Abstract
BACKGROUND AND PURPOSE: The management of intracranial pseudoaneurysms is controversial. The purpose of this study was to provide a preliminary evaluation of the clinical efficacy of a Willis covered stent specially designed for the intracranial vasculature in the management of a pseudoaneurysm of the cranial internal carotid artery (CICA).
MATERIALS AND METHODS: Eight patients with pseudoaneurysms of the CICA were treated with use of the Willis covered stent. The flexibility of the entire stent system was gauged from the resistance met when reaching the target lesion and was categorized as no resistance, no apparent resistance, or resistance that could be overcome. The apposition of the Willis stent after deployment was scored as excellent with no endoleak, good with a small endoleak, or bad with an apparent endoleak. Follow-up angiography was performed 3 to 12 months after placement of the stent, and angiographic assessments were categorized as endoleak, stenosis of the covered segment of vessel, or occlusion of parent arteries. Follow-up clinical evaluations were also performed, and outcomes were graded as full recovery, improvement, unchanged, and aggravation.
RESULTS: Endovascular treatment was technically successful in all aneurysms without procedural-related complications, and all of the stents were easily navigated to the targeted lesions in the CICA. Complete resolution of the pseudoaneurysm was observed in 6 patients immediately after the procedure, and a minimal endoleak into the aneurysm persisted in 2 patients. No morbidity or mortality and no technical adverse event occurred. A follow-up angiogram confirmed complete reconstruction of the internal carotid artery, with no recurrent aneurysmal filling and no occurrence of stenosis in the area of the stent. By the final follow-up visit, 4 patients had fully recovered, 3 had improved, and 1 patient's condition was unchanged.
CONCLUSION: On the basis of our preliminary experience, the Willis covered stent specially designed for the intracranial vasculature can manage a CICA pseudoaneurysm safely and effectively, but longer follow-up and expanded clinical trials are needed.
Pseudoaneurysm (PA) of the cranial internal carotid artery (CICA) C2-C7 segments (Bouthillier classification) is a rare but serious complication after a blunt or penetrating traumatic injury, previous dissection of the vessel, inflammation, or previous surgery.1,2 From a historical perspective, these pseudoaneurysms have been managed with anticoagulants, surgical repair, coils, bare stent placement with or without coil embolization, and a coronal covered stent. Traditional surgical repair of carotid pseudoaneurysms is often technically demanding and is associated with a high rate of morbidity and mortality. Endovascular stent grafts have been used to treat both posttraumatic and postoperative carotid pseudoaneurysms. An endovascular approach to these pseudoaneurysms limits the risk of operative damage to surrounding structures and the potential for substantial blood loss. The purpose of the present study was to evaluate the clinical efficacy of a balloon-expandable covered stent specially designed for the intracranial vasculature, (Willis covered stent; MicroPort, Shanghai, China), in the treatment of CICA pseudoaneurysms.
Materials and Methods
Patients
The Institutional Review Board at The Sixth Affiliated People's Hospital of Shanghai Jiao Tong University approved this pilot study, and we obtained informed consent from all the patients before commencement of the study. Only patients with pseudoaneurysms involving the intracranial internal carotid artery (Bouthellier C2-C7 segment) were included in this study. Eight patients with pseudoaneurysm of the CICA were treated with Willis covered stents between April 2005 and September 2006. All subjects were male in the age range of 11 to 60 years (mean age, 37 ± 16 years). Four cases were secondary to motor vehicle crashes, and the remaining 4 cases occurred after treatment of a carotid cavernous fistula (CCF) by endovascular balloon embolization. The demographics and clinical presentation data of these patients are summarized in the following Table. All 8 patients had at least 1 control angiogram performed at least 3 months after treatment. The aneurysm involved the C3 segment of the internal carotid artery (ICA) in 1 patient, C4 in 3 patients, C5 in 1 patient, C6 in 2 patients, and C7 in 1 patient.
