Abstract
SUMMARY: Reversible cerebral vasoconstriction syndromes (RCVS) typically affect the bilateral medium-sized intracerebral arteries and their branches. We describe a woman with RCVS restricted to the ipsilateral hemisphere after carotid endarterectomy. Serial CT angiography proved useful in documenting vasoconstriction. Perfusion MR imaging showed hypoperfusion in the deep watershed regions of the ipsilateral cerebral arteries but relatively normal perfusion in superficial cortical regions. Diffusion MR imaging showed progressive borderzone infarcts. These novel imaging findings provide insights into the pathophysiology of stroke in RCVS.
Reversible cerebral vasoconstriction syndromes (RCVS) are characterized by recurrent “thunderclap” headaches, focal neurologic deficits, and multifocal segmental arterial narrowing.1–3 The clinical and radiologic abnormalities usually resolve within days to weeks. Some patients can develop permanent deficits from ischemic or hemorrhagic stroke. The ischemic stokes are typically bilateral, symmetric, and located in borderzone arterial territories2,4; however, their mechanism is not clearly understood. We recently encountered a patient with RCVS developing after carotid endarterectomy (CEA) whose unique neuroimaging features provided insights into the pathophysiology of this syndrome.
Case Report
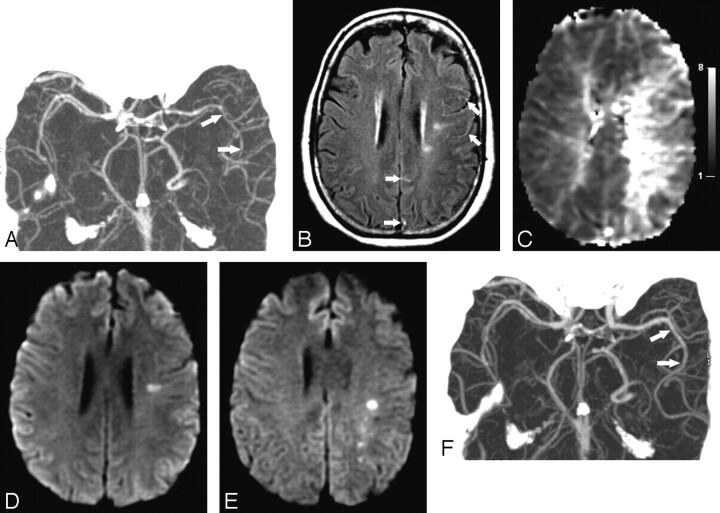
A 54-year-old woman developed a sudden severe thunderclap headache 2 days after CEA for symptomatic left internal carotid artery stenosis. Similar headaches recurred every 2–3 days, frequently exacerbated by Valsalva maneuver. Head CT and brain MR imaging scans showed a subacute left posterior frontal lobe infarction (present before the CEA) but no new lesions. She then developed 2 episodes of aphasia and right-handed numbness and was admitted. Blood pressure was 122/80 mm Hg. Findings of her neurologic examination were normal, except for symmetric hyper-reflexia without clonus. Head and neck CT angiography (CTA) showed that the left internal carotid artery was widely patent in its extracranial segment but showed narrowing in its supraclinoid segment. In addition, there was attenuation of the left middle and anterior cerebral arteries (MCAs and ACAs) and their branches (Fig 1A). These abnormalities were not present on the preoperative CTA. Transcranial Doppler sonography studies showed elevated left MCA blood-flow velocities (200 cm/s). On brain MR imaging, fluid-attenuated inversion recovery (FLAIR) sequences showed multiple punctate and curvilinear hyperintensities in the sulci overlying the left cerebral hemisphere, consistent with slow flow within dilated MCA cortical branches (Fig 1B). Perfusion MR imaging showed hypoperfusion (increased mean transit time, reduced cerebral blood flow) in the left hemisphere, predominantly affecting the internal watershed regions of the left MCA and ACA, with relative sparing of the superficial cortical regions (Fig 1C). Diffusion-weighted MR imaging showed the previously identified left posterior frontal lobe stroke but no new areas of infarction (Fig 1D). Findings of CSF examination were normal (2 white blood cells, 2 red blood cells, 37 mg/dL proteins, no xanthochromia). On day 2 after admission, the patient developed 3 episodes of transient right-handed numbness followed by persistent right-handed weakness. Pharmacologically induced hypertension therapy was instituted in an effort to improve cerebral perfusion, yet her symptoms did not improve. Rather, the longest spell of right-handed weakness occurred at a systolic blood pressure of 140 mm Hg. A repeat MR imaging on day 3 after admission showed new borderzone infarctions located within the areas of left hemisphere hypoperfusion (Fig 1E). The patient was treated with intravenous fluids, intravenous magnesium, oral nimodipine, and aspirin. Her neurologic deficits resolved completely, and she was discharged. A follow-up CTA after 2 months showed complete resolution of the cerebral arterial vasoconstriction (Fig 1F).
