Abstract
BACKGROUND AND PURPOSE: Animal models with appropriate volume are crucial for preclinical assessment of aneurysm therapies. Our purpose was to control the aneurysm volume by adjusting the position of ligation during creation of elastase-induced aneurysms in rabbits.
MATERIALS AND METHODS: Sixty elastase-induced aneurysms in rabbits were created. Two different methods were used for creation, including group 1 (n = 30) by using a lower ligation (from the origin of the right common carotid artery [RCCA] to the ligation point, 10 mm) and group 2 (n = 30) by using a higher ligation (from the origin of the RCCA to the ligation point, 15 mm). Aneurysm sizes (neck diameter, width, and height) and volumes in the 2 groups were measured and calculated, and they were compared by using the Student t test.
RESULTS: The mean aneurysm neck diameter, width, and height for group 2 were significantly larger than those of group 1 (3.3 ± 0.8 versus 2.7 ± 0.6 mm, P < .001; 3.7 ± 0.7 versus 2.5 ± 0.7 mm, P < .001; 9.0 ± 1.7 versus 7.3 ± 1.9 mm, P < .001, respectively). The aneurysm volume in group 2 was significantly larger than that in group 1 (102.4 ± 54.8 mm3 versus 36.6 ± 26.8 mm3, P < .001).
CONCLUSION: The aneurysm volume of elastase-induced models in rabbits can be controlled by adjusting the position of the ligation. Using a higher ligation can create relatively more voluminous aneurysms, compared with using a lower ligation.
As endovascular treatment for intracranial aneurysm continued growing, more and more aneurysm models were widely used. As one of the most popular models,1-12 the elastase-induced aneurysm model in rabbits has been used for testing various endovascular devices.13-19 One potential disadvantage of this model is the apparent lack of volume control when it is used for testing of devices aimed at comparison of effects between small and large aneurysms. Creation of elastase-induced aneurysms involves isolation of the common carotid artery (CCA) lumen by distal ligation and temporary proximal balloon occlusion.7 During this procedure, elastase is incubated above the occlusion balloon. In this study, we report that the volume of the aneurysm model can be controlled by adjusting the position of the ligation.
Materials and Methods
Aneurysm Creation
Elastase-induced saccular aneurysms were created in 60 New Zealand white rabbits. All procedures were approved by the Institutional Animal Care and Use Committee at our institution.
Two groups were studied in a prospective fashion, including group 1 (n = 30) by using a lower ligation (from the origin of the right CCA [RCCA] to the ligation point, 10 mm) and group 2 (n = 30) by using a higher ligation (from the origin of the RCCA to the ligation point, 15 mm). Aneurysms were created mainly according to a method previously described with some modifications.7 Details of the modifications follow.
An 8-cm right paramidline incision was made from the thyroid cartilage to the sternum. Both the right sternomastoid muscle and the right sternothyroid muscle were dissected. The attachments of the right pectoralis tenuis muscle to the top of the sternum were dissected and reflected. After removing surrounding tissue gently, the origin of the RCCA at its junction with the subclavian and brachiocephalic arteries was completely exposed. A 5F sheath (Cordis Endovascular, Miami Lakes, Fla) was advanced retrograde in the RCCA. The inflated balloon was placed completely in the brachiocephalic and right subclavian arteries in all rabbits. After 20 minutes of incubation of the elastase solution, the balloon and sheath were removed, and the RCCA was ligated at a lower level (10 mm, group 1, n = 30) and at a higher level (15 mm, group 2, n = 30) cephalad to the RCCA origin. The proximal RCCA was transected just cephalad to site of ligation. The skin was closed with a 4–0 vicryl running suture.
Follow-Up Angiography
Intra-arterial digital subtraction angiography (IADSA) was performed at least 3 weeks after creation because our previous study indicated that the elastase-induced aneurysms remain stable beyond 3 weeks.8 The animals were anesthetized as described previously. Using a sterile technique, the surgeon performed exposure of the right common femoral artery and placed a 5F vascular sheath. Heparin (100 U/kg) was administered by the sheath. A 5F catheter (Envoy, Cordis Endovascular) was advanced into the brachiocephalic artery, and DSA was performed. An external sizing device was in place during IADSA. The sizes of the aneurysms (including neck diameter, width, and height) were measured and were determined in reference to the external sizing device. The volume of the aneurysm before embolization was approximately calculated on the basis of the supposition that the aneurysm was cylindric, by using the following formula: volume of the aneurysm = 3.14 × (width /2) × (width /2) × height. Mean aneurysm sizes (including average and SD) were calculated.
Statistical Analysis
Aneurysm sizes between the 2 groups were compared by using the Student t test.