Summary of eight patients with intracranial pseudoaneurysms treated with the Willis covered stent
| Case No./Age /Sex | Presenting Symptoms | Cause | Aneurysm Location | Aneurysm Size (mm) | Stent Size (mm) | Resistance Reaching Target Lesion | Immediate Angiography, Poststenting | Follow-Up (mos) | Follow-Up Angiography | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/48/M | Headache, diplopia, decreased visual acuity | Post-balloon occlusion of CCF | Right C3 | 10 × 8 | 4 × 10 | None | PA resolution, parent artery patency | 12 | PA resolution, parent artery patency | Improvement |
| 2/38/M | Left eye blindness | Trauma | Left C4 | 15 × 12 | 4 × 10 | No apparent resistance | PA resolution, parent artery patency | 5 | PA resolution, parent artery patency | Unchanged |
| 3/34/M | Palsy | Post-balloon occlusion of CCF | Right C4 | 15 × 12 | 5 × 13 | None | PA resolution, small CCF | 6 | PA resolution, CCF alleviation | Improvement |
| 4/50/M | Palsy | Post-balloon occlusion of CCF | Right C4 | 23 × 16 | 4 × 13 | None | PA resolution, parent artery patency | 6 | PA resolution, parent artery patency | Full recovery |
| 5/35/M | Epistaxis | Trauma | Left C5 | 22 × 18 | 4 × 10 | None | Minimal endoleak into PA, parent artery patency | 6 | Residual cavity shrinkage, parent artery patency | Full recovery |
| 6/23/M | No symptoms | Post-balloon occlusion of CCF | Left C4 | 15 × 12 | 4 × 13 | None | PA resolution, parent artery patency | 3 | PA resolution, parent artery patency | Full recovery |
| 7/60/M | Headache, Ptosis | Trauma | Left C6 | 30 × 15 | 3.5 × 13 3.5 × 10 | None | Minimal endoleak into PA, parent artery patency | 3 | Residual cavity shrinkage, parent artery patency | Improvement |
| 8/11/M | Decreased visual acuity | Trauma | Right C7 | 18 × 10 | 3.5 × 10 | None | PA resolution, parent artery patency | 3 | PA resolution, parent artery patency | Full recovery |
Note:—CCF indicates carotid cavernous fistula; PA, pseudoaneurysm.
The Willis Covered Stent
The Willis covered stent had been developed by both our institute and the MicroPort Medical Company. It is specifically designed for use in the intracranial vasculature and consists of 3 parts: a bare stent, an expandable polytetrafluoroethylene (ePTFE) membrane, and a balloon catheter. The bare stent was constructed from a strand of cobalt chromium super alloy wire, which was 0.06 mm in diameter. The wire was cut into a sinusoidal wave pattern with the use of a laser. To enhance the flexibility of the stent, multiple stent bodies were connected to the next at the 2 asymmetrical points between the crest waves. The ePTFE membrane, which was in a tubular configuration with a thickness of 30 to 50 μm, was glued along the length of the stent struts with use of organic agglomerate. To facilitate the membrane gluing along the stent, the diameter of tubular membrane is generally 0.05 mm, which is wider than that of the inflated stent. To prevent the balloon from scaling the inner wall of the stent on withdrawal, the balloon was made into 5 valvae, instead of the commonly used 3 valvae. The whole body of the stent was radiopaque under fluoroscopy to facilitate precise placement of the stent. The stent can be manufactured in any diameter from 3 to 5 mm and in any length from 7 to 15 mm. The stent was mounted on a deflated balloon catheter with an outside diameter of 3.8F (1.27 mm) (Fig 1).
Fig 1.
