Abstract
BACKGROUND AND PURPOSE: Time-resolved (TR) MR angiography (MRA) using parallel imaging techniques is proving to have clinical utility for improving MRA spatial and temporal resolution and separating arterial from venous anatomy. The purpose of this study was to evaluate TR MRA of the intracranial vessels at different integrated parallel acquisition technique (IPAT) factors.
MATERIALS AND METHODS: 3D TR MRA using time-resolved echo-shared angiographic technique was performed with different IPAT factors (0, 2, 3) at 1.5T, resulting in temporal resolutions of 4.0, 1.7, and 1.3 seconds, respectively. We studied 14 subjects, comprising 12 patients with various pathologic conditions and 2 healthy subjects. The brain volume was covered by 36 partitions, and a bolus of 5 mL of gadopentate dimeglumine was administered. Signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), the number of frames that distinguished between arterial and venous phases, the conspicuity of the vasculature, and artifacts were analyzed.
RESULTS: There was no significant difference in SNR between IPAT factors 0 and 2. Moreover, SNR was significantly lower with IPAT 3 than with IPAT 0 or 2. Smaller vessel segments (M3 and P3) were rated significantly inferior with TR MRA IPAT 2 or 3 compared with MRA without IPAT. For larger proximal vessels (A1 and A2 segments of anterior cerebral artery, M1 and M2 segments of middle cerebral artery, P2 segment of posterior cerebral artery, and basilar artery), there was no difference between TR MRA IPAT 0 and 2.
CONCLUSION: To obtain arterial and venous information in a clinical setting, intracranial TR MRA is best performed with an IPAT factor of 2 with at least 5 mL of contrast.
Time-resolved MR angiography (TR MRA) has particular advantages for MR imaging of intracranial vascular malformations because it provides hemodynamic information about the arterial and venous anatomy.1,2 The new MR acceleration techniques parallel imaging and time-resolved echo-shared angiographic technique (TREAT) have recently become available, resulting in a ≤4-fold increase in imaging speed.3,4 The use of parallel imaging techniques has allowed an additional reduction of acquisition time and, hence, an improvement in temporal resolution while maintaining the spatial resolution. This reduction is inversely proportional to the increase in the acceleration factor. However, this increase also results in a decreased signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR).5
The choice of acceleration factor, which is essential to optimize the sequence, and the benefit of acceleration for diagnosis have not previously been addressed in a clinical setting. We report the results of a study to compare intracranial high-resolution TR MRA, using integrated parallel acquisition technique (IPAT) at 1.5T with 2 different acceleration factors.
Materials and Methods
Approval for this study was obtained from the Institutional Board of Research Associates. We studied 14 subjects (7 women and 7 men) with a mean age of 51 years (range, 20 to 85 years), comprising 12 patients with various pathologic conditions (primary brain tumors [n = 6], demyelinating disease [n = 3], postoperative [n = 3]) and 2 healthy subjects. 3D TR MRA using TREAT with 2 different IPAT acceleration factors (2 and 3) (TR, 2.3 ms; TE, 0.8 ms; flip angle, 25°; bandwidth, [BW], 900 Hz/pixel) and without an acceleration factor (TR, 3.3 ms; TE, 1.1 ms; flip angle, 25°; BW, 400 Hz/pixel) were performed at 1.5T (Avanto; Siemens Medical Systems, Erlangen, Germany) with 8-channel phased array head coils. Zero-fill interpolation was performed. These were patients receiving contrast, and the 3D TR MRA was acquired during the contrast injection. Parameters were optimized for each of the 3 sequences. The most optimal flip angle, TR, TE, and BW for each 3D TR MRA acquisition was obtained in an iterative manner. The brain volume was covered by 36 partitions with a thickness of 3 mm in the axial plane with IPAT factors of 0, 2, and 3, resulting in temporal resolutions of 4.0, 1.7, and 1.3 seconds, respectively. One frame of 36 dynamic images every acquisition time was repeated 20 times. The pulse sequence parameters were optimized to obtain a fair comparison (Table 1). A bolus of 5 mL of gadopentate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ) was administered for each TR MRA at 3 mL/s followed by a 30-mL saline flush.
