Abstract
BACKGROUND AND PURPOSE: Complex CSF diseases may be underdiagnosed or poorly understood on conventional CT or MR imaging. Although intrathecal CT cisternography with water-soluble iodinated contrast medium has been used, very few studies have dealt with the intrathecal use of gadopentate dimeglumine (Gd-DTPA), though it appears superior to CT. We report our experience with the intrathecal use of Gd-DTPA for MR cisternography and ventriculography in pediatric patients referred for study and treatment of complex CSF-related diseases.
MATERIALS AND METHODS: Ten patients (range, 1 month–16 years of age) were selected after we obtained specific informed consent. Intrathecal gadolinium injection was performed via transfontanelle ventriculostomy, ventriculoperitoneal shunt reservoir, or lumbar puncture. Cases included spontaneous CSF leaks (n = 1), complex traumatic frontoethmoidal fractures with suspected CSF leak (n = 2), multiloculated congenital or acquired hydrocephalus (n = 3), intraventricular tumor (n = 1), suspected postoperative arachnoiditis (n = 1), complex midline defect (n = 1), and acquired orbital meningoencephalocele (n = 1).
RESULTS: No patient showed biologic, behavioral, or neurologic alterations. In complex hydrocephalus or intraventricular cysts, ventriculography Gd-DTPA MR imaging helped to differentiate isolation of a ventricle or noncommunicating cyst in all 4 patients. In suspected posttraumatic CSF leaks, the procedure established with precision the place of the leak in 1 patient and excluded it in the other. In 1 patient who underwent surgery for spinal cord neoplasm, the procedure excluded arachnoiditis. In the other 3 patients with complex CSF-related diseases, the procedure showed distinctive radiologic findings for the understanding and treatment of the disease. Altogether, in 8 patients, imaging findings influenced or changed clinical decisions and surgical planning.
CONCLUSIONS: Our preliminary results showed no side effects and potential useful clinical applications in the evaluation of CNS diseases involving the ventricular system or the subarachnoid space in selected pediatric patients.
MR imaging has proved invaluable in anatomic depiction of the CSF spaces and the surrounding neural and non-neural tissue, though there are still some clinical situations (ie, CSF-flow alterations, communicating or noncommunicating cyst masses bordering CSF pathways, or craniospinal CSF leaks) in which further imaging tests may be required for a definitive diagnosis. Both radionuclide cisternography and CT with intrathecal water-soluble iodinated contrast medium (CT myelography) have been used for this purpose but still have limitations and use ionizing radiation.1,2
Gadopentate dimeglumine (Gd-DTPA) was the first contrast medium approved for intravenous use in MR imaging studies, and a high level of safety and tolerance has been proved in its clinical use.3,4 Despite good anatomic demonstration of brain and spine tissue and CSF, there are still a number of pathologic conditions in which CSF contrast enhancement may be beneficial, including assessment of continuity and outline of subarachnoid spaces, abnormal CSF collections and leakage, arachnoid cysts,5 or as a means to study CSF dynamics. Several studies in animal models reported the benefits of intrathecal gadolinium-enhanced MR imaging for the study of CSF pathway disorders and outlined the tolerance and dose limits.6–8 Different experimental protocols were designed and applied to animal models and tested with identical results (gadodiamide; Omniscan, Nycomed Amersham, Oslo, Norway; gadopentetate dimeglumine, Magnevist, Shering; Berlin, Germany).9–11 Data from acute and subacute physiologic and neurohistologic studies of the central nervous system provided evidence that when intrathecal gadolinium was used in doses sufficient to improve MR imaging of the CSF compartment, it was likely to be accompanied by a low incidence of acute changes in neural function or structure.12
After the first report of Gd-DTPA for ventriculography in 2 adult patients with meningeal carcinomatosis,3 several other cases were reported in personal communications and different publications.4,13 After a pilot study in human patients that included only adults, the safety and feasibility of a low dose of intrathecal gadolinium administration was shown.14 Finally, a multicenter study was developed that included young patients. This cooperative study demonstrated the general safety in all ranges of age with potential useful clinical applications.4 To our knowledge, no series of solely pediatric patients has been reported so far. Our purpose was to report the experience in our institution with the use of low-osmolality Gd-DTPA MR cisternography and ventriculography in the pediatric population. Currently, intrathecal use of Gd-DTPA in America is not approved by the US Food and Drug Administration and is used off-label.