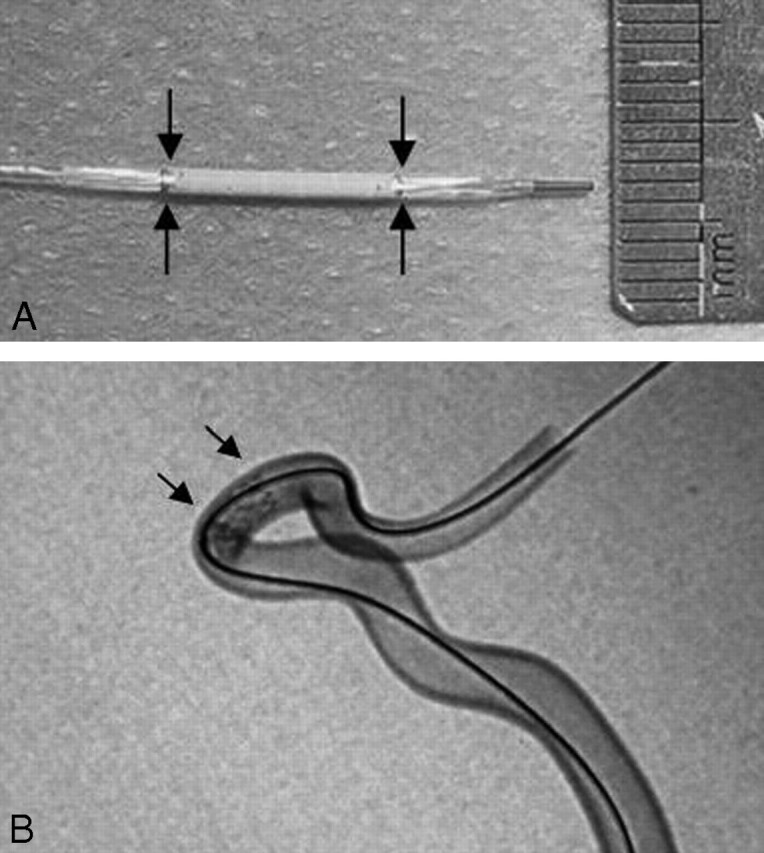
The Willis covered stent. A, The covered stent is attached to the balloon catheter, with the arrows demonstrating the 2 ends of the covered stent. The diameter of the entire system is 3.8F (1.27 mm) when it is not expanded. B, The covered stent is expanded completely against the wall of the model glass tube similar to the siphon segment of the internal carotid artery (arrows).
Endovascular Procedure
We positioned a 6F Envoy guiding catheter (Cordis, Miami Lakes, Fla) in the diseased ipsilateral ICA. After getting a good tolerance to the balloon occlusion test (BOT), we navigated a microguidewire 300 cm in length and 0.014 inch in diameter (Transcend Floppy; Boston Scientific, Natick, Mass) into a distal branch of the middle cerebral artery with the patients under general anesthesia. Using roadmap guidance, we then advanced a delivery system with the Willis covered stent over the microguidewire until the proximal and distal edges of the stent bridged the orifice of the pseudoaneurysm. Multiple control angiograms were obtained to confirm that the stent fully covered the pseudoaneurysm orifice. Under fluoroscopic control, the Willis covered stent was then inflated with 5 atm of pressure. Angiography was performed immediately after deflation of the balloon to confirm correct placement of the stent and satisfactory occlusion of the aneurysm. If we observed an endoleak, we performed redilation of the proximal edge of the covered stent by applying 5 to 6 atm of pressure. Redilation of the stent was conducted with greater pressure than in the original dilation to ensure maximum expansion of the stent, thereby improving its apposition and eliminating the endoleak. Angiography was performed immediately after the procedure, and a head CT scan was performed for evaluation of possible complications. The patients were discharged from the hospital 1 week after the procedure, and follow-up angiography was performed at least 3 months later.
Before the procedure, the patients took aspirin (100 mg/day) and clopidogrel (75 mg/day) for 3 consecutive days, and if the procedure was performed emergently, the patient took aspirin 300 mg and clopidogrel 300 mg once right before the procedure. They received a bolus of 5000 IU heparin at the start of the procedure, followed by a continuous infusion of 2000 IU/h, with the aim of keeping the activated clotting time above 300 s. Heparin was given for 48 hours after the procedure, and the patients were instructed to take aspirin (100 mg/day) and clopidogrel (75 mg/day) orally for 6 months to avoid thrombosis and stenosis in the area of the covered stent segment.
