Abstract
BACKGROUND AND PURPOSE: 3D time-of-flight (TOF) MR angiography (MRA) is insensitive to slowflow; however, the use of MR imaging contrast agents helps to visualize slow-flow vessels and avoids overestimation of vascular occlusion. The purpose of this study was to correlate pre- and postcontrast 3D TOF MRA with the results of conventional angiography during endovascular reperfusion therapy and to determine the accuracy of postcontrast 3D TOF MRA.
MATERIALS AND METHODS: Thirteen patients who underwent endovascular reperfusion therapy for acute ischemic stroke were retrospectively analyzed. MR imaging techniques included single-slab 3D TOF MRA with and without contrast, as well as perfusion-weighted imaging. Angiography during reperfusion therapy was used as a standard of reference. Affected arteries were divided into segments either proximal or distal to the lesion, and pre- and postcontrast MRA signals were graded as absent, diminished or narrowed, or normal.
RESULTS: In 2 of 5 patients with arterial stenosis and 6 of 8 patients with complete occlusion, MRA signal intensity proximal to each lesion was absent, indicating a proximal pseudo-occlusion on precontrast MRA. Postcontrast MRA demonstrated an arterial signal intensity proximal to the stenotic or occlusive lesions in all 13 patients. Arterial signal intensity distal to the occlusion was identified on postcontrast MRA in 7 of 8 patients having complete occlusion, and the extent of occlusion on postcontrast MRA was similar to results of conventional angiography.
CONCLUSION: In this small series, postcontrast 3D TOF MRA more accurately delineated the extent of stenotic or occlusive arterial lesions than precontrast MRA.
3D time-of-flight (TOF) MR angiography (MRA), the most common MRA technique in an acute stroke setting, is useful for evaluating intracranial steno-occlusive disease.1 However, TOF is insensitive to slow flow or in-plane flow because of saturation of the MR imaging signal intensity,2 which can lead to overestimating the severity of the stenosis or to a false diagnosis of vascular occlusion.3 The use of MR imaging contrast agents shortens the T1 relaxation time of blood, decreases the saturation effects, and increases the steady-state signal intensity of blood.4 Although previous reports mention that postcontrast 3D TOF MRA demonstrates slow-flow vessels, thus avoiding overestimation of vascular obstruction,5,6 accuracy of postcontrast 3D TOF MRA has not been assessed on the basis of the results of conventional angiography.
The purpose of this study was to correlate pre- and postcontrast 3D TOF MRA with the results of conventional angiography during endovascular reperfusion therapy and to determine the accuracy of postcontrast 3D TOF MRA.
Patients and Techniques
MR Imaging
All patients had an MR imaging study on admission within the first 6 hours after stroke onset. MR imaging studies were performed with a 1.5T MR imager (Gyroscan Intera, Philips Medical Systems, Best, the Netherlands) by using a standard head coil. Our routine imaging protocol for acute stroke includes diffusion-weighted imaging (DWI), 3D TOF MRA, and fluid-attenuation inversion recovery sequences. DWI was performed with a single-shot spin-echo echo-planar imaging technique with a diffusion sensitivity of b = 1000 s/mm2; 3649/91, TR/TE; 20 sections with 6-mm thickness and 1-mm gap; 23-cm FOV; and a 160 × 79 acquisition matrix reconstructed to a 256 × 256 matrix. The imaging parameters for the conventional single-slab 3D TOF sequence were 25/2.3/13–30°, TR/TE/flip angle; and 160 × 128 × 50 mm acquired volume with a 256 × 192 × 50 matrix, which was then reconstructed to a 512 × 512 × 100 matrix. The acquisition time was 6 minutes 31 seconds.
The 3D TOF MRA slab was obliquely prescribed to include the intradural vertebral artery up to the ambient segment of the posterior cerebral artery and the cavernous internal carotid artery (ICA) up to the proximal portion of the insular segment (M2) of the middle cerebral artery (MCA) in 1 slab. When an infarction of the MCA territory was found on DWI, the 3D TOF MRA slab was selected to cover the cavernous ICA up to the proximal portion of the MCA cortical segment (M4). If less than one third of the MCA territory showed an abnormal signal intensity on DWI, or lacunar infarcts were unlikely on the basis of the DWI and MRA, perfusion-weighted imaging (PWI) was performed.
PWI used a single-shot gradient-echo echo-planar sequence with 1500/29/90°, TR/TE/flip angle; 19 sections with 5-mm thickness and 1-mm gap; 23 × 16 cm FOV; and a 128 × 89 acquisition matrix reconstructed to a 128 × 128 matrix. We collected 950 images over 70 seconds during intravenous administration of a 0.2-mL/kg bolus of gadodiamide hydrate (Omniscan, Daiichi Pharmaceutical, Tokyo, Japan) injected at the rate of 4 mL/s. The hypoperfused area was determined on the basis of the mean transit time map generated from PWI postprocessing.
