Abstract
BACKGROUND AND PURPOSE: Stent-assisted revascularization increases prevailing recanalization rates (≅50%–69%) for vessel occlusions recalcitrant to thrombolytics. Although balloon-mounted coronary stents can displace thrombus (via angioplasty) and retain clot along vessel walls, intracranial self-expanding stents are more flexible and exert less radial outward force during deployment, increasing deliverability and safety. To understand the effectiveness of self-expanding stents for recanalization of acute cerebrovascular occlusions, we retrospectively reviewed our preliminary experience with these stents.
MATERIALS AND METHODS: Eighteen patients (19 lesions) presenting with a clinical diagnosis of acute stroke underwent catheter-based angiography documenting focal occlusion of an intracranial artery. A self-expanding stent was delivered to the occlusion and deployed. Stent placement was the initial mechanical maneuver in 6 cases; others involved a combination of pharmacologic and/or mechanical maneuvers prestenting. GP IIb/IIIa inhibitors were administered in 10 cases intraprocedurally or immediately postprocedurally to avoid acute in-stent thrombosis.
RESULTS: Stent deployment at the target occlusion (technical success) was achieved in all cases. Thrombolysis in Cerebral Ischemia (TICI)/Thrombolysis in Myocardial Ischemia (TIMI) 2/3 recanalization (angiographic success) was achieved in 15 of 19 lesions (79%). All single-vessel lesions (n = 8) were recanalized, but only 7 of 11 combination internal carotid artery and middle cerebral artery lesions were recanalized. No intraprocedural complications occurred. Seven in-hospital deaths occurred: stroke progression, 4; intracranial hemorrhage, 2; respiratory failure, 1. Seven patients had ≥4-point National Institutes of Health Stroke Scale improvement within 24 hours after the procedure, 6 had modified Rankin Score (mRS) ≤3 at discharge, and 4 had mRS ≤3 at 3 months. Overall, revascularization and improvement in clinical outcome were more likely to occur in women.
CONCLUSION: Feasibility of self-expanding stents for treatment of acute symptomatic intracranial occlusions is shown. For single-vessel lesions, stent placement with concomitant administration of IIb/IIIa inhibitors contributed to the achievement of recanalization rates exceeding those currently reported for other means of thrombolysis.
Intra-arterial (IA) therapies for acute stroke have evolved over the past decade. Despite the promising results of PROACT II,1 which demonstrated a 66% recanalization rate, substantially higher recanalization rates with IA pharmacologic thrombolysis have not been achieved over the past 7 years. The Food and Drug Administration recently approved a clot retrieval device (Merci retriever X5, X6; Concentric Medical, Mountain View, Calif).2,3 Unfortunately, when used alone, the clot retriever is successful in only approximately 50% of cases, and multiple passes with this device are often required to achieve successful recanalization.3 IA thrombolytics administered concomitantly enhance the procedural success of this device but may increase the risk of hemorrhagic transformation of the reperfused infarction. There have been several reports of coronary stent implantation used for mechanical thrombolysis of recalcitrant occlusions.4–9 In a recent report, stent placement with balloon-mounted or self-expanding coronary stents was shown to be an independent predictor for recanalization of both intracranial and extracranial cerebrovascular occlusions.5 In another recent report, recanalization rates of 79% were achieved using balloon-mounted stent technology.7
Self-expanding stents designed specifically for the cerebrovasculature can be delivered to target areas of intracranial stenosis with a success rate of >95% and an increased safety profile of deliverability because these stents are deployed at significantly lower pressures than balloon-mounted coronary stents.10,11 There has been an anecdotal report of the use of a self-expanding stent in the setting of acute symptomatic intracranial occlusion.12 In addition, a recent comparison of self-expanding and balloon-mounted stents in an animal model of acute embolic occlusion has shown no difference in the 2 stent groups with respect to recanalization rates.13 Therefore, to determine the feasibility and potential effectiveness of self-expanding stents for recanalization of acute symptomatic intracranial occlusions, we conducted a retrospective review of the preliminary experience at 4 centers at which patients were treated with this technology.
Materials and Methods
Four university centers participated in a retrospective review of patients presenting with stroke as a result of acute intracerebral arterial occlusion in whom stent-assisted recanalization was performed by using an intracranial stent (Neuroform 3 or Wingspan; Boston Scientific, Natick, Mass). The participating centers and patient enrollment are as follows: University at Buffalo (n = 8), Cleveland Clinic (n = 3), Barrow Neurologic Institute (n = 1), and University of Pittsburgh (n = 6). Cases were identified through a search of the endovascular data base at each of the 4 institutions. Clinical charts were reviewed for patient demographics, medical comorbidities, location of occlusion, time to angiography, use of adjunctive therapies (pharmacologic thrombolysis, glycoprotein [GP] IIb/IIIa inhibitors, angioplasty, clot retrieval) and admission and 24-hour National Institutes of Health Stroke Scale (NIHSS) 0 scores, and modified Rankin Scale scores (mRS) at discharge and at 3 months after the procedure. Angiographic images were reviewed retrospectively. Patients were treated between August 31, 2005, and February 3, 2006.
Institutional review board approval was obtained to review the clinical data. The use of these self-expanding intracranial stent platforms in the setting of acute stroke is considered an off-label application. The use of GP IIb/IIIa inhibitors to prevent acute in-stent thrombosis is based on studies reported in the coronary literature14–19 and is also considered an off-label application in this setting.