Evaluation
We assessed the flexibility of both the Willis covered stent and the delivery system of the stent by grading their ability to pass through tortuous vessels (indicating flexibility). The system received flexibility ratings of 1) no resistance with the covered stent reaching the targeted area smoothly, 2) no apparent resistance with the covered stent reaching the targeted area through adjusting the microguidewire, or 3) resistance that could be overcome through adjusting both the microguidewire and the guiding catheter for the covered stent to reach the targeted area. The apposition of the Willis covered stent to the vascular wall after deployment was categorized as 1) excellent with no endoleak, 2) good with small endoleak that caused delayed visualization and delayed evacuation of the aneurysmal cavity, or 3) bad with apparent endoleak that caused synchronized visualization of the aneurysmal cavity at immediate angiography after the deployment. Endoleak, stenosis of the covered segment of vessel, and occlusion of parent arteries were evaluated by follow-up angiography. Follow-up clinical evaluations were also performed to examine outcome, which was categorized as 1) recovery, 2) improvement, 3) invariable with no changes in the symptoms and signs after placement of the covered stent, 4) aggravation with preliminary symptoms, or 5) aggravation with symptoms secondary to occlusion of branches in the area of the CICA covered segment.
Results
Immediate Postprocedural Results
Endovascular treatment was technically successful in all aneurysms without procedural-related complications. All of the stents were easily navigated to the target lesion, with no apparent resistance, to bridge the orifice of the pseudoaneurysm. Complete resolution of the pseudoaneurysm with no endoleak (excellent) was achieved in 4 patients immediately after the procedure (Fig 2). In 4 patients, transient endoleaks into the aneurysm sac were observed immediately after the deployment of the Willis covered stent. In 2 patients, the endoleaks were easily avoided by reinflation of the balloon in the proximal ends of the stent (excellent). In the remaining 2 patients, the endoleak into the aneurysm persisted despite balloon reinflation. In one of these patients (case 7), the endoleak was reduced dramatically by application of a second Willis covered stent to appose the proximal end of the first stent. The remaining patient (case 5) exhibited a minimal endoleak at the orifice of the ophthalmic artery and was left for follow-up.
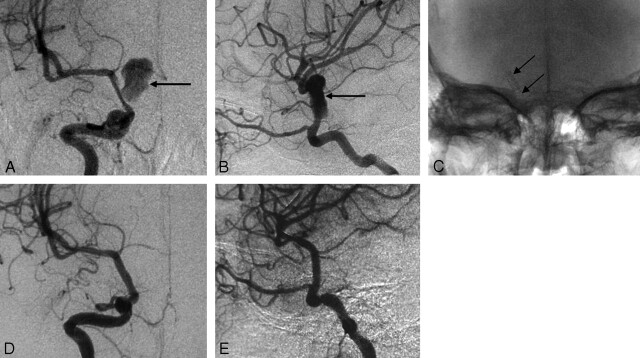
Fig 2.
Case 8, a 11-year-old boy with a pseudoaneurysm secondary to a traumatic internal carotid artery. A,B, Anteroposterior and lateral cerebral angiograms show a narrow-necked pseudoaneurysm on the right C7 segment (arrow), with the stenosis of the parent artery. C, A plain film after stent placement clearly shows the position of the covered stent (arrow). D,E, Anteroposterior and lateral cerebral angiograms show complete resolution of the aneurysm immediately after stent placement, with obliteration of the stenosis of the parent artery.