Results
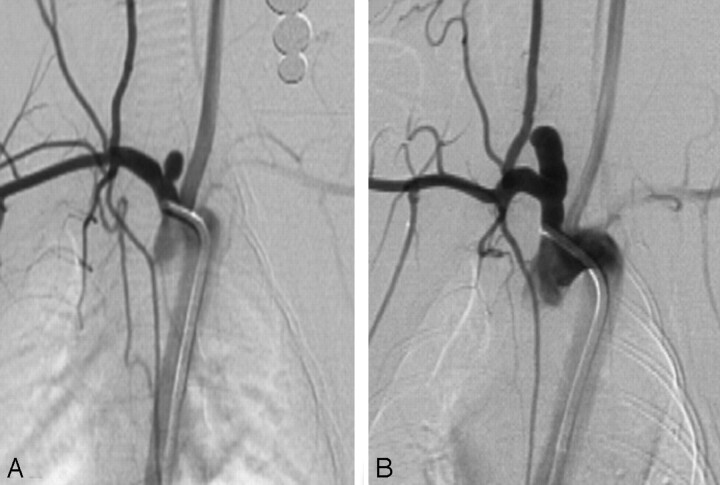
Aneurysm sizes are shown in the Table. Representative images are shown in Fig 1.
Aneurysm sizes in Groups 1 and 2
| Group 1 (n = 30) | Group 2 (n = 30) | Pvalue | |
|---|---|---|---|
| Neck diameter (mean ± SD, mm) | 2.7 ± 0.6 | 3.3 ± 0.8 | <.001 |
| Width (mean ± SD, mm) | 2.5 ± .7 | 3.7 ± 0.7 | <.001 |
| Height (mean ± SD, mm) | 7.3 ± 1.9 | 9.0 ± 1.7 | <.001 |
| Volume (mean ± SD, mm3) | 36.6 ± 26.8 | 102.4 ± 54.8 | <.001 |
Fig 1.
Representative images. A, Lower ligation showing smaller aneurysm. B, Higher ligation showing larger aneurysm.
Aneurysm Neck Size
Mean neck sizes for groups 1 and 2 were 2.7 ± 0.6 mm (range, 1.2–3.9 mm) and 3.3 ± 0.8 mm (range, 1.7–4.4 mm), respectively (P < .001). Neck size in group 2 was significantly larger than that of group 1.
Aneurysm Width
Mean widths for groups 1 and 2 were 2.5 ± 0.7 mm (range, 2.6–4.7 mm) and 3.7 ± 0.7 mm (range, 2.8–5.9 mm), respectively (P < .001). Aneurysm width in group 2 was significantly larger than that of group 1.
Aneurysm Height
Mean heights for groups 1 and 2 were 7.3 ± 1.9 mm (range, 3.3–10.7 mm) and 9.0 ± 1.7 mm (range, 6.7–15.3 mm), respectively (P < .001). Aneurysm height in group 2 was significantly larger than that of group 1.
Aneurysm Volume
Mean aneurysm volumes for groups 1 and 2 were 36.6 ± 26.8 mm3 (range, 3.7–115.8 mm3) and 102.4 ± 54.8 mm3 (range, 33.4–312.4 mm3), respectively (P < .001). Aneurysm volume in group 2 was significantly larger than that of group 1.
Discussion
Elastase-induced aneurysms in rabbits represent an important advance in aneurysm model research.2,7,9,13-21 However, unlike surgical models in which the size of the aneurysm cavity and neck can be controlled by choice of vein pouch and suture technique,4 control of elastase-induced aneurysm volume is difficult.9,10,22,23 In this prospective study, we demonstrated that different sizes of aneurysms could be achieved by adjusting the level of ligation during creation. Lower ligation induced a smaller aneurysm, whereas higher ligation induced a larger aneurysm.
We can only conjecture about the reason that the higher position of the ligation induced larger aneurysms. It is possible that higher ligation leaves more space in the blinded end of the origin of RCCA. Conversely, less space was left in lower ligation aneurysms because the temporary inflated balloon position was the same (in all brachiocephalic and right subclavian arteries)22 and no factors impacted the surface of wall of RCCA. Consequently, the longer segment of the distal RCCA was exposed to elastase in the higher ligation group, and the higher ligation induced longer aneurysms. In the meantime, our results indicated that not only the height of the higher ligation was larger than that of the lower ligation but also the width and neck sizes in the higher ligation group were also larger than those in the lower ligation group. The exact mechanism remains unknown, probably because more shear stress was applied to the vessel wall in the higher ligation group; on the contrary, there was less shear stress on the vessel wall in the lower ligation group. Therefore, the wall of the RCCA was less digested and dilated from the lower ligation compared with that of the higher ligation.
This study showed that larger aneurysms were created by using higher ligation; on the other hand, smaller aneurysms were induced by using lower ligation. The difference in volumes with different methods indicates the potential use of this finding for investigators who use the rabbit elastase–induced aneurysm model for preclinical testing of aneurysm-occlusion devices, which requires a certain volume in the aneurysm models.
Even though we found differences in mean volume of aneurysms, investigators cannot expect that all aneurysms made with higher ligation will be large aneurysms or that lower ligation will induce small aneurysms all the time. These data reflect differences in mean dimensions, with some overlap between groups.