Patients and Methods
Ten pediatric patients (1 month–16 years of age; sex, 8 boys, 2 girls) were referred to our institution after diagnosis of complex CSF-related diseases in whom the standard MR imaging studies were insufficient for a definitive diagnosis (Table). The patients presented clinically with a variety of cranial or spinal signs and symptoms for which an MR ventriculogram, myelogram, or cisternogram was requested by surgical or clinical pediatric neurologic services or a pediatric ear, nose, and throat service. Three children experienced CSF leakage (2 posttraumatic and 1 spontaneous) with rhinorrhea or otorrhea; 4 patients with intraventricular pathology included 3 cases of multiloculated congenital hydrocephalus and 1 case of intraventricular tumor. One patient developed progressive paraparesis after spinal cord tumor resection; another patient presented with complex midline defect; and finally 1 patient was referred because of progressive exophthalmos, in whom an acquired intraorbital meningoencephalocele in the context of osteopetrosis was suspected. Detailed and specific informed consent was obtained after a thorough discussion of the risks (headache, contrast medium reaction, short- and long-term central nervous system [CNS] injuries, seizures, and infection) and benefits, and it was placed in the patient's chart.
Demographics, clinical status, and surgical data
| Patient | Age/Sex | Diagnosis | Administration Procedure | Surgical Implications |
|---|---|---|---|---|
| 1 | 15 d/F | IIIv cystic tumor and asymmetric hydrocephalus | TF | Ex-vacuo ventriculomegaly and ependymal cyst diagnosed; no surgery |
| 2 | 1 m/M | Multicystic hydrocephalus | TF | Surgical planning |
| 3 | 2 m/M | Isolated lateral ventricle and interhemispheric cyst | TF | Agenesis of foramen of Monro diagnosed; surgical planning |
| 4 | 16 y/M | Posttraumatic rhinorrhea | LP | CSF leakage discarded; avoid surgery |
| 5 | 11 y/M | Progressive paraparesis after medullar tumor resection | LP | Lumbar arachnoiditis; avoid surgery |
| 6 | 17 y/M | Posttraumatic CSF leakage | LP | Surgical planning |
| 7 | 7 y/M | Orbital encephalocele; osteopetrosis | LP | Surgical planning |
| 8 | 22 m/F | Hypertelorism; midline defect | LP | No associated encephalocele; surgery delayed |
| 9 | 9 m/M | Multicystic hydrocephalus | VP shunt | Excluded IIIv diagnosed; surgical planning |
| 10 | 8 y/M | Spontaneous multiple CSF fistulae | LP | Surgical planning; LuP shunt |
| Transient juvenile osteoporosis | Avoid open surgery |
Note:—IIIv indicates 3rd ventricle; TF, transfontanelle; LP, lumbar puncture; VP, ventriculoperitoneal; LuP, lumboperitoneal; d, days; m, month; y, year; F, female; M, male.
Under sterile conditions, an intrathecal single dose of gadolinium (gadopentetate dimeglumine; Magnograf, Juste, Spain) diluted in autologous 5 mL of CSF was injected via lumbar puncture (20- to 22-gauge needle), transfontanelle ventriculostomy, or through the reservoir of the shunt system. Doses ranged from 0.8 to 2 mL, corresponding with 0.28-μmol/g brain to 0.71 μmol/g brain. Briefly, under sterile conditions, 3–5 mL of the patient's CSF was withdrawn, mixed with a single dose of gadolinium, and injected into the subarachnoid space (L3–4 or L4–5 level) or the desired lateral ventricle; and then the needle was removed. Nonanesthesized patients were positioned prone, 30°–40° with the head down for 30–90 minutes. Anesthetized low-weight patients were positioned upside down for 2–3 minutes. This maneuver tended to maximize accumulation of contrast medium in the basal cisterns and attempted to improve its passage into any suspected CSF fistula or cyst-containing lesions. Slow injection into the patient's diluted CSF was used as a precaution against hypertonic reactions of the CNS to the introduction of gadolinium contrast agent, according to previous recommendations.15 This maneuver has also been reported to reduce the possibility of acute calcium ion deficiency–related neural dysfunction on the surface of the CNS.