Follow-Up Angiographic Results
Control angiography performed 3 to 12 months after the procedure confirmed excellent results (complete occlusion) in 6 patients (Fig 3) as well as a good (small endoleak) result in 2 patients. All CICAs were reconstructed with no recurrent aneurysmal filling and no occurrence of a hemodynamically significant stenosis. No morbidity or mortality occurred in any of the patients during or after the treatment, including the follow-up period. No technical adverse event such as perforation of a vessel or thromboembolism occurred. In one of the 2 patients with an initial endoleak (case 7), follow-up cerebral angiography at 3 months after the procedure revealed shrinkage of the residual cavity. In the remaining patient (case 5), follow-up cerebral angiography 2 months after stent placement showed slight enlargement of the endoleak with retention of contrast medium at the orifice of the ophthalmic artery, which suggested the presence of a residual cavity. Follow-up cerebral angiography at 6 months demonstrated shrinkage of the residual cavity with patency of the parent vessel (Fig 4).
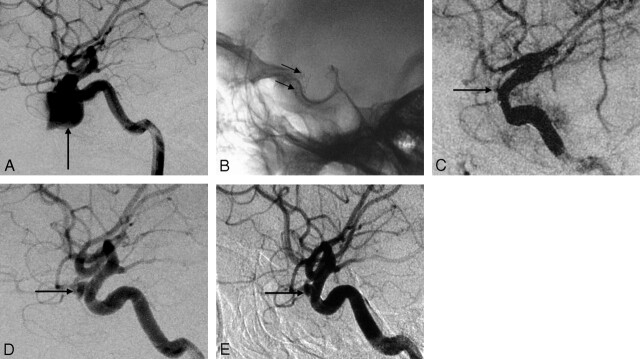
Fig 3.
Case 6, a 23-year-old man with a pseudoaneurysm secondary to post-balloon embolization of a CCF. A, Lateral cerebral angiogram reveals a wide-necked pseudoaneurysm (black arrow) on the left C4 segment, with stenosis at the proximal part of the parent artery (empty arrow). B, Plain film after stent placement clearly shows the covered stent bridging the pseudoaneurysm and the stenosis (arrows). C, Lateral cerebral angiogram shows complete resolution of the aneurysm immediately after the stent placement. D, Cerebral angiography 3 months after the procedure shows total obliteration of the aneurysm with patency of the parent artery.
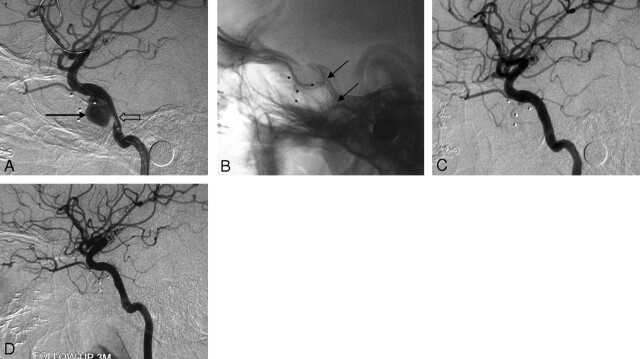
Fig 4.
Case 5, a 35-year-old man with massive epistaxis. A, Lateral cerebral angiogram shows a giant pseudoaneurysm on the left C5 segment (arrow). B, The Willis covered stent can be clearly seen in the plain film (arrows) after stent placement. C, Cerebral angiogram immediately after stent placement demonstrates a minimal endoleak into the pseudoaneurysm (arrow) in the orifice of the ophthalmic artery (arrow). D, Follow-up cerebral angiogram 2 months after the procedure demonstrates that retention of contrast medium at the orifice of the ophthalmic artery is increased (arrow), which suggests the existence of a residual cavity. E, Follow-up cerebral angiogram 6 months after the procedure demonstrates obvious shrinkage of the residual cavity (arrow) with patency of the parent artery.
Follow-Up Clinical Results
Clinical follow-up data were obtained from all 8 patients at 5.50 ± 2.98 months (range, 3–12 months) after stent placement. The patients’ symptoms resolved at variable times after stent placement, which ranged from 2 weeks to 2 months. No occurrence of ischemia was reported by any of the patients. Clinical evaluations performed at the final follow-up visit showed full recovery in 4 patients, improvement in 3 patients, and no change in 1 patient. All patients were alive at the time of this report.