Table 1:
Parameters for time-resolved MR angiography at different IPAT factors
| Parameter | IPAT 0 | IPAT 2 | IPAT 3 |
|---|---|---|---|
| TR (msec) | 3.3 | 2.3 | 2.3 |
| TE (msec) | 1.1 | 0.8 | 0.8 |
| BW (msec) | 400 | 900 | 900 |
| TA (seconds) | 4.0 | 1.7 | 1.3 |
| Total TA (seconds) | 84 | 38 | 31 |
| Section thickness (mm) | 3 | 3 | 3 |
| Flip angle (°) | 20–25 | 20–25 | 25 |
| FOV | 167 × 250 | 167 × 250 | 167 × 250 |
| Matrix (pixels) | 128 × 192 | 128 × 192 | 128 × 192 |
| Voxel size | 1.3 × 1.3 × 3 | 1.3 × 1.3 × 3 | 1.3 × 1.3 × 3 |
Note:—BW indicates bandwidth; IPAT, integrated parallel acquisition technique; TA, acquisition time, FOV, field of view.
Signal intensity of the proximal M1 segments of the middle cerebral artery (MCA) was measured on the source images by a neuroradiologist (J.-Y.G.) who defined regions of interest (ROIs) within these segments. The size of the ROIs was adapted to the size of the vessel lumen and for any given subject remained unchanged for all image sets. Signal intensity (SI) of background was measured in the posterior temporal regions, and noise was measured by taking the standard deviation (SD) of air outside the patient. The relative SNR and CNR for each M1 segment of the middle cerebral artery was calculated for the 3 pulse sequences as follows: SNR∼SI vessel/SD noise; CNR∼(SI vessel − SI background)/SD noise.
All MR image sets were consensually evaluated by 2 neuroradiologists (J.-Y.G., M.L.). They evaluated in separate sessions individually and then reviewed the images again together in a second session to come to a consensus. Segments A1 and A2 of the anterior cerebral artery, M1, M2, and M3 of the MCA, the basilar artery (BA), and P2 and P3 of the posterior cerebral artery were rated for confidence of visualization on the following 3-point scale: 1, not visible; 2, partially visible; 3, clearly visible. Furthermore, to determine whether arterial-to-venous separation was achievable, one score was evaluated corresponding to the number of arterial phases without venous phases. Wrap artifact was noted.
Mixed model analysis of variance was used to compare IPAT factors with respect to SNR and CNR while accounting for the correlation among observations obtained from the same subject. SNR and CNR were used as dependent variables in separate univariate analyses. In each case, IPAT factor was treated as a fixed classification factor, and the covariance structure was modeled by assuming observations to be correlated only when derived for the same patient and by allowing the error variance to differ across IPAT factor levels. The IPAT factor levels were pairwise compared with respect to each of the ordinal end points (Score, A1, A2, M1, M2, M3, AB, P2, P3) using Wilcoxon matched-pairs signed-ranks tests. All reported P values are 2-sided and were declared statistically significant at the 5% level. The statistical computations were carried out using SAS for Windows version 9.0 (SAS Institute, Cary, NC).
Results
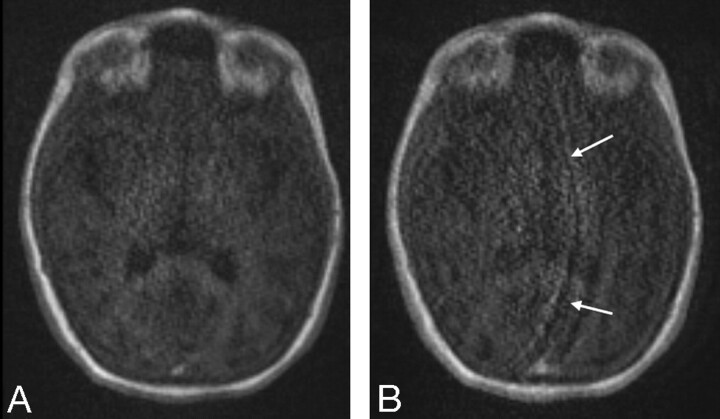
The protocol was fully implemented in the 14 subjects, all of whom tolerated the MR examination. With TR MRA with IPAT 3, 2 cases of wrap artifact were noted (Fig 1).
Fig 1.
Wrap artifacts with IPAT 2 and 3. Note the wrap artifacts (arrows) due to the use of the higher IPAT 3 (B) reconstruction algorithm compared with the moderate IPAT 2 factor (A).
Analysis of the vessel SNR and CNR is summarized in Table 2. The results indicate an increase in both vessel SNR and CNR with TR MRA without IPAT compared with TR MRA with IPAT 2 and 3. The lowest SNR and CNR were found on the TR MRA with IPAT 3. The difference was significant only between TR MRA with IPAT 0 and IPAT 3.
