Abstract
BACKGROUND AND PURPOSE: Intracranial solitary fibrous tumors (ISFTs) are rare mesenchymal neoplasms originating in the meninges. The aim of this study was to describe the CT, MR imaging, and angiographic features of the solitary fibrous tumor and to identify imaging characteristics.
MATERIALS AND METHODS: We retrospectively reviewed CT, MR, and angiographic findings in 6 cases of ISFT. We evaluated the size, shape, and location of the tumor; the internal content and margin of the lesion; the pattern of enhancement; and the change of the adjacent structures. Density on noncontrast CT scans, signal intensity on MR images, and angiographic features were also documented.
RESULTS: Each lesion appeared as a discrete extra-axial mass (size, 3–7 cm; mean, 5 cm). Five lesions were entirely solid, and 1 had peritumoral cyst. All 5 of the noncontrast CT scans showed hyperattenuated masses, and the tumors exhibited marked heterogeneous enhancement. No lesion contained calcification, and 2 cases showed bone invasions. On the MR images, 4 lesions showed mixed signal intensity on T2-weighted imaging. All of the lesions revealed marked heterogeneous enhancement. All of the tumors had thickening of the meninges adjacent to the tumor. Angiography showed delayed tumor blushing in all, and 3 of them had dysplastic dilation of the tumor vessels.
CONCLUSION: Although there are no pathognomonic imaging findings, some imaging features, such as the “black-and-white mixed” pattern on T2-weighted images and marked heterogeneous enhancement, might be helpful in the diagnosis of intracranial solitary fibrous tumor.
Solitary fibrous tumor (SFT), a rare mesenchymal neoplasm affecting mainly the visceral pleura, was first described as a primary spindle-cell tumor of the pleura by Klemperer and Rabin in 1931.1 After increased recognition of this lesion outside of the thorax, it has also been described in a number of head and neck locations, including the orbit, nasal cavities, paranasal sinuses, thyroid glands, parotid glands, and buccal and parapharyngeal spaces.2–9 Primary SFT involving the central nervous system (CNS) was first reported in 1996 by Carneiro et al,10 who described 7 cases of meningeal SFT that could be distinguished from fibrous meningioma on morphologic and immunohistochemical grounds. Since then, more than 60 cases of CNS SFT including the meninges and the spinal cord have been described in the pertinent literature.11
For its rarity and resemblance to other more common brain tumors, such as meningioma and hemangiopericytomas, intracranial SFT (ISFT) is often poorly recognized and remains a diagnostic challenge.12–14 Moreover, there is only limited description of imaging features of SFT affecting the intracranial region.15,16 The purpose of this study was to describe the MR imaging, CT imaging, and angiographic features of pathologically proven ISFT and to identify imaging characteristics.
Methods
Between 1995 and 2005, 6 consecutive patients with pathologically proven ISFT were treated at our institute. The patients included 4 men and 2 women who were 25–62 years old with a mean age of 50 years. We retrospectively reviewed CT (n = 5) and MR (n = 6) images obtained from these 6 patients.
CT scans were obtained using a spiral CT scanner (HiSpeed; GE Healthcare, Milwaukee, Wis). Scanning parameters were 120 kVp, 200 mA, and a 0.8-second scan time. Four patients underwent unenhanced and enhanced scans, and 1 patient only underwent unenhanced CT scanning. The enhanced scans were obtained with the injection of 150 mL of iopromide (Ultravist 300; Schering, Seoul, Korea); 100 mL was injected as a bolus before scanning, and 50 mL was infused as a drip during scanning.
The MR examinations were performed on a 1.5T MR unit (Signa Advantage Horizon; GE Medical Systems). Axial T1-weighted and T2-weighted images were obtained with the following sequences: 466–516/11–14/2 (TR/TE/NEX) for T1-weighted image; 4016–4000/96–104/1 (TR/TE/NEX) for T2-weighted image; FOV, 18–20 cm; section thickness, 5 mm with a 1-mm gap; and matrix size, 256 × 256. Contrast-enhanced T1-weighted spin-echo images were obtained in the axial, sagittal, and coronal planes after intravenous injection of 0.1 mmol/kg of gadolinium dimeglumine.