Discussion
Conservative strategies for treating pseudoaneurysms in the CICA have included anticoagulation therapy as well as surgical and endovascular approaches. Anticoagulation alone is generally ineffective and may worsen the lesion.3,4 Surgical treatments for pseudoaneurysms have entailed direct clipping, wrapping, trapping, ligation of the carotid artery, and bypass for those patients in whom BOT is not well tolerated.5 Direct surgical access to a poorly endothelialized pseudoaneurysm carries a very high rate for morbidity, especially in the proximal intracranial segment of the ICA. Typically, these lesions are composed of only fibrous tissue and do not contain normal vessel wall elements. These aneurysms also lack a real neck. Therefore, the dissection and preparation of the aneurysmal sac for clipping involves an extremely high risk of perioperative rupture.6 This complication carries a poor prognosis, because it is difficult to gain proximal control without sacrificing the parent vessel, which often leads to serious ischemic consequences. Proper securing of these lesions in surgery might require special trimming of the base of the aneurysm and the use of encircling clips. The surgeon must also be ready for reconstructive or bypass surgery, and perioperative carotid angiography and balloon occlusion must be available. All of these procedures are technically demanding and require exceptional skills and experience from the entire team. Loss of patency of the parent vessel can be unintentional and can go unrecognized during surgery, as a result of either incorporation of too much vessel wall inside the clip blades or by use of an encircling clip that is too tight.7 Therefore, direct surgical repair of the artery is usually not practical.
Endovascular treatment of a pseudoaneurysm in the CICA may include either sacrifice or preservation of the carotid artery. If a BOT is well tolerated, then trapping or parent artery occlusion is an option. Several studies have indicated that the clinical outcome is better in patients who are treated by sacrifice of the carotid artery and that these patients have a lower rate of complications.8,9 However, ischemic complications including cerebral infarct after occlusion of the ICA occur in 5% to 22% of patients, despite a normal BOT.10–12 Some reports have also demonstrated formation or growth of a cerebral aneurysm after permanent occlusion of the carotid artery.13–15 If the patient cannot tolerate BOT, preserving the patency of the carotid artery as much as possible is a more desirable goal. Although coil embolization and stent-assist embolization have been used for the preservation of carotid artery patency, pseudoaneurysms are more likely to recanalize because of the lack of a true wall.16–19
In 1998, Van Nieuwenhove et al20 reported the first successful treatment of an iatrogenic intracranial pseudoaneurysm in the C4 segment with use of autologous vein-covered stents. Saatci et al21 reported successful treatment of traumatic aneurysms located at the C2-C7 segment in 17 patients with the use of Jomed coronary covered stent grafts (Jomed International, Helsingborg, Sweden). There have only been 25 reported cases of intracranial pseudoaneurysms treated with covered stents and only 4 kinds of covered stents (ie, autologous vein-covered stents, Symbiot covered stent, Jostent covered stent, and carotid Wallstent) mentioned in the literature since 1990.2,20–23 The Jostent covered stent (Abbott Vascular, Redwood City, Calif) is a composite balloon-expandable stent with an ultrathin layer of ePTFE sandwiched between 2 stainless steel stents, and this device is manually compressed over a conventional angioplasty balloon.2,24 The Symbiot covered stent (Boston Scientific, Natick, Mass) is a small, nitinol self-expandable stent that was originally designed for vascular use in coronary saphenous vein grafts.2,24 Autologous vein-covered stents were first used for the treatment of native and saphenous vein graft coronary perforation.20 The carotid Wallstent (Boston Scientific) is applied to extracranial carotid diseases. Although the Jostent, Symbiot grafts, autologous vein-covered stents, and carotid Wallstents have been used for intracranial vascular application, they can only be used for selected cases. A significant limitation to the use of these stents intracranially is their large profile and inflexibility, which makes it difficult for them to negotiate tortuous vascular segments at the skull base. Also, none of these covered stents have been specifically designed for intracranial use. Therefore, the clinical application of the currently available covered stents in the treatment of intracranial aneurysm is greatly limited because of their lack of flexibility and relatively poor apposition.