Table 2:
The mean ± standard deviation of signal- (SNR) and contrast-to-noise ratios (CNR) at different IPAT factors
| IPAT 0 | IPAT 2 | IPAT 3 | |
|---|---|---|---|
| SNR | 108.6 ± 63.9 | 86.8 ± 49.8 | 77.1 ± 47.5 |
| CNR | 103.5 ± 61.8 | 80.8 ± 37.3 | 68.5 ± 35.8 |
Note:—IPAT indicates integrated parallel acquisition technique.
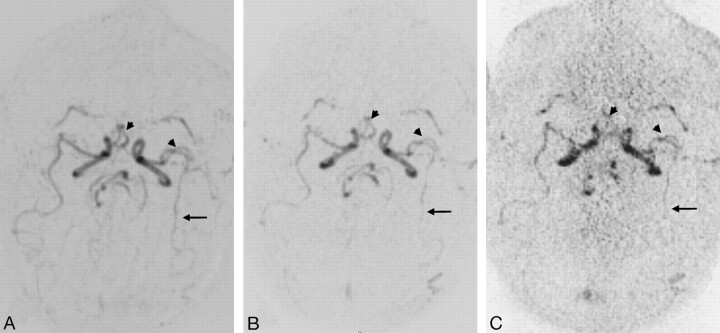
The rating for the conspicuity of larger proximal vessels, such as A1, A2, M1, M2, BA, and P2, was not significantly different between TR MRA IPAT 0 and 2 (Fig 2, Table 3). For IPAT 3, all segments were significantly less well visualized compared with IPAT 0, and all segments except M3 and P3 were significantly less well visualized compared with IPAT 2. Visualization of distal and/or smaller vessel segments such as M3 and P3 was significantly superior with TR MRA without IPAT compared with TR MRA with IPAT 2 and 3.
Fig 2.
Axial TR MRA maximum intensity projection reconstructions without IPAT (A) and with IPAT 2 (B) and 3 (C). Note the better visualization of distal MCA (arrows) without IPAT and the similar conspicuity of proximal arterial branches (head arrows) without IPAT (A) compared with IPAT 2 (B). In contrast to the MRA without and with IPAT 2, the noise was slightly increased at IPAT 3 (C).
Table 3:
The mean ± standard deviation of each ordinal assessment at different IPAT factors
| IPAT | Arterial Phases | A1 | A2 | M1 | M2 | M3 | BA | P2 | P3 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.8 ± 1.0 | 2.7 ± 0.7 | 2.9 ± 0.3 | 2.9 ± 0.3 | 2.6 ± 0.7 | 2.3 ± 0.9 | 3.0 ± 0.1 | 2.8 ± 0.6 | 2.1 ± 0.8 |
| 2 | 2.6 ± 0.7 | 2.2 ± 0.8 | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.4 ± 0.8 | 1.6 ± 0.8 | 2.9 ± 0.3 | 2.3 ± 0.8 | 1.3 ± 0.8 |
| 3 | 3.7 ± 0.7 | 1.2 ± 0.4 | 1.3 ± 0.5 | 1.9 ± 0.7 | 1.7 ± 0.7 | 1.1 ± 0.3 | 2.0 ± 0.6 | 1.2 ± 0.4 | 1.0 ± 0.1 |
Note:—IPAT indicates integrated parallel acquisition technique; A1 and A2, A1 and A2 segments of anterior cerebral artery; M1, M2, and M3, M1, M2, and M3 segments of middle cerebral artery; P2 and P3, P2 and P3 segments of posterior cerebral artery; BA, basilar artery.
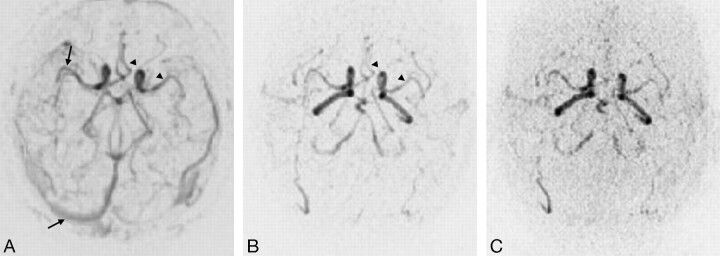
The score for the average number of arterial phases was 0.8 ± 1.0, 2.6 ± 0.7, and 3.7 ± 0.7 for IPAT 0, 2 and 3, respectively. A significant difference was demonstrated among all 3 MRA (Fig 3, Table 4).
Fig 3.
Axial TR MRA maximum intensity projection reconstructions without IPAT (A) and with IPAT 2 (B) and 3 (C). Note the simultaneous visualization of arterial and venous vessels (arrows) with TR MRA without IPAT (A), whereas the TR MRA with IPAT (B, C) provides the visualization of one arterial phase without venous opacification and the similar conspicuity of proximal arterial branches (head arrows) without IPAT (A) compared with IPAT 2 (B).