After transferring the patients to the MR imaging suite, we performed MR imaging. Children under 7 years of age were positioned on the MR imaging table under general anesthesia and monitored as in any standard anesthesia procedure in our MR imaging suite. Breathing and anesthesia were controlled via a mechanical MR imaging–compatible ventilator. Throughout the entire MR imaging session, all vital signs, including body temperature, respiratory rate, heart rate, blood pressure, blood oxygen saturation level, and expiratory CO2 levels, were constantly supervised by a pediatric anesthetist. Expiratory CO2 levels were kept between 5%–6%, and blood oxygen saturation was kept at 95% while the child was anesthetized. In these children, lumbar puncture or intraventricular injection was also performed with the child under general anesthesia. Immediate and delayed (range, 15 minutes–2 hours) T1-weighted imaging (500/15/2, TR/TE/NEX; 3-mm sections) with fat-saturation technique in case of study the skull base and nasal cavity and T2-weighted imaging (fast spin-echo [FSE]; 3500–4000/40/8–10, TR/TE/echo train) were performed in 2 or 3 orthogonal planes on a superconductive high-field MR imaging unit operating at 1.5T. Decisions concerning when and whether to perform delayed imaging were taken at the discretion of the neuroradiologist (A.M.). In older children with suspected CSF leaks (patients 4, 6, and 10), we asked the patients to avoid nasal fluid discharge the morning before the procedure. We also asked if they felt the sensation of fluid discharge the day of the procedure. If not, as it has happened in patient 10 once, we postponed the radiologic procedure until the appropriate clinical status. Furthermore, we performed the radiologic procedure in these patients in a prone head-down position. Nonanesthesized patients were monitored by electrocardiogram.
Heart rate, blood pressure, and blood oxygen saturation levels were checked during the lumbar puncture injection of the contrast and during the radiologic procedure. In all of the children, blood standard biochemistry was studied before and after the radiologic procedure. The study included glucose concentration, creatine level, liver enzyme concentration, creatine phosphokinase level, lipase level, lactate dehydrogenase concentration, hemoglobin level, and red, white, and platelet cell counts. Patients were hospitalized for an observation period of 24–48 hours after the examination. Results of the biochemistry studies of each patient before and after the procedure were analyzed by both neurosurgeons and the neuroradiologist for meaningful changes.
Images were recorded and examined by an experienced pediatric neuroradiologist (A.M.) with no attempt to compare them with radionuclide cisternography and CT myelography because our diagnostic decisions were not made on the basis of a clinical trial, and preliminary reports showed potential superiority of this method compared with other methods that were riskier or more invasive.1
Results
None of the patients showed significant clinical disturbances other than minor postural headache (2 older children) after the puncture, which resolved within the first 48 hours with conservative bed rest and conventional analgesics. We observed no gross behavioral changes, neurologic alterations, seizure activity, or changes in the standard biologic parameters following the procedure.
In the 4 patients with multiloculated hydrocephalus, gadolinium-enhanced MR imaging helped in preoperative surgical planning (Fig 1, patient 3), mainly because enhanced CSF allows differentiation between communicating and noncommunicating ventricle outpouching, isointense intraventricular cysts, and passage of enhanced fluid through the foramen of Monro. In 1 patient with a complex dysraphic state, which affected the frontoethmoidal region, presumptive ethmoidal encephalocele could be excluded (Fig 2, patient 8). In 1 patient with post-traumatic CSF fistula (patient 4), intrathecal gadopentetate dimeglumine could be traced through the dural defect, which was successfully repaired by using intraoperative findings observed on MR imaging. In another patient with the same presurgical diagnosis, intrathecal Gd-DTPA did not demonstrate CSF leak (patient 6), and eventually allergic rhinitis was diagnosed as a putative cause of situational rhinorrhea. Of interest, these 2 patients underwent intrathecal CT cisternography with water-soluble iodinated contrast medium, but the results of this test were inconclusive.
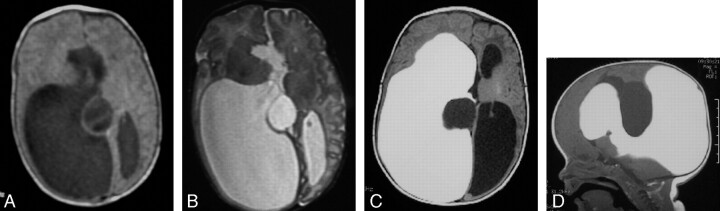
Fig 1.