A drawback of this study was the relatively low number of subjects in each group, and we are doing more studies for further conformation.
Conclusion
The volume of the elastase-induced aneurysm in rabbits can be controlled by adjusting the position of ligation. Using a higher ligation can create relatively more voluminous aneurysms, compared with a lower ligation.
Footnotes
This work was supported by National Institutes of Health grant (NS42646).
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, April 29–May 5, 2006; San Diego, Calif.
References
- 1.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: Part III. Pathology. Surg Neurol 1979;11:299–304 [PubMed] [Google Scholar]
- 2.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology 1999;213:223–28 [DOI] [PubMed] [Google Scholar]
- 3.Sorteberg A, Sorteberg W, Rappe A, et al. Effect of Guglielmi detachable coils on intra-aneurysmal flow: experimental study in canines. AJNR Am J Neuroradiol 2002;23:288–94 [PMC free article] [PubMed] [Google Scholar]
- 4.Guglielmi G, Ji C, Massoud TF, et al. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology 1994;36:547–50 [DOI] [PubMed] [Google Scholar]
- 5.Stehbens WE. Histological changes in chronic experimental aneurysms surgically fashioned in sheep. Pathology 1997;29:374–79 [DOI] [PubMed] [Google Scholar]
- 6.Reul J, Weis J, Spetzger U, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. AJNR Am J Neuroradiol 1997;18:35–42 [PMC free article] [PubMed] [Google Scholar]
- 7.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award: creation of saccular aneurysms in the rabbit—a model suitable for testing endovascular devices: American Roentgen Ray Society. AJR Am J Roentgenol 2000;174:349–54 [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara NH, Cloft HJ, Marx WF, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR Am J Neuroradiol 2001;22:698–703 [PMC free article] [PubMed] [Google Scholar]
- 9.Short JG, Fujiwara NH, Marx WF, et al. Elastase-induced saccular aneurysms in rabbits: comparison of geometric features with those of human aneurysms. AJNR Am J Neuroradiol 2001;22:1833–37 [PMC free article] [PubMed] [Google Scholar]
- 10.Kallmes DF, Fujiwara NH, Berr SS, et al. Elastase-induced saccular aneurysms in rabbits: a dose-escalation study. AJNR Am J Neuroradiol 2002;23:295–98 [PMC free article] [PubMed] [Google Scholar]
- 11.Ding YH, Dai D, Lewis DA, et al. Intra-venous digital subtraction angiography: an alternative method to intra-arterial digital subtraction angiography for experimental aneurysm imaging. Neuroradiology 2005;47:792–95. Epub 2005 Aug 25 [DOI] [PubMed] [Google Scholar]
- 12.Ding YH, Dai D, Danielson MA, et al. Long-term patency of elastase-induced aneurysm model in rabbits. AJNR Am J Neuroradiol 2006;27:139–41 [PMC free article] [PubMed] [Google Scholar]
- 13.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217–22 [DOI] [PubMed] [Google Scholar]
- 14.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol 2002;23:1580–88 [PMC free article] [PubMed] [Google Scholar]
- 15.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand White rabbit model. AJNR Am J Neuroradiol 2003;24:591–96 [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiwara NH, Kallmes DF. Healing response in elastase-induced rabbit aneurysms after embolization with a new platinum coil system. AJNR Am J Neuroradiol 2002;23:1137–44 [PMC free article] [PubMed] [Google Scholar]
- 17.Marx WF, Cloft HJ, Helm GA, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intra-aneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001;22:323–33 [PMC free article] [PubMed] [Google Scholar]
- 18.De Gast AN, Altes TA, Marx WF, et al. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 2001;49:690–96 [DOI] [PubMed] [Google Scholar]
- 19.Ding YH, Dai D, Lewis DA, et al. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol 2005;26:1757–63 [PMC free article] [PubMed] [Google Scholar]
- 20.Dai D, Ding YH, Danielson MA, et al. Modified histologic technique for processing metallic coil-bearing tissue. AJNR Am J Neuroradiol 2005;26:1932–36 [PMC free article] [PubMed] [Google Scholar]
- 21.Dai D, Ding YH, Lewis DA, et al. A proposed ordinal scale for grading histology in elastase-induced, saccular aneurysms. AJNR Am J Neuroradiol 2006;27:132–38 [PMC free article] [PubMed] [Google Scholar]
- 22.Ding YH, Dai DY, Lewis DA, et al. Can neck size in elastase-induced aneurysms be controlled? A prospective study. AJNR Am J Neuroradiol 2005;26:2364–67 [PMC free article] [PubMed] [Google Scholar]
- 23.Ding YH, Danielson MA, Kadirvel R, et al. Modified technique to create morphologically reproducible elastase-induced aneurysms in rabbits. Neuroradiology 2006;48:528–32. Epub 2006 May 18 [DOI] [PubMed] [Google Scholar]