Table 4:
Significance levels (P values) for the pairwise comparison of IPAT factor levels with respect to each end point
| End Point | IPAT Factor Levels Compared |
||
|---|---|---|---|
| 0 vs 2 | 0 vs 3 | 2 vs 3 | |
| SNR | .186 | .042* | .146 |
| CNR | .155 | .020* | .074 |
| Score | .004* | .004* | .014* |
| A1 | .059 | .004* | .009 |
| A2 | .371 | .004* | .004* |
| M1 | .371 | .009* | .009* |
| M2 | .371 | .006* | .009* |
| M3 | .022* | .009* | .100 |
| AB | .999 | .006* | .006* |
| P2 | .059 | .004* | .006* |
| P3 | .022* | .009* | .371 |
Note:—IPAT indicates integrated parallel acquisition technique; SNR, signal-to-noise ratio; CNR, contrast-to-noise ratio; A1 and A2, A1 and A2 segments of anterior cerebral artery; M1, M2, and M3, M1, M2, and M3 segments of middle cerebral artery; P2 and P3, P2 and P3 segments of posterior cerebral artery; BA, basilar artery. Statistically significant results are marked with asterisks.
Discussion
Our results indicate that, by using a combination of parallel imaging (IPAT 2) and a view sharing technique (TREAT), the vascular system of the brain can be visualized with a 1.7-second temporal resolution and acceptable spatial resolution. We have also shown that, with a very small dose of intravenous contrast agent (5 mL), TR MRA is a technically feasible method for examining the intracranial vasculature. Compared with the technique without parallel imaging, temporal resolution was increased by 42% (ie, from 4 seconds to 1.7 seconds). In our experience, a temporal resolution within the region of 1.7 seconds, providing an average of about 3 arterial phases, is sufficient for current clinical practice to distinguish between arterial and venous drainage; however the current lower temporal resolution compared with routine DSA studies (usually with a 0.3–0.5 second temporal resolution) does not yet allow this technique to substitute for DSA. Combining higher field MR (3T) with newer contrast agents (with higher relaxivity) will probably allow increases in temporal resolution in the near future to allow direct comparison with routine DSA.
Parallel imaging is a recent major development in MR imaging. Multiple and integrated panoramic coils are used to obtain multiple datasets to reduce scan time. The individual datasets are combined to generate the final image. In our study, the parallel acquisition strategy in the implementation of parallel imaging applied to produce the final reconstructed image used generalized autocalibrating partially parallel acquisitions (GRAPPA), which combines individual coil raw data before a Fourier transform. A major drawback of parallel imaging is a reduction of the achievable SNR, especially if conventional receiver coils are used.5,6 Consequently, with the image parameters used and with the hardware configuration available, the acceleration factor could not be increased without compromising image quality. We have demonstrated that the use of IPAT 3 with the shorter acquisition time prevents interpretation because of the decreased SNR and CNR. For the larger proximal vessels, this decrease did not prevent interpretation, but the confidence level of visualization of smaller distal vessels was significantly better with TR MRA with no IPAT than with IPAT 2 and 3.
The combination of TR MRA (TREAT) with parallel imaging enables a smaller volume of contrast agent to be used. TREAT updates the center of k-space more often than the periphery of k-space. As a result, TREAT acquires a wider spectrum of lines in each timeframe. In contrast to parallel imaging, it does not affect the SNR, because the same amount of data are used to calculate the 3D datasets.7 It is therefore possible to inject a smaller volume compared with the routine volume.8,9 Because the technique requires only 5 mL of contrast agent, it can be added to a routine high-resolution MRA (with or without contrast), and dynamic susceptibility contrast perfusion MR imaging can be performed in the same session.