Postcontrast MRA using the same location and coverage as precontrast MRA was performed immediately after PWI. Data were transferred to an independent workstation, and subvolumes were generated. Pre- and postcontrast MR angiograms were displayed by using a maximum intensity projection (MIP) algorithm. Postcontrast MRA required additional subvolumes to erase the superficial middle cerebral vein, other prominent cortical veins, dural sinuses, and nasal mucosa. Because the cavernous ICA was surrounded by enhanced cavernous sinus and was obscured on MIP images, sagittal multiplanar reconstruction images were also used for the evaluation.
If there was a PWI/DWI mismatch, percutaneous transluminal cerebral balloon angioplasty (PTCBA) or intra-arterial thrombolysis was indicated. The patients and their relatives were informed about the hazards of reperfusion therapy as well as its potential benefit and, thus, the risk of not being treated. From April 2004 to November 2005, a total of 13 patients agreed to have interventional reperfusion therapy and were included in this study. The 13 patients comprised 5 women and 8 men ranging from 51 to 88 years of age (mean, 67 years of age).
Reperfusion Therapy
All 13 patients underwent reperfusion therapy under local anesthesia via a femoral artery. Heparin (3000 U) was injected intravenously to avoid thrombus formation during the procedure. A guidewire was navigated across the occlusion or stenosis and advanced into the distal segment. In 9 patients who underwent PTCBA, a balloon catheter (Gateway, Boston Scientific/Target Therapeutics, Fremont, Calif) was guided over the wire and then inflated to the nominal pressure for 30 seconds. In the remaining 4 patients, a microcatheter (Renegade, Boston Scientific/Target Therapeutics) was inserted into and distal to the thrombus and 72,000–240,000 U of urokinase was slowly injected by hand. Selective angiography during PTCBA or thrombolysis was also performed to clarify the extent of the lesion and as a standard of reference for image analysis.
Image Review
Two senior neuroradiologists (H.I. and M.M.), who were blinded to the results of MRA, analyzed and interpreted the conventional angiography results on a PACS station through consensus. The vessel patency on conventional angiography was graded according to the Thrombolysis in Myocardial Infarction (TIMI) criteria7: TIMI grade 0 flow indicates complete occlusion; TIMI grade 1 flow denotes some penetration of the obstruction by contrast material, but no perfusion of the distal vascular bed; TIMI grade 2 flow denotes perfusion of the entire distal vascular bed, but with delayed flow compared with that of a normal artery; and TIMI grade 3 flow denotes full perfusion with normal flow. The extent of complete obstruction was determined by superselective angiography beyond the lesion or angiography after PTCBA.
The results of pre- and postcontrast MR angiography were also evaluated by consensus by 2 senior neuroradiologists (H.I. and M.M.), who knew the results of conventional angiography. Arterial sections were divided into 2 segments on the basis of the results of the conventional angiography, either proximal or distal to the lesion. Arterial signal intensity on MRA was graded as 0 for absent, 1 for diminished or narrowed, or 2 for normal.
Results
Arterial signal intensity on pre- and postcontrast MRA is listed in the Table.
Correlation between catheter and MR angiographic findings
| Catheter Angiography |
TOF MR Angiography |
||||||
|---|---|---|---|---|---|---|---|
| Proximal Side |
Distal Side |
||||||
| Patient | Age/ Sex | TIMI Grading | Pre | Post | Pre | Post | |
| 1 | 61/M | VA stenosis* | 2 | 2 | 2 | 2 | 2 |
| 2 | 71/M | VA stenosis* | 1 | 2 | 2 | 2 | 2 |
| 3 | 88/F | M1 stenosis | 1 | 2 | 2 | 2 | 2 |
| 4 | 51/M | C2 stenosis | 1 | 0 | 2 | 0 | 2 |
| 5 | 85/M | BA embolism | 1 | 0 | 2 | 2† | 2 |
| 6 | 49/M | M1 occlusion | 0 | 2 | 2 | 0 | 1 |
| 7 | 68/F | M2 occlusion | 0 | 2 | 2 | 0 | 2 |
| 8 | 59/F | VA occlusion* | 0 | 0 | 2 | 0 | 2 |
| 9 | 72/M | C4 occlusion | 0 | 0 | 2 | 0 | 2 |
| 10 | 73/M | M2 occlusion | 0 | 0 | 2 | 0 | 2 |
| 11 | 77/M | M1 occlusion | 0 | 0 | 2 | 0 | 2 |
| 12 | 63/F | M2 occlusion | 0 | 0 | 2 | 0 | 2 |
| 13 | 61/F | M2 occlusion | 0 | 0 | 2 | – | – |
Note:—VA indicates vertebral artery; Pre, precontrast; Post, postcontrast; BA, basilar artery; M1, horizontal segment of the MCA; M2, insular segment of the MCA; C2, C2 segment of the ICA; C4, C4 segment of the ICA;
, with contralateral VA occlusion;
, cross-flow via posterior communicating artery; –, vessel distal to the occlusion was not included in the scanning range.
