5T might increase the sensitivity for the detection of intratumoral hemorrhage in patients with pituitary macroadenomas. Further investigation is required.33
Conclusion
T2*-weighted GE MR imaging could detect an intratumoral hemorrhage in patients with pituitary macroadenomas as various “dark” appearances, such as “rim,” “mass,” “spot,” and “diffuse.” This technique can clearly detect clinical (classic) and subclinical apoplexy, identify cryptic and diffuse hemorrhage, and evaluate cysts from an old hemorrhage. T2*-weighted GE MR imaging may be useful for the assessment of recent and old intratumoral hemorrhagic events in patients with pituitary macroadenomas.
Footnotes
This study was partly supported by grants from Japan's Ministry of Education, Science, Sports and Culture.
References
- 1.Wakai S, Yamakawa K, Manaka S, et al. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery 1982;10:437–44 [DOI] [PubMed] [Google Scholar]
- 2.Mohr G, Hardy J. Hemorrhage, necrosis, and apoplexy in pituitary adenomas. Surg Neurol 1982;18:181–89 [DOI] [PubMed] [Google Scholar]
- 3.Kyle CA, Laster RA, Burton EM, et al. Subacute pituitary apoplexy: MR and CT appearance. J Comput Assist Tomogr 1990;14:40–44 [DOI] [PubMed] [Google Scholar]
- 4.Piotin M, Tampieri D, Rüfenacht DA, et al. The various MRI patterns of pituitary apoplexy. Eur Radiol 1999;9:918–23 [DOI] [PubMed] [Google Scholar]
- 5.Lubina A, Olchovsky D, Berezin M, et al. Management of pituitary apoplexy: clinical experience with 40 patients. Acta Neurochir (Wien) 2005;147:151–57; discussion 157 [DOI] [PubMed] [Google Scholar]
- 6.Semple PL, Webb MK, de Villiers JC, et al. Pituitary apoplexy. Neurosurgery 2005;56:65–72; discussion 72–73 [DOI] [PubMed] [Google Scholar]
- 7.Ram Z, Hadani M, Berezin M, et al. Intratumoural cyst formation in pituitary macroadenomas. Acta Neurochir (Wien) 1989;100:56–61 [DOI] [PubMed] [Google Scholar]
- 8.Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy: presentation, surgical management, and outcome in 21 patients. Neurosurgery 1990;26:980–86 [PubMed] [Google Scholar]
- 9.Kurihara N, Takahashi S, Higano S, et al. Hemorrhage in pituitary adenoma: correlation of MR imaging with operative findings. Eur Radiol 1998;8:971–76 [DOI] [PubMed] [Google Scholar]
- 10.Nishi T, Goto T, Takeshima H, et al. Tissue factor expressed in pituitary adenoma cells contributes to the development of vascular events in pituitary adenomas. Cancer 1999;86:1354–61 [DOI] [PubMed] [Google Scholar]
- 11.Unger EC, Cohen MS, Brown TR. Gradient-echo imaging of hemorrhage at 1.5 Tesla. Magn Reson Imaging 1989;7:163–72 [DOI] [PubMed] [Google Scholar]
- 12.Atlas SW, Mark AS, Grossman RI, et al. Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology 1988;168:803–07 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka A, Ueno Y, Nakayama Y, et al. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke 1999;30:1637–42 [DOI] [PubMed] [Google Scholar]
- 14.Alemany Ripoll M, Stenborg A, Sonninen P, et al. Detection and appearance of intraparenchymal haematomas of the brain at 1.5 T with spin-echo, FLAIR and GE sequences: poor relationship to the age of the haematoma. Neuroradiology 2004;46:435–43 [DOI] [PubMed] [Google Scholar]
- 15.Arnould MC, Grandin CB, Peeters A, et al. Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. AJNR Am J Neuroradiol 2004;25:939–44 [PMC free article] [PubMed] [Google Scholar]
- 16.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004;292:1823–30 [DOI] [PubMed] [Google Scholar]
- 17.Yanagawa Y, Tsushima Y, Tokumaru A, et al. A quantitative analysis of head injury using T2*-gradient-echo imaging. J Trauma 2000;49:272–77 [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Fei Z, Zhang J, et al. Management of nonfunctioning pituitary adenomas with suprasellar extensions by transsphenoidal microsurgery. Surg Neurol 1999;52:380–85 [DOI] [PubMed] [Google Scholar]
- 19.Alleyne CH, Barrow DL, Oyesiku NM. Combined transsphenoidal and pterional craniotomy approach to giant pituitary tumors. Surg Neurol 2002;57:380–90; discussion 390 [DOI] [PubMed] [Google Scholar]
- 20.Abe T, Matsumoto K, Kuwazawa J, et al. Headache associated with pituitary adenomas. Headache 1998;38:782–86 [DOI] [PubMed] [Google Scholar]
- 21.Levy MJ, Matharu MS, Meeran K, et al. The clinical characteristics of headache in patients with pituitary tumours. Brain 2005;128:1921–30 [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Bae HJ, Ko SB, et al. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry 2004;75:423–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonneville F, Cattin F, Marsot-Dupuch K, et al. T1 signal hyperintensity in the sellar region: spectrum of findings. Radiographics 2006;26:93–113 [DOI] [PubMed] [Google Scholar]
- 24.Romano A, Chibbaro S, Marsella M, et al. Carotid cavernous aneurysm presenting as pituitary apoplexy. J Clin Neurosci 2006;13:476–79 [DOI] [PubMed] [Google Scholar]
- 25.Rilliet B, Mohr G, Robert F, et al. Calcifications in pituitary adenomas. Surg Neurol 1981;15:249–55 [DOI] [PubMed] [Google Scholar]
- 26.Molitch ME, Thorner MO, Wilson C. Management of prolactinomas. J Clin Endocrinol Metab 1997;82:996–1000 [DOI] [PubMed] [Google Scholar]
- 27.Hagiwara A, Inoue Y, Wakasa K, et al. Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 2003;228:533–38 [DOI] [PubMed] [Google Scholar]
- 28.Messori A, Polonara G, Mabiglia C, et al. Is haemosiderin visible indefinitely on gradient-echo MRI following traumatic intracerebral haemorrhage? Neuroradiology 2003;45:881–86 [DOI] [PubMed] [Google Scholar]
- 29.Ostrov SG, Quencer RM, Hoffman JC, et al. Hemorrhage within pituitary adenomas: how often associated with pituitary apoplexy syndrome? AJR Am J Roentgenol 1989;153:153–60 [DOI] [PubMed] [Google Scholar]
- 30.Pierallini A, Caramia F, Falcone C, et al. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging—initial experience. Radiology 2006;239:223–31 [DOI] [PubMed] [Google Scholar]
- 31.Igarashi T, Saeki N, Yamaura A. Long-term magnetic resonance imaging follow-up of asymptomatic sellar tumors—their natural history and surgical indications. Neurol Med Chir (Tokyo) 1999;39:592–98; discussion 598–99 [DOI] [PubMed] [Google Scholar]
- 32.Sanno N, Oyama K, Tahara S, et al. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol 2003;149:123–27 [DOI] [PubMed] [Google Scholar]
- 33.Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005;26:662–65 [PMC free article] [PubMed] [Google Scholar]