A covered stent can be placed in the parent artery to bridge the orifice of the aneurysm, which occludes blood flow into the aneurysmal lumen. This procedure has the following advantages: 1) the procedure is not performed within the aneurysmal sac, and the risk of procedural-related rupture of aneurysms or rebleeding is minimal; 2) it is relatively simple and fast; 3) the patency of the parent artery is maintained; and 4) the mass effects associated with this procedure are less severe than those associated with coil embolization. Nevertheless, delivery of stents to the tortuous CICA requires low-profile stents that are flexible, highly elastic, and have the ability to effectively adhere to the vascular wall.
The Willis stent covered with ePTEF on the abluminal side and with an outer diameter of 3.8F is specifically designed for use in the intracranial vasculature.25 It is quite flexible and easy to negotiate into the tortuous CICA. In our study, the Willis covered stents were deployed successfully with no procedure-related complications. Angiography showed good efficacy in the occlusion of the pseudoaneurysms, with no evidence of stenosis of the parent arteries either after the procedure or at early follow-up. During clinical follow-up, all of the patients were symptomatically improved with no signs of neurologic ischemia.
The present report described single-center experience with the Willis covered stent in the treatment of pseudoaneurysms of the CICA. Long-term durability of the Willis covered stents in the prevention of stroke and the long-term patency of the parent artery is not yet established. Nevertheless, the Willis covered stents might provide a viable solution for pseudoaneurysms of the CICA. They may also serve in the relevant treatment of patients at high risk for stroke and those with postsurgical traumatic aneurysm. It is important to note that poststenting stenosis, vasospasm of the cerebral arteries resulting from the balloon-expandable stent, and closure of side branches stemming from the segment of the artery where the stent graft is covered are still being observed. Therefore, a longer follow-up and meta-analysis are needed to establish the true efficacy of the Willis covered stent system in the management of pseudoaneurysms of the CICA.
In conclusion, although additional clinical trials and expanded follow-up studies are needed, our preliminary results indicate that this new intracranial covered stent is safe and effective in the management of a CICA pseudoaneurysm, and the patency of the parent artery is preserved.
Acknowledgments
The authors thank Tina Kiss and Zhi-Shing Zee, MD, from the Department of Radiology, USC University Hospital, Los Angeles, Calif, for their kindness in revising the language during preparation of the manuscript.
Footnotes
We declare that we have no conflict of interest in this work.
This study was supported by the National Natural Scientific Fund of China (contract no. 30570540), the Shanghai Important Subject Fund of Medicine (contract no. 05 III 023), and the Program for Shanghai Outstanding Medical Academic Leader (contract no. LJ 06016).
References
- 1.Biondi A. Intracranial aneurysms associated with other lesions, disorders or anatomic variations. Neuroimaging Clin N Am 2006;16:467–82, viii [DOI] [PubMed] [Google Scholar]
- 2.Alexander MJ, Smith TP, Tucci DL. Treatment of an iatrogenic petrous carotid artery pseudoaneurysm with a Symbiot covered stent: technical case report. Neurosurgery 2002;50:658–6211841739 [Google Scholar]
- 3.Fabian TC, Patton JH Jr, Croce MA, et al. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg 1996;223:513–22; discussion 522–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 1999;47:845–53 [DOI] [PubMed] [Google Scholar]