Preprocedure Imaging
Patients were selected for intervention after their presentation with acute onset of stroke symptoms (within 8 hours) with an NIHSS score of ≥8 (except one patient, as explained in Results) and cranial CT imaging lacking evidence of hemorrhage or evidence of early cerebral infarction greater than one third of the affected branch distribution. After angiographic evidence of acute intracranial vessel occlusion responsible for the neurologic presenting symptoms, the patient underwent IA recanalization strategies at the discretion of the operator (multicenter retrospective review). If focal occlusion of a medium or large intracranial vessel was identified, stent placement was used after failure of mechanical or pharmacologic thrombolysis or as an initial mechanical maneuver.
Flow through the occluded vessel was assessed using the accepted Thrombolysis in Cerebral Ischemia (TICI)/Thrombolysis in Myocardial Ischemia (TIMI) grading systems20–23 as follows: grade 0, no flow; grade 1, minimal flow (very slow), without significant flow distal to the occlusion site; grade 2, near-normal flow, with flow distal to the occlusion, but not filling the distal branches normally (TICI 2a and 2b were grouped together due to sample size); grade 3, normal flow. Pretreatment and posttreatment grades were retrospectively assigned upon review of the angiographic images by consensus of the interventionists at the participating institutions.
Stent Sizing
Before a decision was made to proceed with stent placement, the length of the occlusive lesion was measured by performing catheter-based angiography immediately proximal to the occlusion and microcatheter angiography immediately distal to the occlusion. The length of the occluded segment was defined by the area without perfusion of contrast. Occlusive lesions limited in length to 16 mm were considered for treatment with the self-expanding stents. The Wingspan and Neuroform 3 stents are currently available in lengths of up to 20 mm for vessel diameters 2.5–4.5 mm, and a slight margin is necessary for stent coverage proximal and distal to the lesion. The stents were sized 0.5–1 mm greater than the diameter of the native parent vessel just proximal to the occlusion to increase the radial outward force of the stent against the occlusion.
Stent Placement
Standard femoral artery access was obtained, and a 6F or larger bore guide catheter was placed in the target vessel, just proximal to the occlusion. After the presence of a medium or large intracranial vessel occlusion was confirmed angiographically, heparin was administered to maintain an activated coagulation time in the range of 250 seconds.
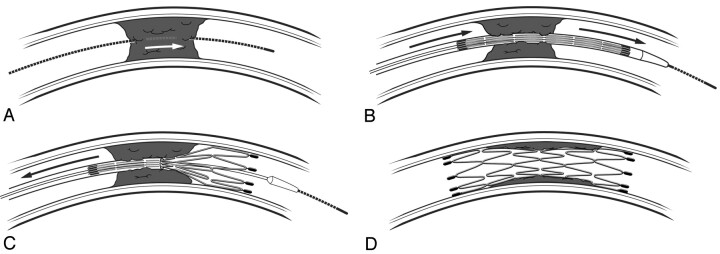
To minimize the release of distal emboli, the occlusion was crossed with a 0.014-inch steerable wire, which was then gently advanced through the clot (Fig 1A). A low-profile microcatheter was then advanced over this wire, just distal to the occlusion. Superselective microcatheter angiography was then performed to confirm that the microcatheter was distal to the occlusion and within an appropriate intravascular position. An exchange wire was brought through the microcatheter and anchored distal to the occlusion. The microcatheter was then removed, and the stent delivery catheter was delivered over the exchange wire, using road-mapping technique for guidance.
Fig 1.
Steps involved in sirolimus-eluting stent delivery and deployment. A, A steerable wire is softly advanced through the occlusive clot. B, Placement of stent across occlusion. C, Deployment of stent, thus trapping the occlusion.D, Recanalization.
To minimize the release of debris, the stent was deployed first distal to the occlusion (thus trapping any debris that might be later released between the stent and the vessel wall) (Fig 1B), then through the occlusion (Fig 1C), and finally just proximal to the occlusion (Fig 1D), thus traversing the entire lesion. Toward that end, efforts were made to assure complete apposition of the stent to the vessel wall. If a portion of the self-expanding stent was not fully expanded, poststent angioplasty (dilation) was performed with an undersized balloon (up to, but not exceeding, the reference vessel diameter) by using slow-inflation techniques (approximately 1 atm per 30 seconds). After the stent was deployed, the delivery system was withdrawn; angiography of the stented region was obtained.
GP IIb/IIIa Inhibition
Before stent deployment, but after crossing the occlusion with a microwire, a GP IIb/IIIa inhibitor was administered to prevent acute stent thrombosis (these patients were not pretreated with adequate antiplatelet medications, such as clopidogrel, because of the acute nature of their conditions). According to the protocol at the institution, a single bolus dose of eptifibatide (180 μg/kg) or abciximab (0.25 mg/kg, followed by an infusion to achieve 60%–80% platelet inhibition based on aggregometry) was administered. In cases of difficult stent placement or lengthy procedure time where there was concern for either iatrogenic or reperfusion hemorrhage, GP IIb/IIIa inhibition was delayed until a CT scan was obtained immediately after the procedure. If diffuse extravasation of contrast material, subarachnoid hemorrhage, or intraparenchymal hematoma (>1 cm) was seen, inhibitors were not used for fear of catastrophic hemorrhage.