Complex hydrocephalus (patient 3). Axial T1-weighted spin-echo (SE) (A) and T2-weighted fast spin-echo (FSE) (B) images showing unilateral ventriculomegaly and midline cyst at the neonate stage and signs of corpus callosum agenesis. Axial (C) and sagittal (D) T1-weighted SE images at 1 month of life, after Gd-DTPA injection via transfontanelle ventriculostomy, show isolated right ventricular enlargement with lack of communication either with the left ventricle or with the midline cyst. Uniportal endoscopic approach was elected for the treatment of both the interhemispheric cyst and the isolated right ventricle.
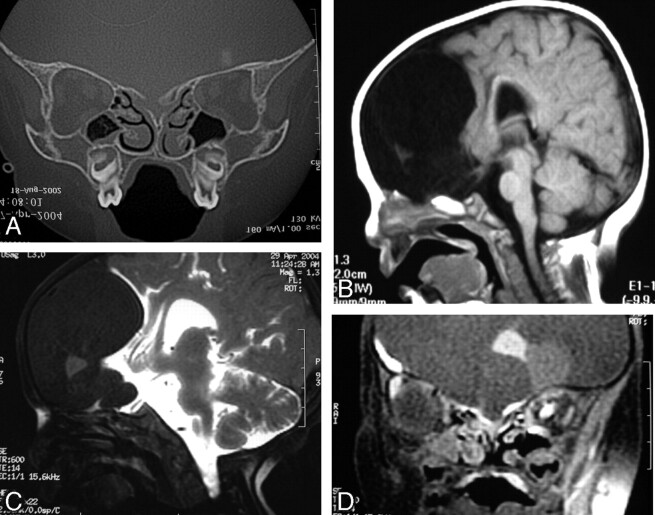
Fig 2.
Dysraphic state with hypertelorism, intracranial suspected dermoid tumor, and bifid nose (patient 8). Coronal CT scan through the cribriform plate (A) and sagittal SE T1-weighted MR image through the anterior cranial fossa (B) do not exclude associated encephalocele. Intrathecal Gd-DTPA-enhanced T1-weighted SE fat-saturated images in sagittal (C) and coronal (D) planes show integrity of the anterior cranial fossa and absence of meningoencephalic herniation. Surgery was delayed without the fear of unnoticed sac rupture.
In 1 patient with progressive exophthalmos in whom a CT scan showed an orbital mass within the roof and conventional MR imaging could not distinguish another potential contralateral orbital mass within the roof with certainty, intrathecal-enhanced Gd-DTPA MR imaging demonstrated an acquired meningocele, which ultimately was promoted by his previously unrecognized osteopetrosis, and excluded contralateral orbital roof meningocele (Fig 3, patient 7). In another patient, CSF leakage could be ascertained from cerebellopontine angle cisterns to the membranous labyrinth (Fig 4, patient 10). Finally, arachnoiditis could be excluded as causative of symptoms in a young patient who had previously undergone surgery for spinal cord ependymoma (patient 5) and developed progressive paraparesis and sphincter incontinence, because intrathecal gadolinium contrast could be followed, eliminating arachnoid adhesions. Previous conventional intravenous gadolinium-enhanced MR imaging of the spine had ruled out tumor recurrence but was unable to differentiate between arachnoiditis and postoperative status.
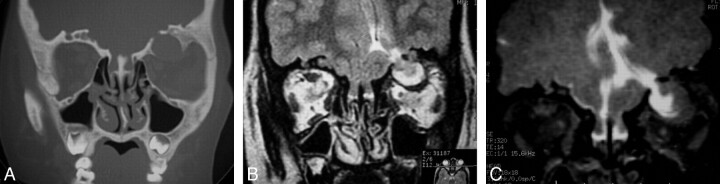
Fig 3.
Acquired orbital meningoencephalocele in a patient with osteopetrosis (patient 7). Coronal CT scan (A) through the orbits shows intraosseous spheric defect on the roof of the left orbit with intracranial canal connection. Notice additional intraosseous orbital defects in the roof of the right orbit. Coronal FSE T2-weighted image (B) shows an encephalocele contained within the roof of the left orbit and raises questions concerning the contents of the right osseous roof defects. Intrathecal Gd-DTPA-enhanced coronal T1-weighted SE fat-saturated image (C) shows free CSF communication between the brain and left orbital roof defect and an encephalocele contained within the cavity. No additional CSF leaks are seen within the bony defects on the right orbit roof, excluding a bilateral surgical subfrontal approach.