Stenosis (TIMI Grades 1 and 2)
Angiographically, 1 patient was graded as TIMI 2, and 4 patients were graded as TIMI 1. In 2 of these 5 patients, arterial signal intensity faded on the proximal side of each lesion, showing proximal pseudo-occlusion on precontrast MRA (Fig 1). Arterial signal intensity distal to the lesions was not identified in these 2 patients. In the remaining 3 patients, arterial signal intensity was identified both proximal and distal to the lesions. Postcontrast MRA delineated an arterial signal intensity proximal and distal to each lesion, which enabled us to precisely evaluate the extent of stenosis in all 6 patients.
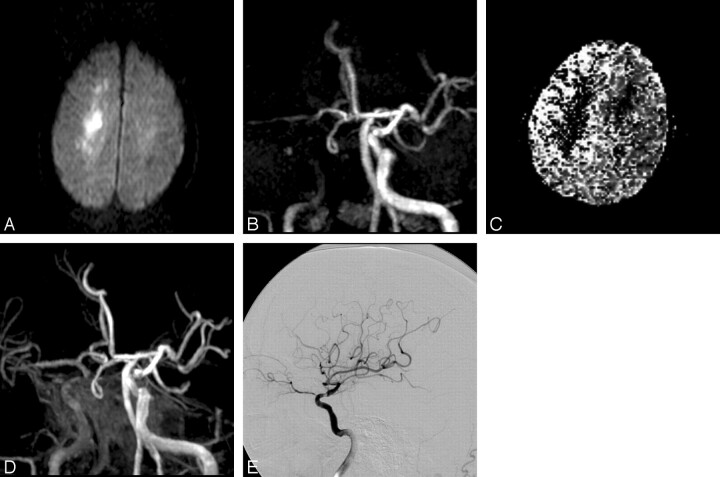
Fig 1.
A 51-year-old man with progressive left-sided weakness, dysarthria, and impaired consciousness (patient 4). A, Axial DWI confirms infarction in the centrum semiovale. B, On 3D TOF MR angiogram (anteroposterior [AP] view), occlusion of the C4 segment of the right ICA is suspected. Antegrade filling of the MCA via an anterior communicating artery is identified. C, Relative mean transit time map shows a region of delayed flow in the right hemisphere. D, Depiction of the cavernous portion of the ICA is improved on postcontrast 3D TOF MR angiogram (AP view), which shows severe stenosis at the C2 portion of the ICA. Some contrast enhancement from the cavernous sinus is evident. E, Right internal carotid angiogram immediately after MR imaging examination shows severe stenosis at the C2 portion and delayed flow in the MCA territory, but no antegrade filling into the anterior cerebral artery territory (TIMI grade 1).
Occlusion (TIMI Grade 0)
Angiographically, 8 patients were graded as TIMI 0. In 2 of these 8 patients, arterial signal intensity was identified proximal to each lesion (Fig 2); however, in the remaining 6 patients, arterial signal intensity faded on the proximal side of the lesions, causing suspicion of more proximal occlusions on precontrast MRA. Arterial signal intensity distal to the lesion was not identified on precontrast MRA in any of these 8 patients. Postcontrast MRA delineated arterial signal intensity just proximal to the lesions in all 8 patients. Arterial signal intensity distal to lesions was identified (normal or narrowed) on postcontrast MRA in 7 of 8 patients (Fig 2). In these patients, the extent of occlusion on postcontrast MRA was similar to that found on superselective angiography during PTCBA or thrombolysis. In the remaining 1 patient, the vessel distal to the occlusion was not included in the scanning range of MRA.