- 5.Kadyrov NA, Friedman JA, Nichols DA, et al. Endovascular treatment of an internal carotid artery pseudoaneurysm following transsphenoidal surgery. Case report. J Neurosurg 2002;96:624–27 [DOI] [PubMed] [Google Scholar]
- 6.Charbel FT, Gonzales-Portillo G, Hoffman W, et al. Distal internal carotid artery pseudoaneurysms: technique and pitfalls of surgical management. Two technical case reports. Neurosurgery 1999;45:643–48; discussion 648–49 [DOI] [PubMed] [Google Scholar]
- 7.Vanninen RL, Manninen HI, Rinne J. Intrasellar iatrogenic carotid pseudoaneurysm: endovascular treatment with a polytetrafluoroethylene-covered stent. Cardiovasc Intervent Radiol 2003;26:298–301 [DOI] [PubMed] [Google Scholar]
- 8.van der Schaaf IC, Brilstra EH, Buskens E, et al. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 2002;33:313–18 [DOI] [PubMed] [Google Scholar]
- 9.Lubicz B, Leclerc X, Gauvrit JY, et al. Giant vertebrobasilar aneurysms: endovascular treatment and long-term follow-up. Neurosurgery 2004;55:316–23; discussion 323–26 [DOI] [PubMed] [Google Scholar]
- 10.Origitano TC, al-Mefty O, Leonetti JP, et al. Vascular considerations and complications in cranial base surgery. Neurosurgery 1994;35:351–62; discussion 362–63 [DOI] [PubMed] [Google Scholar]
- 11.Eckert B, Thie A, Carvajal M, et al. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR Am J Neuroradiol 1998;19:577–82 [PMC free article] [PubMed] [Google Scholar]
- 12.Carter BS, Ogilvy CS, Putman C, et al. Selective use of extracranial-intracranial bypass as an adjunct to therapeutic internal carotid artery occlusion. Clin Neurosurg 2000;46:351–62 [PubMed] [Google Scholar]
- 13.Batjer H, Mickey B, Samson D. Enlargement and rupture of distal basilar artery aneurysm after iatrogenic carotid occlusion. Neurosurgery 1987;20:624–28 [DOI] [PubMed] [Google Scholar]
- 14.Timperman PE, Tomsick TA, Tew JM Jr, et al. Aneurysm formation after carotid occlusion. ANJR Am J Neuroradiol 1995;16:329–31 [PMC free article] [PubMed] [Google Scholar]
- 15.Li MH, Li WB, Pan YP, et al. Persistent primitive trigeminal artery associated with aneurysm: report of two cases and review of the literature. Acta Radiol 2004;45:664–68 [DOI] [PubMed] [Google Scholar]
- 16.Bush RL, Lin PH, Dodson TF, et al. Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther 2001;8:53–61 [DOI] [PubMed] [Google Scholar]
- 17.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery 2006;59:291–300; discussion 291–300 [DOI] [PubMed] [Google Scholar]
- 18.Ahn JY, Han IB, Kim TG, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol 2006;27:1514–20 [PMC free article] [PubMed] [Google Scholar]
- 19.Auyeung KM, Lui WM, Chow LC, et al. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol 2003;24:1449–52 [PMC free article] [PubMed] [Google Scholar]
- 20.Van Nieuwenhove Y, Van den Brande P, van Tussenbroek F, et al. Iatrogenic carotid artery pseudoaneurysm treated by an autologous vein-covered stent. Eur J Vasc Endovasc Surg 1998;16:262–65 [DOI] [PubMed] [Google Scholar]
- 21.Saatci I, Cekirge HS, Ozturk MH, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol 2004;25:1742–49 [PMC free article] [PubMed] [Google Scholar]
- 22.Felber S, Henkes H, Weber W, et al. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery 2004;55:631–38; discussion 638–39 [DOI] [PubMed] [Google Scholar]
- 23.Schonholz C, Krajcer Z, Carlos Parodi J, et al. Stent-graft treatment of pseudoaneurysms and arteriovenous fistulae in the carotid artery. Vascular 2006;14:123–29 [DOI] [PubMed] [Google Scholar]
- 24.Maras D, Lioupis C, Magoufis G, et al. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol 2006;29:958–68 [DOI] [PubMed] [Google Scholar]
- 25.Li MH, Gao BL, Wang YL, et al. Management of pseudoaneurysms in the intracranial segment of the internal carotid artery with covered stents specially designed for use in the intracranial vasculature: technical notes. Neuroradiology 2006;48:841–46 [DOI] [PubMed] [Google Scholar]