CT and MR imaging studies were reviewed by 2 neuroradiologists. Findings were recorded by consensus. We investigated CT and MR imaging characteristics, with emphasis on the location, size, internal content, and margin of the lesion; pattern of enhancement; and the change of the adjacent structures. The size of the lesion was measured at its greatest diameter. As for the internal content, the lesions were divided into solid and cystic, where the “cystic” lesions were defined as those containing typical fluid-like attenuation or signal intensity on CT or MR imaging and also showing rim-like enhancement on contrast-enhanced CT and MR imaging. Density on precontrast CT scans and signal intensity on T1- and T2-weighted MR images were also documented subjectively, as were enhancement patterns on contrast-enhanced CT and MR imaging. The pattern of enhancement was categorized as homogeneous or heterogeneous.
Digital subtraction angiography was performed in 5 patients, and preoperative embolization was performed for preoperation devascularization via the transfemoral approach with spherical particles (Embospheres, 150–250 μm, BiosphereMed, Marlborough, Mass; or BeadBlock, BioCure, Norcross, Ga).
Results
The patients’ most common presenting symptom was headache. Five patients had long-standing nonspecific headache (2 months to 1 year), 1 patient had left-side weakness, and 1 patient had memory disturbances over 2 years. Preoperative imaging diagnosis was meningioma for all of the lesions.
All of the lesions appeared as a discrete extra-axial mass on CT and MR imaging. Four lesions were thought to be arising from the tentorium. Of these, 2 lesions were located in the temporal lobe above the tentorium, and 2 were in the cerebellum beneath the tentorium. One lesion was in the anterior temporal lobe and attached to the ipsilateral clinoid process. This mass was also partially attached to the tentorium. One lesion was located in the parietal convexity. The lesion size ranged from 3 cm to 7 cm in the greatest diameter, with a mean of 5 cm. Five lesions were solid, and 1 had a partly cystic portion, or peritumoral cyst. The margins of all of the masses were well defined with a lobulating contour.
On precontrast CT scans (n = 5), the attenuation of all of the lesions was heterogeneous hyperattenuated compared with the adjacent brain parenchyma (Fig 1). Four patients underwent contrast-enhanced study, and the tumor exhibited marked heterogeneous enhancement (Fig 1). No lesion contained calcification, and 2 lesions showed bone infiltrations at the time of the diagnosis.
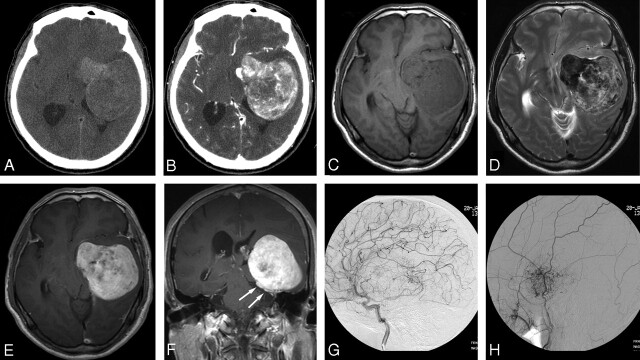
Fig 1.
A 62-year-old man with headache and memory disturbance for 2 years. A, Noncontrast CT shows a heterogenous hyperattenuated multilobulated tumor in left middle cranial fossa. B, Contrast-enhanced CT, intense but inhomogeneous contrast enhancement is noted. C, T1-weighted axial MR image, a large lobulated mass is seen in the left paraclinoid portion to the tentorium. D, T2-weighted axial MR image reveals 2 different signal intensity portions of the mass, hyposignal intensity and hypersignal intensity to gray matter. E and F, Gadolinium-enhanced T1-weighted axial and coronal MR images show marked and heterogenous enhancement. The tumor is partially implanted on the surface of the tentorium (arrows). Memory disturbance might be because of the mass effect on the limbic system. G, Selective injection of the left internal carotid artery (capillary phase); the tumor is supplied at its periphery by pial branches. H, Selective injection of the left external carotid artery; there is tumor blushing with dysplastic dilation of the tumor vessels. There is no demonstrable significant arteriovenous shunt or early venous drainage.