Poststenting
Immediately after the stent procedure, patients received either aspirin (650 mg) administered through an orally placed gastric tube or orally administered aspirin (325 mg) and clopidogrel (300–600 mg) (according to institutional protocol). CT scans were obtained within 24 hours after the procedure to evaluate for hemorrhage and infarction. Patients were discharged on a maintenance dose of aspirin (325 mg daily) and either clopidogrel (75 mg daily for 1 month) or ticlopidine (250 mg twice daily for 1 month).
Definitions and Statistical Analysis
Technical success of the procedure was defined as safe deployment of the stent at the site of vessel occlusion with full coverage of the lesion. Angiographic success was defined as restoration of TIMI/TICI 2 or 3 flow through the stented segment. Improvement in neurologic function was assessed using the NIHSS and defined as a reduction of ≥4 points on this scale. In addition, clinical outcome was assessed using the mRS, and favorable outcomes were limited to scores ≤3 at the time of discharge and 3 months after the procedure.
Baseline characteristics for recanalization versus nonrecanalization, as well as NIHSS clinical improvement, were compared by using the Fisher exact test for categoric variables and the Student t test for continuous variables. α levels of P ≤ .05 were considered statistically significant.
Results
The mean age of the 18 patients, 8 men and 10 women, from the participating institutions was 75.1 ± 11.4 years (range, 56–93 years). The median admission NIHSS score was 18 ± 5.9 (range, 2–23; SD 5.9). The patient who had an admission NIHSS score of 2 was treated because he suffered crescendo transient ischemic attacks during the administration of IV heparin and oral antiplatelet medications. These attacks were the result of his acute basilar artery occlusion. Because the patient failed this maximal medical therapy and because of the exceedingly poor prognosis associated with posterior circulation occlusive disease, we felt that he was an appropriate candidate for stent placement.
Occlusion sites were the M1 middle cerebral artery (MCA) segment (n = 4), M2 MCA segment (n = 1), vertebrobasilar system (VBS) (n = 3), combination of M1 and M2 MCA (n = 4), combination of internal carotid artery (ICA) bifurcation and M1 MCA (n = 3), combination of ICA terminus and ICA bifurcation and M1 MCA (n = 2), and combination of ICA terminus and M1 MCA (n = 2). ICA bifurcation refers to clot extending from the ICA into the branches (M1 origin or A1 origin; in this series, only the M1), whereas the terminus refers to solely the distal ICA. One patient had bilateral MCA occlusions, requiring 2 independent revascularization procedures; these procedures were performed during the same sitting, and each lesion necessitated stent placement to achieve recanalization after other maneuvers were unsuccessful. This resulted in a total of 19 lesions for stent placement in the 18 patients. Neuroform 3 stents were used in 16 lesions and Wingspan stents in 3 lesions. One case included in this review has been reported elsewhere.12
GP IIb/IIIa inhibitors were administered intraprocedurally in 10 of 19 cases. IA thrombolysis was performed in 12 lesions. IV thrombolysis was performed in 5 lesions. Angioplasty as an attempt at mechanical thrombolysis was performed in 10 lesions. Clot retrieval with the Merci retriever was attempted in 9 lesions. As mentioned, intracranial stent placement was performed in each of the 19 lesions. In 6 of these lesions, stent placement was the initial mechanical maneuver, 2 of which were subsequently angioplastied. In 4 lesions, both stent placement and angioplasty (2 before, 2 after) were performed; in 3 lesions, stent placement was performed after Merci clot retrieval; and in 6 lesions, both angioplasty (as a separate mechanical maneuver before stent placement) and clot retrieval were performed in addition to intracranial stent placement. Patients who had attempted recanalization with angioplasty before stent placement did not have repeat angioplasty after stent placement.
Technical success was achieved in all cases, and angiographic success was achieved in 79% of cases (15 of 19 lesions). Table 1 summarizes the predictors of patient outcome, improvement in neurologic outcome (as assessed by a reduction of ≥4 points in the 24-hour NIHSS score), based on the clinical characteristics and maneuvers used for vessel recanalization. Of the 7 patients (39%) in whom the score decreased, 2 received Wingspan stents and 5 received Neuroform stents. Neurologic improvement was achieved more frequently in women than in men. Four of 8 patients with solitary lesions achieved a ≥4-point reduction of admission score; 3 of 10 patients (3 of 11 lesions) with extensive lesions achieved the same improvement in NIHSS. Of note is that patients with a history of hyperlipidemia showed poor improvement in NIHSS score in 24 hours relative to those without such comorbidity (P = .05).