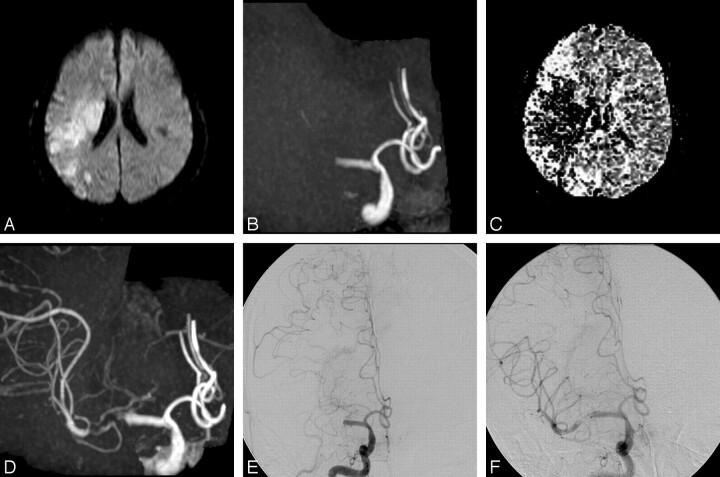
Fig 2.
A 49-year-old man with sudden-onset left-sided paralysis, aphasia, and impaired consciousness (patient 6). A, Axial DWI obtained at the time of MRA confirms infarction of right MCA territory. B, 3D TOF MR angiogram (AP view) shows occlusion of the M1 segment of the right MCA. C, Relative mean transit time map shows a region of delayed flow in the right hemisphere. D, Postcontrast 3D TOF MR angiogram (AP view) shows improved depiction of the distal M1 portion and M2 branches. Some contrast enhancement from the cavernous sinus is evident. E, Right internal carotid angiogram immediately after MR imaging examination shows total occlusion (TIMI grade 0) of the M1 segment of the right MCA. Retrograde opacification of the MCA branches via pial collateral vessels extending from the anterior cerebral artery is noted; however, the vessel just distal to the occlusion is not delineated. F, Right internal carotid angiogram after the first trial of angioplasty by using the 9-mm-long balloon catheter at the occluded portion shows antegrade filling into the distal M1 portion and M2 branches through the residual stenosis. Note that postcontrast MR angiogram (D) precisely demonstrates the extent of the occlusion that is shown on catheter angiography during PTCBA procedures (F).
Discussion
The purpose of MR imaging in an acute stroke setting is to demonstrate the extent of irreversible ischemic brain damage, tissue at risk, and the causative arteriopathy of ischemic stroke and its extent. Although an excellent correlation between the findings of MRA and those of conventional angiography has been reported for detecting intracranial vascular alterations,8 unenhanced MRA has some limitations in diagnostic accuracy because of its relative insensitivity to slow flow.2 This series included only 13 patients; however, it is evident that precontrast TOF MRA did not always show the correct lesion location and character. Angiography demonstrated a delayed washout of contrast proximal to lesions, suggesting slow-flow, which is thought to be related to signal-intensity loss on TOF MRA. The use of MR imaging contrast agents shortens the T1 relaxation time of blood, decreases the saturation effects, increases the steady-state intensity of blood, and improves the delineation of slow flow.4
This study suggests that postcontrast TOF MRA correctly identifies stenosis or occlusion and its location. If we aim to correlate arterial occlusion sites to long-term infarct size or to the effect of reperfusion therapy, we should not determine the occlusion site on the basis of precontrast TOF MRA alone. We should refer to conventional angiography or postcontrast TOF MRA to locate accurately the arterial occlusion sites. CT angiography may also be accurate for determining a vascular occlusion site.9 A study by Tomanek et al,10 however, did not substantiate our results: Precontrast TOF MRA showed higher sensitivity and specificity than postcontrast TOF MRA. This may be a reflection of their method of MRA review. They reviewed film copies of axial collapsed MIP images and source images for both nonenhanced and enhanced 3D TOF and nonenhanced MIP images. Enhanced MIP images were not prepared for review.
Arterial signal intensity distal to lesions was identified on postcontrast MRA in all except 1 patient in whom the vessel distal to the lesion was out of the scanning range. In cases of complete occlusion, delineation of the distal portion of the occluded vessel segment depends on leptomeningeal retrograde filling11 or antegrade filling via anterior and/or posterior communicating arteries (ie, if there is distal carotid occlusion, the M1 segment may be reconstituted by cross-filling via anterior and/or posterior communicating arteries). For interventionists trying to recanalize a completely occluded vessel and especially when advancing the guidewire beyond the lesion, knowing the configuration of the distal patent vessel and the extent of the occlusion in advance is useful. Unfortunately, opacification by conventional angiography of a vessel distal to an occlusion varies depending on collateral flow and may not be a reliable method for evaluating a distal segment.12 Therefore, because postcontrast MRA gives important information about the distal portion of an occluded vessel segment, it will be a more promising tool for an interventionist.