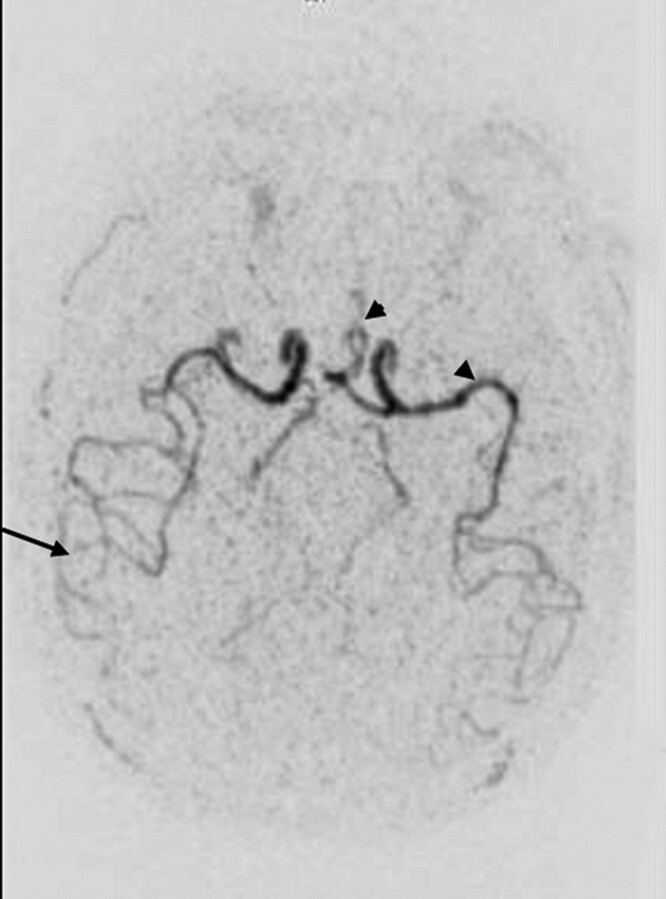
Our technique has several limitations. The first concerns the spatial resolution and the sequence; nonisotropic voxels are not able to provide several construction planes. Another limitation is the conspicuity of small distal vessels. Further studies are needed to assess the diagnostic benefits achieved with a greater volume of contrast media (Fig 4) or with different contrast agents with higher relaxivity.
Fig 4.
Axial TR MRA MIP reconstructions with IPAT 2 after an injection of 10 mL of contrast media. The increased volume of contrast media provides a better visualization of both proximal (head arrows) and distal (arrows) arterial vessels.
The acceleration strategy for time-resolved imaging, including parallel imaging (IPAT 2) and temporal echo sharing, provides the best compromise. We recommend that this protocol be added to and supplement current high-resolution, non–time-resolved MRA techniques to provide temporal information similar to that available from conventional DSA. This will have clinical application for numerous intracranial pathologic conditions, such as arteriovenous malformation, acute ischemic stroke, and brain tumors.
References
- 1.Griffiths PD, Hoggard N, Warren DJ, et al. Brain arteriovenous malformations: assessment with dynamic MR digital subtraction angiography. AJNR Am J Neuroradiol 2000;21:1892–99 [PMC free article] [PubMed] [Google Scholar]
- 2.Wetzel SG, Bilecen D, Lyrer P, et al. Cerebral dural arteriovenous fistulas: detection by dynamic MR projection angiography. AJR Am J Roentgenol 2000;174:1293–95 [DOI] [PubMed] [Google Scholar]
- 3.Fink C, Puderbach M, Ley S, et al. Time-resolved echo-shared parallel MRA of the lung: observer preference study of image quality in comparison with non-echo-shared sequences. Eur Radiol 2005;15:2070–74 [DOI] [PubMed] [Google Scholar]
- 4.Nael K, Michaely HJ, Villablanca P, et al. Time-resolved contrast enhanced magnetic resonance angiography of the head and neck at 3.0 Tesla: initial results. Invest Radiol 2006;41:116–24 [DOI] [PubMed] [Google Scholar]
- 5.Madore B, Pelc NJ. SMASH and SENSE: experimental and numerical comparisons. Magn Reson Med 2001;45:1103–11 [DOI] [PubMed] [Google Scholar]
- 6.Ohliger MA, Grant AK, Sodickson DK. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn Reson Med 2003;50:1018–30 [DOI] [PubMed] [Google Scholar]
- 7.Cashen TA, Kroeker R, Leloudas N, et al. Comparison of temporal and spatial undersampling techniques for time-resolved contrast-enhanced MR angiography [abstract]. Proceedings of the 13th Scientific Meeting and Exhibition of ISMRM; Miami, Florida; May 7–13, 2005. Berkeley, Calif: International Society for Magnetic Resonance in Medicine;2005. .
- 8.Tsuchiya K, Katase S, Yoshino A, et al. MR digital subtraction angiography of cerebral arteriovenous malformations. AJNR Am J Neuroradiol 2000;21:707–11 [PMC free article] [PubMed] [Google Scholar]
- 9.Mori H, Aoki S, Okubo T, et al. Two-dimensional thick-slice MR digital subtraction angiography in the assessment of small to medium-size intracranial arteriovenous malformations. Neuroradiology 2003;45:27–33 [DOI] [PubMed] [Google Scholar]