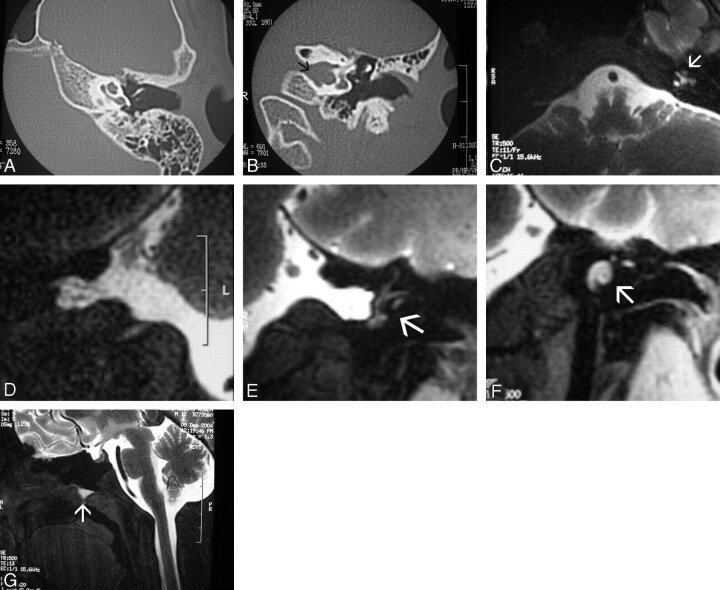
Fig 4.
Spontaneous otorrhea and dizziness (patient 10). A and B, Axial and coronal CT scans through the left middle ear and osseous labyrinth show absence of osseous cochlear dysplasia (A) and a bulbous appearance of the internal auditory canal (arrow in B), with no middle ear or mastoid cavity filling. C, Intrathecal Gd-DTPA-enhanced axial T1-weighted SE fat-saturated MR image through the upper medulla oblongata shows abnormal filling of the left cochlear structure (arrow). D–F, Coronal T1-weighted SE fat-saturated MR image through the cerebro-pontine angle cisterns. Spot view of the right side (D) shows CSF filling up to the lamina cribrosa. However, further membranous labyrinth structures (semicircular canals) are filled on the left side (arrow in E), as well as the cochlear duct (arrow in F). Additional CSF deposit is seen in the floor of the sphenoidal sinus (arrow in G) (the patient was positioned in a prone head-down position). This patient was eventually diagnosed with juvenile transient osteoporosis because of an upper limb pathologic fracture.
Discussion
Despite advances in imaging and the availability of several different and potentially useful diagnostic techniques (from conventional cranial radiography to polytomography, radionuclide cisternography, thin-section CT, intrathecal water-soluble iodinated contrast agent–enhanced CT cisternography, and MR imaging with T2-weighted sequences), accurate demonstration of the site of the CSF leakage, CSF dynamics in cyst-containing masses (such as arachnoid cysts) or along CSF pathways (hydrocephalus), diagnostic differentiation between intra- or extra-axial masses, or even complex arachnoid adhesions still remains a challenge for both radiologists and surgeons.
Intravenous administration of gadolinium as a contrast agent in MR imaging has been widely used worldwide. Its safety and tolerance have been well established in extensive clinical trials with low incidence of minor side effects.1–5,10,13,15
Under this premise, experimental models of CSF fistula were developed to evaluate gross clinical response to the intrathecal gadolinium, which was found a promising technique, apparently well tolerated in murine and primate models.6,16–18 On the basis of a study for neural tolerance in rats after cisternal injection and assuming that humans have the same lethal dose for 50% of the animals and dose producing 50% morbidity, safety doses were calculated.17,18 Toxicologic and pharmacokinetic studies were also designed and realized by Schering, with high margins of tolerance and excretion largely complete after 24 hours of administration. Although for reasons unrelated to the preclinical safety profile, the company decided not to pursue the development of Magnevist for subarachnoid administration.