On MR imaging, 4 lesions were composed of 2 portions with different signal intensity: a portion of low or isosignal intensity on T1-weighted images and high signal intensity on T2-weighted images and a portion of high or isosignal intensity on T1-weighted images and low signal intensity on T2-weighted images (Fig 1). Another lesion with cysts had a solid portion that had high signal intensity on T1-weighted images and low signal intensity on T2-weighted images. The cystic portion showed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The other lesion of homogenous low signal intensity on T1-weighted images showed 2 portions of high and low signal intensity on T2-weighted images (Fig 2).
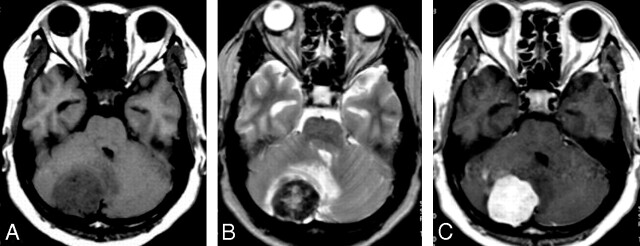
Fig 2.
A 52-year-old man with headache and dizziness for 6 months. A, T1-weighted axial MR image, a homogeneous low signal intensity mass is noted in the right cerebellum. B, T2-weighted axial MR image shows 2 different signal intensity portions of the mass: the peripheral hyposignal intensity and the central hypersignal intensity to gray matter. Mass effect on the fourth ventricle is also noted due to peritumoral edema. C, Gadolinium-enhanced T1-weighted axial MR image shows marked homogenous enhancement.
All of the solid portions of the 6 lesions revealed marked heterogeneous (n = 5) or homogeneous enhancement (n = 1), whereas the cystic portion demonstrated peripheral rim enhancement. All of the tumors showed thickening of the adjacent meninges (a dural “tail”) or tentorium, and there was 1 case with involvement of the superior sagittal sinus.
Digital subtraction angiography was performed in 5 patients, and preoperative tumor embolization was performed in 3 of these patients. Angiography showed prominent tumor staining with dilated vessels. One lesion had a pial feeder (temporal branch of posterior cerebral artery), 1 lesion had an external carotid artery feeder (superficial temporal artery), and 3 lesions had a dual supply of internal carotid artery and external carotid artery, mainly external carotid artery feeders with some pial supply. All of the lesions showed delayed tumor blushing, and 3 of them had dysplastic dilation of the tumor vessels (Fig 1). However, there was no significant arteriovenous shunt or early venous drainage.
All of the patients underwent surgery, and 3 of them were treated with radiation therapy. In 4 patients, the outcome was favorable, with no recurrence during follow-up, which ranged from 34 to 45 months after surgery. In 2 patients, there was no evidence of tumor recurrence at 9 months and 14 months after surgery.