Table 1:
Predictors of improvement in neurologic outcome
| NIHSS Improved ≥4 Points in 24 Hours |
P | ||
|---|---|---|---|
| No | Yes | ||
| Demographics (patients) | n = 11 | n = 7 | |
| Age, years (mean ± SD) | 71.3 ± 12.0 | 81 ± 7.9 | .08 |
| Admission NIHSS (mean ± SD) | 16.5 ± 5.9 | 15.8 ± 5.7 | .84 |
| Time to angiography, minutes (mean ± SD) | 624 ± 918 | 216 ± 123 | .35 |
| Women (men) | 4 (7) | 6 (1) | .05 |
| Comorbidities (patients) | n = 11 | n = 7 | |
| Hypertension | 11 | 6 | .39 |
| Diabetes mellitus | 2 | 1 | .67 |
| Atrial fibrillation | 3 | 4 | .22 |
| Hyperlipidemia | 7 | 1 | .05 |
| Coronary artery disease | 3 | 2 | .68 |
| Location (lesions) | n = 12 | n = 7 | |
| M1 MCA | 2 | 2 | .47 |
| M2 MCA | 0 | 1 | .37 |
| VBS | 2 | 1 | .70 |
| Any solitary lesion | 4 | 4 | .30 |
| M1 MCA + M2 MCA | 2 | 2 | .47 |
| ICA bifurcation + ICA terminus + M1 MCA | 2 | 0 | .39 |
| ICA bifurcation + M1 MCA | 2 | 1 | .70 |
| ICA terminus + M1 MCA | 2 | 0 | .39 |
| Limited to MCA (M1 or M2 MCA or M1+M2) | 4 | 5 | .13 |
| Limited to MCA or VBS | 6 | 6 | .14 |
| Stent used (lesions) | n = 12 | n = 7 | |
| Neuroform | 11 | 5 | .29 |
| Wingspan | 1 | 2 | .29 |
| Adjunctive treatment (lesions) | n = 12 | n = 7 | |
| IIb/IIIa inhibitor | 8 | 2 | .13 |
| IA thrombolysis | 8 | 4 | .52 |
| IV thrombolysis | 4 | 1 | .37 |
| Merci retriever | 4 | 5 | .13 |
| Angioplasty | 7 | 3 | .43 |
Note:—NIHSS indicates National Institutes of Health Stroke Scale; MCA, middle cerebral artery; VBS, vertebrobasilar system; ICA, internal carotid artery; IA, intra-arterial.
Favorable discharge mRS scores correlated with 24-hour NIHSS improvement (r = 0.66), except in 1 patient who did not improve by 4 or more points in NIHSS score and had an mRS score of 1 at discharge (ie, the patient who had the baseline NIHSS score of 2). Other than the aforementioned patient, the mRS was either 5 or 6 if the patient's NIHSS score did not improve by 4 or more points and 1 if the NIHSS did improve. Six patients (33%) had an mRS score of 1 at discharge. Despite these results, the only variable that significantly predicted which patient would improve clinically was sex. Women were more likely to benefit from stent placement, as assessed by an improved NIHSS score after the procedure: 6 of 10 female patients (60%) demonstrated a reduction of NIHSS ≥4 after stent placement, whereas only 1 of 8 male patients (13%) showed the same improvement. Moreover, of the 6 patients with an mRS ≤3 at discharge, 5 were female.
Table 2 summarizes the predictors for recanalization based on the clinical characteristics, lesion location, and maneuvers used for vessel recanalization. Only 3 of 7 lesions involving the ICA were successfully revascularized to TIMI/TICI 2 or 3 after stent placement. Occlusions including both segments of the ICA and M1 were associated with poor revascularization after stent placement. However, although statistical significance was not achieved secondary to a small sample size, all solitary lesions appear to be preferentially amenable to revascularization after stent placement, in that all lesions isolated to 1 vessel (n = 8) were revascularized to TIMI/TICI 2 or 3 flow after stent placement. Overall, lesions localized to the MCA or VBS (n = 12) were associated with recanalization (TIMI/TICI 2 or 3 flow) (P = .01). It is noteworthy that all lesions limited to any portion of the MCA, whether solitary or multifocal, were revascularized to TIMI/TICI 2 or 3 (P = .05). TIMI/TICI 2 or 3 was achieved in all 3 Wingspan cases and in 12 (75%) Neuroform cases, for an overall revascularization rate of 79%. All 10 female patients (n = 10) demonstrated TIMI/TICI 2 or 3 flow (P = .02), whereas only 4 of 8 male patients showed the same increase in revascularization. Seven of 14 patients exhibiting revascularization to TIMI/TICI 2 or 3 flow also improved clinically, as measured by reduction in NIHSS ≥4. Six of 14 patients (43%) in whom angiographic success was achieved had an mRS score ≤3 at discharge.
Table 2:
Predictors of revascularization
| TIMI 2–3 Flow |
P | ||
|---|---|---|---|
| No | Yes | ||
| Demographics (patients) | n = 4 | n = 14 | |
| Age, years (mean ± SD) | 67.5 ± 11.6 | 77.2 ± 10.8 | .14 |
| Admission NIHSS (mean ± SD) | 15.8 ± 5.2 | 16.4 ± 6.3 | .86 |
| Time to angiography, minutes (mean ± SD) | 372 ± 72 | 509 ± 894 | .77 |
| Women (men) | 0 (4) | 10 (4) | .02 |
| Comorbidities (patients) | n = 4 | n = 14 | |
| Hypertension | 4 | 13 | .78 |
| Diabetes mellitus | 1 | 2 | .55 |
| Atrial fibrillation | 2 | 5 | .51 |
| Hyperlipidemia | 3 | 5 | .21 |
| Coronary artery disease | 0 | 5 | .23 |
| Location (lesions) | n = 4 | n = 15 | |
| M1 MCA | 0 | 4 | .35 |
| M2 MCA | 0 | 1 | .79 |
| VBS | 0 | 3 | .47 |
| Any solitary lesion | 0 | 8 | .09 |
| M1 MCA + M2 MCA | 0 | 4 | .35 |
| ICA bifurcation + ICA terminus + M1 MCA | 2 | 0 | .04 |
| ICA bifurcation + M1 MCA | 0 | 3 | .47 |
| ICA terminus + M1 MCA | 2 | 0 | .04 |
| Limited to MCA (M1 or M2 MCA or M1+M2) | 0 | 9 | .05 |
| Limited to MCA or VBS | 0 | 12 | .01 |
| Stent used (lesions) | n = 4 | n = 15 | |
| Neuroform | 4 | 12 | .47 |
| Wingspan | 0 | 3 | .47 |
| Adjunctive treatment (lesions) | n = 4 | n = 15 | |
| IIb/IIIa inhibitor | 3 | 7 | .33 |
| IA thrombolysis | 3 | 9 | .53 |
| IV thrombolysis | 2 | 3 | .27 |
| Merci retriever | 1 | 8 | .33 |
| Angioplasty | 1 | 9 | .25 |
| Clinical outcomes (patients) | n = 4 | n = 14 | |
| mRS score ≤ 3 | 0 | 6 | .16 |
| mRS score ≤ 3 at 3 months | 0 | 4 | .42 |
| NIHSS score reduced by ≥ 4 | 0 | 7 | .11 |
Note:—TIMI indicates Thrombolysis in Myocardial Infarction; NIHSS, National Institutes of Health Stroke Scale; MCA, middle cerebral artery; VBS, vertebrobasilar system; ICA, internal carotid artery; IA, intra-arterial; mRS, modified Rankin Scale.