Following these preliminary studies in animal models,6,7 the use of Gd-DTPA in the human clinical setting was first reported in the study of 2 patients with meningeal carcinomatosis.3 In one previous report, Siebner et al3 foresaw potential applications for assessing the outline and continuity of the subarachnoid space in patients with abnormal CSF collections and CSF leakage, and for assessing patterns of CSF flow and dynamics. In other previous reports, the effects of intraventricular gadolinium (gadopentetate dimeglumine, Magnevist) injection in a murine model produced behavioral and neurologic alterations, including focal seizure activity, ataxia, delayed tremor, and histologic changes (loss of oligodendroglia, hypertrophy of astrocytes, and formation of eosinophilic granules).11 These neuropathologic changes followed an abrupt dose-response effect, with no-effect level below doses of 1.25-μmol/g brain and a maximal effect at 10-μmol/g brain. Even at this maximal dose, the purely astrocytic lesion affecting the cerebellum did not occur elsewhere in the brain.11 Such behavioral and morphologic changes were not seen in the same reported studies when the total dose of intraventricular gadolinium was less than 15 μL (3.3/μmol/g brain).
Similarly, another recent study in an animal model that used low intrathecally administered doses of a low-osmolar different formulation of gadolinium (Omniscan, Nycomed Amersham) did not reveal such alterations.8 Further extensive animal experiments critically analyzed the effects of intrathecal gadolinium at the extrapolated dosage range used in the human studies. The parameters studied were within the limits of normal determinations and internal control studies.12 In addition, these subjects did not show chronic MR imaging alterations of the brain at 1 year after gadolinium-enhanced MR cisternography.14
Soon afterward, a pilot study in human patients was designed to evaluate the safety, characteristics, and clinical response to intrathecal gadolinium (gadopentetate dimeglumine),4 and again no significant gross neurologic abnormalities, CSF changes, electroencephalographic alterations, MR imaging morphologic evidence, or MR signal intensity change related to the intrathecal gadolinium was seen on initial examination or on follow-up clinical-radiologic studies. The estimated intrathecal gadolinium (0.5–2.0 mL as a single dose of total volume) dose per gram of brain in this clinical trial was much less than that used in the previously discussed animal model experiments that produced neurologic and histologic changes at higher doses (ie, 0.07–0.36 μmol/g brain in the human study versus 2.5–15 μmol/g brain in the animal study).
Finally, a cooperative multicenter human study was designed to evaluate the safety and clinical response to single-dose gadolinium intrathecal injection, and the study concluded that intrathecal gadopentetate dimeglumine administration was safe and feasible when a low dose (0.5–1.0 mL of GD; 0.5 mL = 0.18 μmol/g brain) was used.15 Later, the same authors faced a detailed study centered on cisternography to determine potential communication between the CSF pathways and intracranial arachnoid cysts.5 The study succeeded in showing communication or noncommunication of intracranial arachnoid cysts with the CSF pathways and thus bearing on the selection for surgery. It is suggested that symptomatic patients with noncommunicating cysts should undergo surgery, whereas patients with immediate communication of the arachnoid cysts should be treated by mere clinical observation. Those patients with delayed communication or delayed retention of gadolinium should be followed up more closely on the basis of clinical and radiologic sequential studies.
The underlying mechanism of CSF enhancement by gadolinium on T1-weighted images relies on shortening the relaxation times of the water when a diluted paramagnetic contrast agent interacts with neighboring water protons (proton-relaxation enhancement).16,19,20 At lower concentrations of gadolinium, the main effect is on T1, whereas at higher concentrations, the main effect is on T2 and paradoxical phenomenon may appear on T1. The lower dosage of diluted gadolinium used (0.2–2 mL) appears to be adequate for diagnostic subarachnoid enhancement on MR imaging in most clinical situations.1,4
Our report includes a variety of CNS pathologic conditions, mainly in the brain, in a specific population, though within a wide age range. Our standard protocol is similar to that used and recommended by other researchers,1,3,4,10,15 including contrast dose, periprocedural care, and injection rate and mode, aiming to minimize potential CNS irritation and risk of damage. For this reason, we have also used a low-osmolality gadolinium preparation.5 Technically, acquisition sequences included T1-weighted fat-saturated images, particularly when the skull base and nasal cavity were scanned. These sequences are very suitable for this purpose because they suppress the high signal intensity from the fat-containing medullary bone, thus avoiding misinterpretation and pitfalls between the high-signal-intensity CSF containing gadolinium and fat from bone, which might conceivably be confused with leaks. This feature is particularly useful when CSF leaks are studied in posttraumatic status but also in any leak and in the study of the spine.