Discussion
SFTs are rare spindle-cell neoplasms of mesenchymal origin that most frequently arise in the pleural cavity.17 There has been debate over the histogenesis of the SFT as to whether the origin is a mesothelial or mesenchymal cell, but recent immunohistochemical and electron microscopic studies have suggested that they originate from mesenchymal fibroblast-like cells.17–20 Extrapleural SFTs might also support the mesenchymal origin of the tumors. SFTs can mimic other benign or malignant spindle cell tumors, rendering histologic diagnosis difficult.18 This is especially true when SFT occurs in the meninges, which is an unusual site, not only because of its rarity, but also because of the similarity of imaging features to other extra-axial brain tumors of mesenchymal origin. The main differential diagnosis of ISFT includes fibrous meningioma and hemangiopericytoma; it may also mimic schwannoma as a cerebellopontine angle tumor. The natural history of ISFT is not completely defined, because the period of follow-up of the reported cases was short or not specified in most cases.12,21 However, SFTs of the CNS must be differentiated from anaplastic meningioma, hemangiopericytoma, and fibrosarcoma, because they all seem to have a more aggressive course than SFTs.22
The most common presenting symptom was headache (n = 5) in our patients. Symptoms were related to tumor location, which was similar to that of meningioma. Other presenting symptoms were motor weakness (n = 1) and memory disturbances because of the mass effect of the tumor in parietal convexity and paraclinoid portion, respectively. Although any age group can be affected (range, 11–73 years), these tumors usually occur in adults with a mean age of 47.6 years and are equally common in men and women.11 In our study, the age range of the patients at diagnosis was 50 years (range, 25–62 years), and the incidence was higher in men than in women with a sex ratio of 4:2. These demographic discrepancies between the studies were probably caused by a selection bias because of the small number of cases. Meningeal SFTs had a tendency to arise in the posterior fossa and spine.11 However, the supratentorial location was more frequent (4 of 6 cases) than the infratentorial location in our study. Five SFTs (83%) in our 6 patients based to the tentorium broadly or partially. Rarely, parenchymal locations have been described in the brain and spinal cord,21 as well as in intraventricular SFTs.23,24
Macroscopically, the SFT is a well-circumscribed neoplasm usually in contact with the meninges. Microscopically, the SFT is known to have collagenous fibrotic hypocellular areas alternating with others of more dense hypercellularity.10,25 Histopathologically, the SFT may appear very similar, in particular on hematoxylin-eosin stains, to other spindle cell neoplasms, including fibrous meningioma. This is because of the morphology and disposition of the cells and collagen bundles. Because the morphologic features may not be sufficient for making a differential diagnosis in the setting of the meningeal mass, correct characterization of the neoplasm relies mainly on the immunohistochemical profile. CD34, an antigen also present in hematopoietic tissues and endotheliums, shows a diffuse and strong positivity in the SFT in 80%–100% of cases.25 The SFT also shows a positive reaction for vimentin and bcl-2, whereas EMA and S-100 are negative.10,12,14 Although hemangiopericytoma also shows a positive reaction with vimentin and sometimes CD34, the reactivity is mild and patchy for the latter, related in most cases to an endothelial-positive reaction. Fibrous meningioma is usually positive for EMA (80%) and negative (or mildly positive) for CD34.10
In our study, 5 (83%) of 6 SFTs had 2 different portions with low and high signal intensities on T2-weighted imaging. The SFT has been known to be indistinguishable from other meningeal tumors in imaging studies. In fact, all of our cases were diagnosed as meningiomas before they were surgically proved. The reason that we performed cerebral angiography and embolization of the tumor was that we thought the masses were meningiomas. The SFT usually appears as a lesion in contact with the meninges, isosignal intensity on T1-weighted images, and heterogenous hyposignal intensity on T2-weighted images. However, some authors suggest that the embodiment of areas of low and high signal intensity on T2-weighted MR images, the so called patch or “ying-yang” appearance, is characteristic for the SFT.16,26 In our study, 4 lesions were composed of 2 portions with different signal intensities: 1 portion of high or isosignal intensity on T1-weighted images and low signal intensity on T2-weighted images and the other portion of isosignal or low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Another tumor that had homogenous low signal intensity on T1-weighted images also showed 2 separate areas appearing with low signal intensity and high signal intensity on T2-weighted images. This feature on T2-weighted images has the appearance of a “black-and-white” mixed pattern or ying-yang pattern. Nawashiro et al15 suggested that the imaging findings are due to collagenous hypocellular regions and hypercellular regions with “staghorn” vascular pattern capillaries, which, respectively, correspond with the area of low signal intensity and the area of high signal intensity on T2-weighted images. Most solid portions of the lesions showed heterogeneous strong enhancement. Indeed, although the appearance of the tumors on MR imaging scans in our patients was initially indistinguishable from that of a meningioma, mixed signal intensity of the mass on T2-weighted images and heterogeneous enhancement patterns might be a characteristic finding for SFT.