Successful recanalization was best achieved when self-expanding stent delivery was performed in conjunction with angioplasty and Merci clot retrieval, with the addition of adjunctive pharmacologic therapy (Table 3). In this case, TIMI/TICI 3 flow was achieved in all 6 patients in whom angioplasty and clot retrieval were used as an adjunct with self-expanding stent delivery. Sixty-six percent of these patients improved clinically, based on NIHSS.
Table 3:
Recanalization by mechanical maneuver with adjunctive pharmacotherapy (such as IIb/IIIa inhibitors)
| Recanalization | Stent | Angioplasty and Stent | Merci and Stent | Angioplasty, Merci, and Stent |
|---|---|---|---|---|
| Lesions | 6 | 4 | 3 | 6 |
| TIMI/TICI 0/1, n (%) | 2 (33) | 1 (25) | 1 (33) | 0 (0) |
| TIMI/TICI 2, n (%) | 2 (33) | 1 (25) | 1 (33) | 0 (0) |
| TIMI/TICI 3, n (%) | 2 (33) | 2 (50) | 1 (33) | 6 (100) |
| TIMI/TICI 2/3, n (%) | 4 (66) | 3 (75) | 2 (66) | 6 (100) |
| NIHSS improved ≥4 (% patients) | 33 | 0 | 66 | 66 |
Note:—TIMI indicates Thrombolysis in Myocardial Infarction; TICI, Thrombolysis in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale.
Among the patients in our series, there were no intraprocedural complications. A total of 7 patients had acute hemorrhage (either intraparenchymal or subarachnoid) on postprocedural CT scans obtained within 24 hours of the procedure. Of these, 2 of the hemorrhages resulted in progression to death, and 3 were asymptomatic and resolved on subsequent CT scans. Of the remaining 2 patients, neurologic deficits could not be solely related to the hemorrhage because the clots were localized to the region of ischemia, resulting in the patients' presenting stroke symptoms.
Seven in-hospital deaths occurred: 4 due to progression of stroke and withdrawal of care at the family's request, 1 secondary to respiratory failure, and 2 (as mentioned above) secondary to subsequent hemorrhagic conversion. Both patients who succumbed to intracerebral hemorrhage (ICH) received IA tissue plasminogen activator (tPA) as part of the treatment regimen; one had also received IIb/IIIa therapy.
We noted one patient who developed early stent rethrombosis (Fig 2A–D). No delayed in-stent thrombosis was noted on the last angiographic follow-up. Four patients had mRS scores ≤3 at the 3-month clinical follow-up evaluation.
Fig 2.
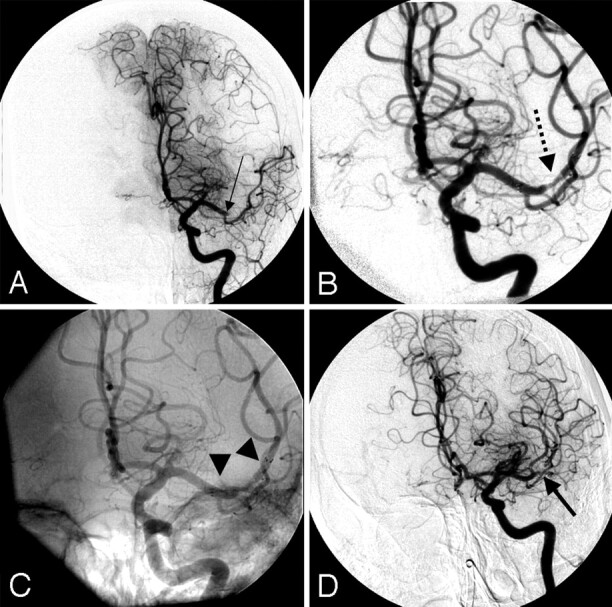
A 69-year-old woman with an NIHSS score of 17 who was noted to have a right hemiplegia and aphasia was given an IV bolus dose of tPA because she presented less than 3 hours from symptom onset. She was transferred on an emergency basis to our institution and referred for IA therapy.
A, Angiography (anteroposterior view) reveals a left M1 MCA occlusion (black arrow) with a large anterior temporal branch.
B, A 3.5- × 20-mm Neuroform 3 stent was deployed across the focal area of occlusion after administering an IV bolus dose of eptifibatide (180 μg/kg). Recanalization (TIMI/TICI 3 flow) was re-established in the posterior division of the MCA (dotted black arrow).