In accordance with other studies, no side or toxic effects, including both clinical and biologic, were recorded in our patients in the present study, except mild transient postural headaches observed following the procedure, occurring in 2 patients and assumed to be caused by minor CSF leakage engendered by the lumbar punctures. This result further supports the safety shown in other reports at the dose used, though our population was small and there are no long-term follow-up studies. Therefore, our study also shows the clinical safety, feasibility, and reliability of low-dose intrathecal/intracisternal low-osmolality gadolinium-enhanced MR imaging as has been reported in previous animal and human studies.1,3,4,7,9,11,13,15
The relative or absolute advantages of gadolinium-enhanced intrathecal/intraventricular administration include an absence of ionizing radiation, the capability of direct multiplanar imaging, an absence of bony artifact, and high spatial and contrast resolution. Some reports and our experience also show the inaccuracy of noninvasive unenhanced T2 heavily weighted MR imaging, so-called MR imaging myelography/cisternography, in critical or postoperative states.21
As in previous studies15,21 for practical and ethical reasons, direct comparison of intrathecal gadolinium-enhanced MR myelography/cisternography with CT myelography/cisternography or radionuclide cisternography was not specifically addressed in our study. However, 2 of our patients suspected of having CSF fistula in a complex posttraumatic status had previously undergone intrathecal CT cisternography with water-soluble iodinated contrast medium. The results of this test were inconclusive; these results often occur in these particular patients because many small bony defects, hard-beam artifacts, and high-attenuation bone/iodine contrast in critical frontoethmoidal areas may preclude a definitive diagnosis. However, Gd-DTPA MR cisternography lacks these constraints when fat-saturated T1-weighted acquisitions are used, making it invaluable in these clinical settings, not only for the visualization of the contrast leak, but also for the exclusion of fistula, as happened in our experience.
Concerning the effectiveness of intrathecal gadolinium-enhanced MR myelography/cisternography in resolving the critical clinical and surgical questions posed by our selected patients, we found it of great practical impact because in 8 of 10 patients, this new additional study influenced or changed clinical decisions or surgical planning. This result is particularly remarkable when considering that 4 surgical candidates were excluded from any surgical procedure on the basis of the new information drawn from the gadolinium-enhanced MR images. This result is very important, considering that these types of procedures were performed in a subset of patients who presented with neurosurgical challenges that were not resolved after an extended array of biologic and radiologic procedures. Therefore, cisternography and ventriculography gadolinium-enhanced MR imaging could be the definitive radiologic procedure in patients in whom the rest of radiologic procedures were inconclusive, in particular patients with CSF flow alterations, communicating or noncommunicating cyst masses bordering CSF pathways, or craniospinal CSF leaks.
Shortcomings and caveats of this report include the limited number of patients, the lack of long-term clinical follow-up of patients, and the uncertainty of the maturing brain in response to intrathecal contrast agents. Also cisternography and ventriculography gadolinium-enhanced MR imaging combines the contraindications and risks of any CNS puncture and MR imaging technology, such as foreign bodies and pacemakers. Also, intrathecal administration of gadolinium-containing contrast media is not currently approved worldwide.
Conclusions
Our preliminary experience with low-osmolality paramagnetic gadolinium as an intrathecal contrast agent in the setting of enhanced MR myelography/cisternography showed no side effects, and potential useful clinical applications were demonstrated in the evaluation of CSF pathways. Cisternography and ventriculography Gd-DTPA–enhanced MR imaging seems a feasible and useful technique for the evaluation of obstructions and communications of the subarachnoid space, spontaneous or traumatic/postsurgical craniospinal CSF leaks, or postsurgical adhesions/arachnoiditis in the pediatric population. However, additional studies are needed to further evaluate the long-term safety of the administration of low doses of intrathecal gadolinium in the maturing CNS.