Vascular enhancement in acute cerebral ischemia is due to slow-flow, indicating the presence of collateral circulation.13,14 Because our study population was limited to patients in whom we performed endovascular reperfusion therapy, we were only able to analyze results from patients with good collateral circulation. According to a previous report, patients presenting with MCA occlusion and no vascular enhancement exhibited impaired diffusion in the entire MCA territory and had a poor prognosis.11 In patients with poor collateral circulation, therefore, the distal portion of an occluded vessel segment might not be delineated on postcontrast MRA.
We did not use multiple overlapping thin-slab acquisitions because slab boundary artifact was inevitable with our equipment. Multiple overlapping thin-slab acquisition minimizes flow saturation and will improve delineation of slow flow,15 but very slow flow will lose its signal intensity on TOF MRA. We expect that adding MR imaging contrast to multiple overlapping thin-slab acquisitions further improves delineation of slow flow.
In conclusion, postcontrast MRA delineates vascular stenosis or occlusion associated with acute ischemic stroke more accurately than precontrast MRA. Postcontrast MRA also provides information about the vessels distal to a lesion, which is useful for planning interventional therapy.
References
- 1.Korogi Y, Takahashi M, Mabuchi N, et al. Intracranial vascular stenosis and occlusion: diagnostic accuracy of three-dimensional, Fourier transform, time-of-flight MR angiography. Radiology 1994;193:187–93 [DOI] [PubMed] [Google Scholar]
- 2.Axel L. Blood flow effects in magnetic resonance imaging. Magn Reson Annu 1986;237–44 [PubMed]
- 3.Stock KW, Wetzel S, Kirsch, et al. Anatomic evaluation of the circle of Willis: MR angiography versus intraarterial digital subtraction angiography. AJNR Am J Neuroradiol 1996;17:1495–99 [PMC free article] [PubMed] [Google Scholar]
- 4.Yano T, Kodama T, Suzuki Y, et al. Gadolinium-enhanced 3D time-of-flight MR angiography: experimental and clinical evaluation. Acta Radiol 1997;38:47–54 [DOI] [PubMed] [Google Scholar]
- 5.Pedraza S, Silva Y, Mendez J, et al. Comparison of preperfusion and postperfusion magnetic resonance angiography in acute stroke. Stroke 2004;35:2105–10 [DOI] [PubMed] [Google Scholar]
- 6.Yang JJ, Hill MD, Morrish WF, et al. Comparison of pre- and postcontrast 3D time-of-flight MR angiography for the evaluation of distal intracranial branch occlusions in acute ischemic stroke. AJNR Am J Neuroradiol 2002;23:557–67 [PMC free article] [PubMed] [Google Scholar]
- 7.The Thrombolysis in Myocardial Infarction (TIMI) trial: Phase I findings—TIMI Study Group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 8.Heiserman JE, Drayer BP, Keller PJ, et al. Intracranial vascular stenosis and occlusion: evaluation with three-dimensional time-of-flight MR angiography. Radiology 1992;185:667–73 [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita T, Ogawa T, Kado H, et al. CT angiography in the evaluation of intracranial occlusive disease with collateral circulation: comparison with MR angiography. Clin Imaging 2005;29:303–06 [DOI] [PubMed] [Google Scholar]
- 10.Tomanek AI, Coutts SB, Demchuk AM, et al. MR angiography compared to conventional selective angiography in acute stroke. Can J Neurol Sci 2006;33:58–62 [DOI] [PubMed] [Google Scholar]
- 11.Pantano P, Toni D, Caramia F, et al. Relationship between vascular enhancement, cerebral hemodynamics, and MR angiography in cases of acute stroke. AJNR Am J Neuroradiol 2001;22:255–60 [PMC free article] [PubMed] [Google Scholar]
- 12.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005;26:1789–97 [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller DP, Yuh WT, Fisher DJ, et al. Arterial enhancement in acute cerebral ischemia: clinical and angiographic correlation. AJNR Am J Neuroradiol 1993;14:661–68 [PMC free article] [PubMed] [Google Scholar]
- 14.Essig M, von Kummer R, Egelhof T, et al. Vascular MR contrast enhancement in cerebrovascular disease. AJNR Am J Neuroradiol 1996;17:887–94 [PMC free article] [PubMed] [Google Scholar]
- 15.Davis WL, Blatter DD, Harnsberger HR, et al. Intracranial MR angiography: comparison of single-volume three-dimensional time-of-flight and multiple overlapping thin slab acquisition techniques. AJR Am J Roentgenol 1994;163:915–20 [DOI] [PubMed] [Google Scholar]