C, An unsubtracted image reveals the proximal and distal edges of the stent (black arrowheads).
D, A follow-up run reveals that the anterior division of the MCA is now also patent, but there are filling defects within the stent (arrow demonstrates filling of branch vessels with a filling defect in one of the branches). The patient improved on the day after the procedure to an NIHSS score of 10.
Discussion
This retrospective multicenter series demonstrates that the use of self-expanding stents is feasible in the setting of symptomatic medium- and large-vessel intracranial occlusions. With stent placement as a first-line mechanical treatment or as a “last-resort” maneuver, TIMI/TICI 2 or 3 revascularization was successfully obtained in 79% of the lesions in which stents were placed. This retrospective review suggests that focal occlusions limited to a single medium or large vessel, particularly solitary occlusions of the MCA or VBS, may be preferentially amenable to stent placement and thus can help clinicians to achieve improved rates of recanalization. In addition, gender may play a role in the success of self-expanding stent implantation: TIMI/TICI 2 or 3 flow was documented in all female patients studied, and female patients were more likely to achieve improved clinical outcomes as measured by NIHSS and mRS scores. Most importantly, our preliminary experience may lead to future pivotal studies that might aid clinicians to better stratify patients most likely to derive maximal clinical benefit from stent placement.
IA Interventional Approaches
Despite increasing utilization of prourokinase or other antithrombotic agents (eg, alteplase and reteplase), recanalization rates remain approximately 60%.1 The major concerns with pharmacologic thrombolysis (alone) have been the rate of hemorrhage, inability to effectively dissolve platelet-rich clots, lengthy times to recanalization, and inability to prevent abrupt reocclusion at the initial site of obstruction.24 In PROACT II, ICH with neurologic deterioration within 24 hours occurred in 10.9% of the prourokinase group and 3.1% of the control group (P = .06), without differences in mortality.1 Abrupt reocclusion of recanalized arteries has been found to occur relatively frequently, even with the addition of angioplasty or snare manipulation for mechanical disruption of thrombus, and seems to be associated with poor clinical outcomes.9,25
The use of other mechanical means has been reported to be effective in recanalization of acute occlusions. In the MERCI trial, overall recanalization rates (TIMI/TICI 2 or 3 flow) of 48% were achieved with the Merci mechanical clot retriever.3 It makes sense that a combination of mechanical and pharmacologic approaches would yield greater benefit. Although the addition of tPA in the MERCI study increased the recanalization rate to 64% (compared with the rate achieved using the retriever without thrombolytics), this was achieved at the expense of an increased rate of hemorrhagic transformation (bleeding into the area of infarction). It may be difficult to compare recanalization rates from the MERCI trial with PROACT, however, because MERCI included carotid T occlusions and basilar occlusions, whereas PROACT included only M1 or M2 occlusions. The cohort of patients with intracranial occlusions treated at our institutions is similar to the MERCI trial population. The results of the Multi-MERCI trial were recently presented.26 Investigators used the newer generation L5 device initially; passes could be made with the first-generation devices (X5, X6) subsequently. Adjuvant therapy with IA tPA was allowed after the Merci retriever was used. Successful recanalization (TIMI 2 or 3) was achieved with the retriever alone in 60 of 111 (54%) “treatable” vessels. Successful recanalization with the retriever plus adjunctive therapy was achieved in 77 of 111 (69%) treatable vessels. These rates trended higher than those for MERCI. However, the rate of hemorrhagic transformation was again increased (compared with the retriever without thrombolytics), at a similar rate as in MERCI, with 10 of 111 patients (9%) experiencing symptomatic ICH. Given the small sample size, we cannot draw any conclusions regarding a relationship between the means of recanalization and hemorrhage occurrence, though a recently published series suggests that the use of more modalities does not necessarily increase the risk of ICH.5 In addition, because of our small sample size, we could not adequately evaluate the influence of the addition of GP IIb/IIIa inhibitors on recanalization rates or ICH after stent placement. As shown in MERCI,2,3,26 the use of adjuvant therapy with clot retrieval was associated with an increased rate of mortality in the present series. Both of our patients with symptomatic hemorrhages that were fatal underwent a Merci retrieval attempt, angioplasty, IA tPA administration, and stent placement; 1 also received IIb/IIIa therapy.
Experience with Balloon-Mounted and Self-Expanding Stents
In a recent investigation in an animal model, both Wingspan self-expanding stents and Liberté balloon-mounted stents (Boston Scientific) were able to re-establish flow through acutely occluded vessels.13 The self-expanding stents performed better than the balloon-mounted stents in terms of navigability to the target site. The self-expanding stents incurred lower rates of vasospasm and side-branch occlusion, which suggests superiority of these stents, over balloon-mounted stents, to maintain branch vessel patency during treatment of acute vessel occlusion. In a previous animal study conducted by the same investigators, intimal proliferation and loss of lumen diameter were seen after the implantation of bare-metal, balloon-expandable stents.27,28 These phenomena are believed to be attributable to intimal injury created during the high-pressure balloon angioplasty that is required for stent deployment.