References
- 1.Aydin K, Guven, Sencer S, et al. MRI cisternography with gadolinium-containing contrast medium: its role, advantages and limitations in the investigation of rhinorrhoea. Neuroradiology 2004;46:75–80. Epub 2003 Nov 13 [DOI] [PubMed] [Google Scholar]
- 2.Wenzel R, Leppien A. Gadolinium-myelocisternography for cerebrospinal fluid rhinorrhoea. Neuroradiology 2000;42:874–80 [DOI] [PubMed] [Google Scholar]
- 3.Siebner HR, Gräfin von Einsiedel H, Conrad B, et al. Magnetic resonance ventriculography with gadolinium DTPA: report of two cases. Neuroradiology 1997;39:418–22 [DOI] [PubMed] [Google Scholar]
- 4.Zeng QY, Xiong L, Jinkins JR, et al. Intrathecal gadolinium-enhanced MR myelography and cisternography: a pilot study in human patients. AJR Am J Roentgenol 1999;173:1109–15 [DOI] [PubMed] [Google Scholar]
- 5.Turgut TE, Ercan N, Kaymaz M, et al. Intrathecal gadolinium (gadopentetate dimeglumine)-enhanced MR cisternography used to determine potential communication between the cerebrospinal fluid pathways and intracranial arachnoid cysts. Neuroradiology 2004;46:744–54 [DOI] [PubMed] [Google Scholar]
- 6.Di Chiro G, Knop RH, Girton ME, et al. MR cisternography and myelography with gadolinium DTPA in monkeys. Radiology 1985;157:373–77 [DOI] [PubMed] [Google Scholar]
- 7.Di Chiro G, Girton ME, Frank JA, et al. Cerebrospinal fluid rhinorrhea: depiction with MR cisternography in dogs. Radiology 1986;160:221–22 [DOI] [PubMed] [Google Scholar]
- 8.Skalpe IO, Tang GJ. Magnetic resonance imaging contrast media in the subarachnoid space: a comparison between gadodiamide injection and gadopentetate dimeglumine in an experimental study in pigs. Invest Radiol 1997;32:140–48 [DOI] [PubMed] [Google Scholar]
- 9.Ibarra R, Jinkins JR, Korvick D, et al. Evaluation of intrathecal gadolinium-enhanced MR cisternography in a rabbit model of traumatic nasoethmoidal CSF fistula. J Magn Reson Imaging 2000;11:20–24 [DOI] [PubMed] [Google Scholar]
- 10.Morris TW, Ekholm SE, Prentice LJ. The effects of gadolinium-DTPA and –DOTA on neural tissue metabolism. Invest Radiol 1991;26:1087–90 [DOI] [PubMed] [Google Scholar]
- 11.Ray DE, Holton JL, Nolan CC, et al. Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats. AJNR Am J Neuroradiol 1998;19:1455–62 [PMC free article] [PubMed] [Google Scholar]
- 12.Toney GM, Chavez HA, Ibarra R, et al. Acute and subacute physiologic and histologic studies of the central nervous system after intrathecal gadolinium injection in the anesthetized rat. Invest Radiol 2001;36:33–40 [DOI] [PubMed] [Google Scholar]
- 13.Jinkins JR, Rudwan M, Krumina G, et al. Intrathecal gadolinium-enhanced MR cisternography in the evaluation of clinically suspected cerebrospinal fluid rhinorrhea in humans: early experience. Radiology 2002;222:555–59 [DOI] [PubMed] [Google Scholar]
- 14.Zeng QY, Xiong L, Jinkins JR, et al. Intrathecal gadolinium-enhanced MR myelography: a pilot study in human patients (abstr). Presented at: Radiological Society of North America Scientific Program 1997; November 30–December 5, 1997; Chicago;1997;205(P):468 [Google Scholar]
- 15.Turgut TE, Ercan N, Krumina G, et al. Intrathecal gadolinium (gadopentetate dimeglumine) enhanced magnetic resonance myelography and cisternography: results of a multicenter study. Invest Radiol 2002;37:152–59 [DOI] [PubMed] [Google Scholar]
- 16.Brash RC. Methods of contrast enhancement for NMR imaging and potential applications. Radiology 1983;147:781–88 [DOI] [PubMed] [Google Scholar]
- 17.Rosen GM, Griffeth LK, Brown MA, et al. Intrathecal administration of nitroxides as potential contrast agents for MR imaging. Radiology 1987;163:239–43 [DOI] [PubMed] [Google Scholar]
- 18.Weinmann HJ, Brasch RC, Press WR, et al. Characteristics of gadolinium DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol 1984;142:619–24 [DOI] [PubMed] [Google Scholar]
- 19.Hinshaw DB, Wong VC. Paramagnetic contrast agents in the evaluation of brain neoplasms. Top Magn Reson Imaging 1989;1:79–93 [PubMed] [Google Scholar]
- 20.Saini S. Advances in contrast-enhanced MR imaging: principles. AJR Am J Roentgenol 1991;156:236–39 [DOI] [PubMed] [Google Scholar]
- 21.Hegarty SE, Millar JS. MRI in the localization of CSF fistulae: is it of any value? Clin Radiol 1997;52:768–70 [DOI] [PubMed] [Google Scholar]