Fig 1.
Neuroimaging findings in a 54-year-old woman with RCVS after CEA. A, CTA on day 1 shows attenuation and segmental narrowing of the left ACAs and MCAs (arrows). These abnormalities were not present on a CTA performed before the endarterectomy. B, FLAIR image from the brain MR imaging on day 1 shows multiple punctate and curvilinear hyperintensities (arrows) overlying the left cerebral hemisphere, suggesting slow blood flow within abnormally dilated arteries. C, Mean-transit-time maps from the perfusion MR imaging on day 1 (gray scale in seconds) show hypoperfusion within the left internal watershed arterial territories and relative sparing of the superficial cortical regions. D, Diffusion-weighted brain MR imaging on day 1 shows a posterior left frontal-lobe infarction, which was present before the CEA, but no new lesion. E, Repeat diffusion-weighted brain MR imaging on day 3 shows new acute infarctions developing within the previously noted regions of subcortical hypoperfusion. F, Repeat CTA performed after 2 months shows complete resolution (arrows) of the arterial vasoconstriction.
Discussion
Our patient developed recurrent thunderclap headaches soon after CEA and proved to have reversible cerebral artery vasoconstriction by CTA, perfusion MR imaging, and transcranial Doppler sonography. These clinical and radiologic features are consistent with the diagnosis of post-CEA RCVS. The abrupt onset of headaches, benign CSF examination findings, and complete clinical and angiographic resolution without immunosuppressive treatment excluded the possibility of primary central nervous system vasculitis—an important mimic of this syndrome.2,3
Transfemoral cerebral angiography is the gold standard for detecting cerebral artery vasoconstriction and ideally should be repeated after weeks to months to document reversibility and confirm the diagnosis. This is the 1st report documenting the utility of serial CTA, a relatively noninvasive imaging technique, in diagnosing cerebral vasoconstriction syndrome. Furthermore, the patient's brain MR imaging findings were novel and provide insights into the pathophysiology of this syndrome. FLAIR images showed sulcal hyperintensities consistent with slow flow within abnormally dilated cortical vessels, a sign that has been associated with cerebral vasoconstriction syndrome.5 Mean transit time was increased, and cerebral blood flow was decreased in the internal watershed regions of the left cerebral hemisphere, whereas perfusion in the superficial cortical regions was relatively normal. The hypoperfused regions later developed ischemic strokes. The topography of these MR imaging lesions is consistent with cortex-sparing arterial-watershed-territory bilateral strokes that have been noted in patients with reversible vasoconstriction syndrome2–4 and suggests that vasoconstriction is the underlying stroke mechanism.