A recent report of patients with acute stroke treated with coronary balloon-expandable stents at 2 University Centers (University of Pittsburgh and University at Buffalo) included 13 men and 6 women with a median baseline NIHSS score of 16 (range, 15–22).7 Eight lesions were located at the ICA terminus, 7 in the M1/M2 MCA segment, and 4 in the basilar artery. The overall recanalization rate (TIMI/TICI 2 or 3) was 79%, which is the highest rate of acute recanalization reported thus far in a clinical series. In addition to yielding higher rates of recanalization, the use of stents may minimize the need for an escalating dose of thrombolytic therapy. This, in turn, will reduce the hemorrhagic complication rate, as demonstrated in the MERCI and multi-MERCI trials.2,3,26,29 Based on the preliminary data presented in this report and that of the MERCI trials, we determined that self-expanding stent implantation, with or without previously attempted embolectomy, might re-establish luminal patency more effectively than embolectomy with or without thrombolytic therapy and might incur a lower rate of hemorrhagic transformation. In the present study, the rate of successful revascularization was highest in patients who had embolectomy, angioplasty, and self-expanding stent implantation but includes the 2 patients (11%) who suffered ICH.
In another recent report, independent predictors for recanalization of occluded vessels among 168 patients with acute stroke included intracranial stent placement (P < .001) and the combination of IV GP IIb/IIIa inhibitors (eptifibatide) and IA thrombolytics (tPA or urokinase) (P < .048) stroke.5 Although in both these clinical studies,5,7 coronary balloon-mounted stents were used to restore the vessel lumen in the setting of acute occlusion, we believe that the self-expanding stent platform is the optimal choice for the intracranial vasculature. Compared with coronary balloon-mounted stents, self-expanding stents designed for use in the intracranial circulation are superior because they are easier to track to the intracranial circulation and safer to deploy in vessels in which the true diameter and degree of intracranial atherosclerotic disease are unclear.11 Moreover, based on previous experience,12,13 currently available self-expanding stents provide enough radial outward force at body temperature to revascularize occluded vessels, with low potential for the negative remodeling and in-stent restenosis that are associated with balloon-mounted stents in nonintracranial vascular beds.30 Because self-expanding stents are not mounted on balloons, they are the most trackable of the stents currently available for the intracranial circulation. Unlike clot retrievers, which lose access to the target (occlusion site) every time they are retrieved (and often necessitate multiple passes), self-expanding stents allow for wire access to the occlusion at all times, increasing the safety profile of the procedure by not requiring repeat maneuvers to gain access to the target site (as is the case for the Merci clot retriever).
Self-expanding stent placement of acute intracranial vessel occlusion may provide a novel means of recanalization after failure of clot retrieval, angioplasty, and/or thrombolytic therapy. The patency rates in this series are encouraging. In 2 cases, persistent obvious underlying stenosis (luminal defect) was identified despite TIMI or TICI 3 revascularization. Although some of the lesions may become reoccluded (in a delayed fashion), further intervention to achieve optimal luminal patency is not recommended in the high-risk setting of an acute stroke resulting from intracranial vessel occlusion. In the setting of acute stroke, restoring flow is of singular importance. In-stent stenosis or delayed stenosis may be treated in a delayed fashion on an elective basis, should the patient achieve a functional recovery from the stroke.
The fear of perforator occlusion from “snow-plowing” of thrombus after stent placement remains a concern. We cannot say whether this is less likely with self-expanding stents than with balloon-mounted stents that deploy at higher pressures because of the small sample size and because a direct comparison of these 2 stent types was not performed. However, if thrombus is occluding the parent vessel proximal to or in the region of the perforators, these perforators will remain occluded. Recanalization with self-expanding stents may provide flow through the parent artery, and restore flow to the perforators, or, alternatively, they may remain occluded. Restoring flow to the main artery, however, will reduce the stroke burden.
Limitations of the Present Study
This study suffers from the limitations of a relatively small sample size with retrospective data. Any findings of statistical significance must be considered with respect to the small sample size. Despite these limitations, the message that these stents, in combination with pharmacologic and mechanical maneuvers, may play a role in recanalization of acute occlusions is becoming apparent. A prospective study comparing stent placement with the best pharmacotherapy for recanalization of focal intracranial arterial occlusion is needed.
Conclusions
Our early experience at multiple centers indicates the feasibility of intracranial self-expanding stents for acute intracranial artery occlusions. TIMI/TICI 2 or 3 revascularization was successfully obtained in 79% of lesions, exceeding the recanalization rates currently reported for other means of thrombolysis. Moreover, focal occlusions limited to single medium or large vessels, particularly solitary occlusions of the MCA or VBS, may be preferentially amenable to stent placement.
Acknowledgments
We thank the endovascular neurosurgery fellows, nurses, and technicians for their role in the care of the patients treated at the centers participating in this study, Wisam Z. Alfay for assistance with obtaining follow-up data, and Paul H. Dressel for preparation of the illustrations.
Footnotes
Financial Disclosures: Dr. Albuquerque received consultant fees from Boston Scientific, Cordis, Micrus and honoraria from Boston Scientific, Cordis. Dr. Hanel received research funding from Boston Scientific, Cordis, Microvention and is a paid scientific advisor for Neurovasx. Dr. Hopkins received research grant support and consultant fees from Boston Scientific, Cordis, EndoTex, and Micrus, is a shareholder for EndoTex, and Micrus, and received honoraria from Bard, Boston Scientific, Cordis, and Medsn. Dr. Levy received research grant support from Boston Scientific, and Cordis, and honoraria from Boston Scientific, and Cordis. The remaining authors have no financial relationships to disclose. The authors received no external funding for the research discussed in this study.