Most SFTs show capillary tumor blush with irregular dysplastic feeding vessels originating from a main trunk. Late arterial- and capillary-phase films show a prominent, homogeneous prolonged vascular blush without arteriovenous shunt or early venous drainage. Except in 1 case that had pial supply only, all of the tumors had external carotid artery branches, and 3 of them had dual supply from the internal carotid artery and the external carotid artery in our study. Because the SFTs are also dural based like most meningiomas, they initially appear to be supplied entirely by meningeal vessels, such as the middle meningeal and occipital arteries; then, large tumors can parasitize adjacent pial branches. Thus, these angiographic findings of the SFT exhibit similar behavior to meningiomas in this sense. In most meningiomas, the central portion is supplied by meningeal vessels, whereas the periphery is vascularized by the anterior, middle, or posterior cerebral arteries.27 Discrimination of SFT from hemangiopericytoma is especially relevant, because it carries significant therapeutic and prognostic implications. It has been reported that the major feeding vessels of hemangiopericytomas are the internal carotid artery branches and posterior cerebral arteries rather than the external carotid artery branches, in contrast to meningiomas, where the major supply is from meningeal arteries that supply the center of the tumor.28 However, it may be difficult to diagnose SFTs from meningiomas or hemangiopericytomas on angiographic findings alone.
Conclusions
The SFT is a rare tumor occurring in the meninges, but SFTs should be included in the differential diagnosis of tumors arising from the meninges. Although there are no pathognomonic imaging findings for SFTs, some imaging features such as the black-and-white mixed pattern on T2-weighted images and marked heterogeneous enhancement might be helpful in the diagnosis of ISFT. Further clinical and pathologic studies are needed to clarify the natural history of this tumor.
References
- 1.Klemperer P, Rabin CB. Primary neoplasms of the pleura: a report of five cases. Arch Pathol 1931;11:385–412 [DOI] [PubMed] [Google Scholar]
- 2.Goodlad JR, Fletcher CD. Solitary fibrous tumor arising at unusual sites: analysis of a series. Histopathology 1991;19:515–22 [DOI] [PubMed] [Google Scholar]
- 3.Dorfman DM, To K, Dickersin GR, et al. Solitary fibrous tumor of the orbit. Am J Surg Pathol 1994;18:281–87 [DOI] [PubMed] [Google Scholar]
- 4.Kim TA, Brunberg JA, Pearson JP, et al. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol 1996;17:1767–72 [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler A, Lapinsky J, Berenholz L, et al. Solitary fibrous tumor of the nasal cavity. Otolaryngol Head Neck Surg 1999;121:826–28 [DOI] [PubMed] [Google Scholar]
- 6.Cameselle-Teijeiro J, Varela-Duran J, Fonseca E, et al. Solitary fibrous tumor of the thyroid. Am J Clin Pathol 1994;101:535–38 [DOI] [PubMed] [Google Scholar]
- 7.Sato J, Asakura K, Yokoyama Y, et al. Solitary fibrous tumor of the parotid gland extending to the parapharyngeal space. Eur Arch Otorhinolaryngol 1998;255:18–21 [DOI] [PubMed] [Google Scholar]
- 8.Shin JH, Sung IY, Suh JH, et al. Solitary fibrous tumor in the buccal space: MR findings with pathologic correlation. AJNR Am J Neuroradiol 2001;22:1890–92 [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong AK, Lee HK, Kim SY, et al. Solitary fibrous tumor of the parapharyngeal space: MR imaging findings. AJNR Am J Neuroradiol 2002;23:473–75 [PMC free article] [PubMed] [Google Scholar]