RCVS can be idiopathic or associated with conditions such as pregnancy (postpartum angiopathy), vasoconstrictive drug use, pheochromocytoma, migraine, thunderclap headache, and primary headache disorders, among others.1,2,6 In their report on 16 patients with RCVS, Call et al1 reported seeing 2 patients who developed the syndrome after CEA; however, no details were provided. Since then, post-CEA vasoconstriction syndrome has been described in at least 3 case reports.7–9 Brick et al7 described a 57-year-old man who developed headaches 8 days after right CEA; carotid angiography showed segmental arterial beading in the right-sided MCA and ACA branches. Lopez-Valdes et al8 described 2 similar patients. The first was a 50-year-old man who, 1 week after right CEA, developed left-sided visual-field deficits and extremity numbness, right-sided headaches and ischemic strokes, and segmental arterial vasoconstriction affecting the bilateral anterior and posterior circulation. The second patient was a 54-year-old woman who underwent a left CEA followed by right CEA 1 week later; on day 8 after the right CEA, she developed severe headaches, right-sided ischemic strokes, and segmental vasoconstriction affecting the bilateral anterior circulation. Dagher et al9 recently described a 62-year-old man who developed weakness in the right arm and expressive aphasia 5 days after left CEA; cerebral angiography showed multifocal segmental arterial vasoconstriction affecting only the ipsilateral ACAs and MCAs.
The relationship between CEA and vasoconstriction is not clear. Bilateral diffuse vasoconstriction is typical of vasoconstriction syndromes and was documented in 2 of the post-CEA cases discussed above. However, both of these patients also had a history of migraine, which is implicated in the pathophysiology of vasoconstriction syndrome. In comparison, vasoconstriction was restricted to the ipsilateral hemisphere in 2 of the 4 patients discussed previously, as well as in our patient. The ipsilateral occurrence suggests a direct mechanistic relationship between CEA and vasoconstriction syndrome. Chronic severe carotid artery stenosis results in disturbances in cerebral autoregulation within ipsilateral cerebral arteries, and the relative hypertension in these arteries after CEA may have a role in precipitating vasoconstriction.
A similar pathophysiology is implicated in post-CEA hyperperfusion syndrome, a recognized complication of CEA in which the relative hypertension after CEA is believed to result in ipsilateral brain edema.10 Post-CEA hyperperfusion syndrome and post-CEA vasoconstriction syndrome have several similarities. They are both characterized by abrupt-onset severe headaches, seizures, and focal neurologic deficits. The clinical outcome is usually benign, but some patients can develop permanent deficits from stroke. Also, the radiologic abnormalities are ipsilateral and reversible. We suggest that the post-CEA hyperperfusion and post-CEA vasoconstriction syndromes are interrelated, similar to the relationship between RCVS and the reversible posterior leukoencephalopathy syndrome (another brain edema syndrome).6 These syndromes likely represent a spectrum of disorders resulting from disturbed cerebral autoregulation.
Acknowledgments
We thank Ona Wu, PhD, for creating the Figures.
References
- 1.Call GK, Fleming MC, Sealfon S, et al. Reversible cerebral segmental vasoconstriction. Stroke 1988;19:1159–70 [DOI] [PubMed] [Google Scholar]
- 2.Singhal AB. Cerebral vasoconstriction syndromes. Top Stroke Rehabil 2004;11:1–6 [DOI] [PubMed] [Google Scholar]
- 3.Singhal AB, Bernstein RA. Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit Care 2005;3:91–97 [DOI] [PubMed] [Google Scholar]
- 4.Singhal AB, Caviness VS, Begleiter AF, et al. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology 2002;58:130–33 [DOI] [PubMed] [Google Scholar]
- 5.Iancu-Gontard D, Oppenheim C, Touze E, et al. Evaluation of hyperintense vessels on FLAIR MRI for the diagnosis of multiple intracerebral arterial stenoses. Stroke 2003;34:1886–91 [DOI] [PubMed] [Google Scholar]
- 6.Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 2004;61:411–16 [DOI] [PubMed] [Google Scholar]
- 7.Brick JF, Dunker RO, Gutierrez AR. Cerebral vasoconstriction as a complication of carotid endarterectomy: case report. J Neurosurg 1990;73:151–53 [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Valdes E, Chang HM, Pessin MS, et al. Cerebral vasoconstriction after carotid surgery. Neurology 1997;49:303–04 [DOI] [PubMed] [Google Scholar]
- 9.Dagher HN, Shum MK, Campellone JV. Delayed intracranial vasospasm following carotid endarterectomy. Cerebrovasc Dis 2005;20:205–06 [DOI] [PubMed] [Google Scholar]
- 10.van Mook WN, Rennenberg RJ, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005;4:877–88 [DOI] [PubMed] [Google Scholar]