References
- 1.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 2.Gobin YP, Starkman S, Duckwiler GR, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke 2004;35:2848–54 [DOI] [PubMed] [Google Scholar]
- 3.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 4.Eckert B, Koch C, Thomalla G, et al. Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion: combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): results of a multicenter study. Stroke 2005;36:1160–65 [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Vora NA, Horowitz MB, et al. Multimodal reperfusion therapy for acute ischemic stroke: factors predicting vessel recanalization. Stroke 2006;37:986–90 [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Jovin TG, Tayal A, et al. Urgent stenting of the M2 (superior) division of the middle cerebral artery after systemic thrombolysis in acute stroke. AJNR Am J Neuroradiol 2006;27:521–23 [PMC free article] [PubMed] [Google Scholar]
- 7.Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery 2006;58:458–63 [DOI] [PubMed] [Google Scholar]
- 8.Levy EI, Ecker RD, Hanel RA, et al. Acute M2 bifurcation stenting for cerebral infarction: lessons learned from the heart. Technical case report. Neurosurgery 2006;58:E588. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Siddiqui AM, Kim SH, et al. Reocclusion of recanalized arteries during intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2004;25:322–28 [PMC free article] [PubMed] [Google Scholar]
- 10.Summary of Safety and Probable Benefit: Wingspan Stent System with Gateway PTA Balloon Catheter. Available at: http://www.fda.gov/cdrh/pdf5/h050001b.pdf. Accessed February 27,2006. .
- 11.Henkes H, Miloslavski E, Lowens S, et al. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology 2005;47:222–28 [DOI] [PubMed] [Google Scholar]
- 12.Sauvageau E, Levy EI. Self-expanding stent-assisted middle cerebral artery recanalization: technical note. Neuroradiology 2006;48:405–08 [DOI] [PubMed] [Google Scholar]
- 13.Levy EI, Sauvageau E, Hanel RA, et al. Self-expanding versus balloon-mounted stent-assisted recanalization following embolic occlusion in the canine model: technical feasibility study. AJNR Am J Neuroradiol 2006;27:2069–72 [PMC free article] [PubMed] [Google Scholar]
- 14.Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med 1994;330:956–61 [DOI] [PubMed] [Google Scholar]
- 15.Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med 1997;336:1689–96 [DOI] [PubMed] [Google Scholar]
- 16.Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study [published erratum appears in Lancet 1997; 350: 744]. Lancet 1997;349:1429–35 [PubMed] [Google Scholar]
- 17.ESPRIT Investigators. Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin Therapy. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial [published erratum appears in Lancet 2001; 357: 1370]. Lancet 2000;356:2037–44 [DOI] [PubMed] [Google Scholar]
- 18.Steinhubl SR, Talley JD, Braden GA, et al. Point-of-care measured platelet inhibition correlates with a reduced risk of an adverse cardiac event after percutaneous coronary intervention: results of the GOLD (AU-Assessing Ultegra) multicenter study. Circulation 2001;103:2572–78 [DOI] [PubMed] [Google Scholar]
- 19.Walter DH, Schachinger V, Elsner M, et al. Platelet glycoprotein IIIa polymorphisms and risk of coronary stent thrombosis. Lancet 1997;350:1217–19 [DOI] [PubMed] [Google Scholar]
- 20.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 21.Higashida R, Furlan A, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol 2003;14:S493–S494 [DOI] [PubMed] [Google Scholar]
- 22.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–137 [DOI] [PubMed] [Google Scholar]
- 23.Sheehan FH, Braunwald E, Canner P, et al. The effect of intravenous thrombolytic therapy on left ventricular function: a report on tissue-type plasminogen activator and streptokinase from the Thrombolysis in Myocardial Infarction (TIMI Phase I) trial. Circulation 1987;75:817–29 [DOI] [PubMed] [Google Scholar]
- 24.Lincoff AM, Topol EJ. Illusion of reperfusion. Does anyone achieve optimal reperfusion during acute myocardial infarction? Circulation 1993;88:1361–74 [DOI] [PubMed] [Google Scholar]
- 25.Ringer AJ, Qureshi AI, Fessler RD, et al. Angioplasty of intracranial occlusion resistant to thrombolysis in acute ischemic stroke. Neurosurgery 2001;48:1282–90 [DOI] [PubMed] [Google Scholar]
- 26.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol 2006;27:1177–82 [PMC free article] [PubMed] [Google Scholar]
- 27.Levy EI, Hanel RA, Howington JU, et al. Sirolimus-eluting stents in the canine cerebral vasculature: a prospective, randomized, blinded assessment of safety and vessel response. J Neurosurg 2004;100:688–94 [DOI] [PubMed] [Google Scholar]
- 28.Levy EI, Hanel RA, Tio FO, et al. Safety and pharmacokinetics of sirolimus-eluting stents in the canine cerebral vasculature: 180-day assessment. Neurosurgery 2006;59:925–33; discussion 933–4 [DOI] [PubMed] [Google Scholar]
- 29.Smith S. for the Multi-MERCI Investigators. Results of the Multi-MERCI trial (abstract P503). Stroke 2006;37:711–12 [Google Scholar]
- 30.Fiorella D, Albuquerque FC, Deshmukh VR, et al. In-stent stenosis as a delayed complication of Neuroform stent-supported coil embolization of an incidental carotid terminus aneurysm. AJNR Am J Neuroradiol 2004;25:1764–67 [PMC free article] [PubMed] [Google Scholar]