- 10.Carneiro SS, Scheithauer BW, Nascimento AG, et al. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol 1996;106:217–24 [DOI] [PubMed] [Google Scholar]
- 11.Caroli E, Salvati M, Orlando ER, et al. Solitary fibrous tumors of the meninges: report of four cases and literature review. Neurosurg Rev 2004;27:246–51 [DOI] [PubMed] [Google Scholar]
- 12.Martin AJ, Fisher C, Igbaseimokumo U, et al. Solitary fibrous tumours of the meninges: case series and literature review. J Neurooncol 2001;54:57–69 [DOI] [PubMed] [Google Scholar]
- 13.Perez-Nunez A, Rivas JJ, Ricoy JR, et al. Solitary fibrous tumor of the tentorium cerebelli. Case report. J Neurosurg Sci 2004;48:59–62 [PubMed] [Google Scholar]
- 14.Nikas DC, De Girolami U, Folkerth RD, et al. Parasagittal solitary fibrous tumor of the meninges. Case report and review of the literature. Acta Neurochir (Wien) 1999;141:307–13 [DOI] [PubMed] [Google Scholar]
- 15.Nawashiro H, Nagakawa S, Osada H, et al. Solitary fibrous tumor of the meninges in the posterior cranial fossa: magnetic resonance imaging and histological correlation—case report. Neurol Med Chir (Tokyo) 2000;40:432–34 [DOI] [PubMed] [Google Scholar]
- 16.Kim KA, Gonzalez I, McComb JG, et al. Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 2004;54:1004–09 [DOI] [PubMed] [Google Scholar]
- 17.Briselli M, Mark EJ, Dickersin GR. Solitary fibrous tumors of the pleura: eight new cases and review of 360 cases in the literature. Cancer 1981;47:2678–89 [DOI] [PubMed] [Google Scholar]
- 18.England DM, Hochholzer L, MacCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640–58 [DOI] [PubMed] [Google Scholar]
- 19.Dervan PA, Tobin B, O’Connor M. Solitary (localized) fibrous mesothelioma: evidence against mesothelial cell origin. Histopathology 1986;10:867–75 [DOI] [PubMed] [Google Scholar]
- 20.Witkin GB, Rosai J. Solitary fibrous tumor of the mediastinum. A report of 14 cases. Am J Surg Pathol 1989;13:547–57 [DOI] [PubMed] [Google Scholar]
- 21.Tihan T, Viglione M, Rosenblum MK, et al. Solitary fibrous tumours in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med 2003;127:432–39 [DOI] [PubMed] [Google Scholar]
- 22.Pizem J, Matos B, Popovic M. Malignant intracranial solitary fibrous tumour with four recurrences over a 3-year period. Neuropathol Appl Neurobiol 2004;30:696–701 [DOI] [PubMed] [Google Scholar]
- 23.Kocak A, Cayli SR, Sarac K, et al. Intraventricular solitary fibrous tumor: an unusual tumor with radiological, ultrastructural, and immunohistochemical evaluation: case report. Neurosurgery 2004;54:213–16 [DOI] [PubMed] [Google Scholar]
- 24.Surendrababu NR, Chacko G, Daniel RT, et al. Solitary fibrous tumor of the lateral ventricle: CT appearances and pathologic correlation with follow-up. AJNR Am J Neuroradiol 2006;27:2135–36 [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JK. Solitary fibrous tumour–everywhere, and a diagnosis in vogue. Histopathology 1997;31:568–76 [DOI] [PubMed] [Google Scholar]
- 26.Tateishi U, Nishihara H, Morikawa T, et al. Solitary fibrous tumor of the pleura: MR appearance and enhancement pattern. J Comput Assist Tomogr 2002;26:174–79 [DOI] [PubMed] [Google Scholar]
- 27.Osborn AG. The external carotid artery. In: Osborn AG, eds. Introduction to Cerebral Angiography.Hagerstown, Md: Harper and Row;1980:49–86 [Google Scholar]
- 28.Marc JA, Takei Y, Schechter MM, et al. Intracranial hemangiopericytomas. Angiography, pathology and differential diagnosis. Am J Roentgenol Radium Ther Neucl Med 1975;125:823–32 [DOI] [PubMed] [Google Scholar]