Abstract
The retrosplenial cortex (RSC) is extensively interconnected with the dorsal hippocampus and has several important roles in learning and memory. Recent work has demonstrated that certain types of context-dependent learning are selectively impaired when the posterior, but not the anterior, region of the RSC is damaged, suggesting that the role of the RSC in memory formation may not be uniform along its rostro-caudal axis. The current experiments tested the idea that the anterior and posterior portions of the rat RSC contribute to different aspects of memory formation. We first confirmed that brief optogenetic inhibition of either the anterior or posterior RSC resulted in decreased local cellular activity as indexed by immediate early gene zif268 expression and that this decrease was restricted to the target region within RSC. We then found that silencing the anterior or posterior RSC during trace fear training trials had different effects on memory: While inhibiting neural activity in the anterior RSC had a selective impact on behavior evoked by the auditory CS, inhibition of the posterior RSC selectively impaired memory for the context in which training was conducted. These results contribute to a growing literature that supports functionally distinct roles in learning and memory for subregions of the RSC.
Subject terms: Fear conditioning, Cortex
Introduction
The retrosplenial cortex (RSC) has several important roles in learning and episodic memory, including learning about discrete stimuli [1] and spatial environments [2, 3]. The role of the RSC in memory is well-conserved across species, ranging from rodents [4] to humans [5, 6] and non-human primates [[7], see [8], for a review]. It has been proposed that the RSC is responsible for binding together information about multiple aspects of memory during both acquisition and consolidation [9], particularly in situations in which information about the environment informs associations between a cue and an outcome (i.e., event-related information).
The RSC is well-positioned for an integrative role in memory as it shares reciprocal connections with several other structures critical for episodic memory including the prefrontal and cingulate cortices, as well as the dorsal hippocampus and thalamus [8, 10–14]. However, memory-related functions of the rat RSC do not appear to be uniform along its anterior-posterior axis. Damage to the posterior region of the RSC (pRSC) disrupts spatial learning [12], but lesions of the anterior RSC (aRSC) have no impact [15, 16]. This effect is likely driven by the extensive direct connections between the pRSC and the dorsal hippocampus [17, 18], a structure critically important for contextual memory. Conversely, the aRSC shares more connections with the anterior cingulate cortex (ACC), a region important for cue-related processing in rodents [19, 20]. Each RSC subregion therefore likely supports distinct aspects of memory.
Associative learning in rodents has increasingly been used to understand both behavioral and neurobiological mechanisms that contribute to memory [21]. In a typical delay conditioning paradigm, a conditional stimulus (CS; e.g., a tone) is paired with an unconditional stimulus (UCS; e.g., a footshock). Following several CS–UCS pairings, later presentations of the CS alone will elicit a conditioned response (e.g., freezing behavior) [22]. Throughout conditioning, necessary information is also learned about the context (the environment in which CS–UCS pairings occur) [23] that helps guide later fear responding. Complete RSC lesions result in impairments in context conditioning during both cued [24] and contextual [25–27] fear conditioning, but it is unclear how RSC subregions contribute to auditory and contextual aspects of memory.
Trace fear conditioning (TFC) provides an ideal method to study distinct aspects of episodic memory as important information is learned about both the event (the cue that predicts the shock) as well as the context in which conditioning occurs [28]. Unlike delay conditioning, in TFC a brief period of time known as the trace interval separates the CS and UCS. TFC requires activity in several brain regions that are not critical for standard delay conditioning including the dorsal hippocampus (DH) and RSC [29, 30]. Due to its interconnectivity with several brain regions needed to support TFC, the RSC is well-positioned to encode and integrate distinct aspects of event- and environment-related information [31–34]. Understanding how the brain encodes distinct aspects of memory will not only provide a deeper mechanistic understanding of memory, but will also shed light on how memory for environment and event-related stimuli contribute to maladaptive processes that underlie several neuropsychiatric disorders.
The current experiments therefore aimed to determine if a functional difference exists between the aRSC and pRSC in TFC, using temporally precise optogenetic inhibition of neural activity. We hypothesized that the RSC subregions would differentially encode event-related and context-related information. We predicted that aRSC activity would be needed to learn about the event (the stimulus) and that pRSC activity would be needed for context learning during trace fear acquisition. We also tested whether the same manipulation applied during the interval between training trials during the acquisition session, when no discrete stimuli were presented, would impact performance during memory retrieval.
Methods
Subjects
Male Long-Evans rats (300–400 g at virus surgery; Harlan, WI) were housed individually in plastic cages with chip bedding and free access to food and water, in accordance with the UWM IACUC. The room where animals were housed was maintained on a 14:10 light/dark cycle.
Surgical procedures
Solution containing AAV9-CAG-ArchT-GFP or AAV9-CAG-GFP recombinant virus (obtained from the University of North Carolina Vector Core; titer: 2 × 1012 molecules/ml) was infused at multiple sites in either the aRSC or pRSC. This virus causes expression of a light-activated proton pump in all cell types throughout the targeted region (including neurons). Rats were anesthetized with isoflurane and placed in a stereotaxic frame. Six 0.5-mm diameter holes were drilled in the skull above either the aRSC or pRSC. Coordinates for the anterior infusions were 0.5 mm lateral, 1.8 mm ventral, and 1.6, 2.6, and 3.6 mm posterior with respect to bregma. Coordinates for the posterior infusions were 1.0 mm lateral, 1.8 mm ventral, and 5.6, 6.6, and 7.6 mm posterior with respect to bregma. Using a 10-ul syringe and a 34-gauge needle (World Precisions Instruments, Sarasota, FL), 0.3 ul of either ArchT or control virus was injected at a rate of 50 nl/min. The needle was left in place for an additional 10 min to allow for diffusion away from the injector. This was repeated once at each of the six injection sites for each animal. The incision was sutured and each animal was given six weeks to allow for optimal expression of opsins.
Following the six-week recovery period, LED implants were mounted to the skull above the infusion site for each rat [as in [35]]. The skull was thinned to create a 2 mm2 translucent area centered above the infusion sites. Light penetration was limited by applying a layer of opaque black lacquer to the exposed skull surrounding the thinned window. Silicon-encased, prewired surface-mount 5050 trichip ultrabright LEDs (Green-521 nm; oznium.com) were affixed with clear superglue centered over the skull window. Encased LEDs were secured to the skull with two screws, cyanoacrylate, and dental cement. Rats were allowed 5–7 days of recovery following LED implantation prior to behavioral procedures.
Behavioral procedure
Following recovery from LED implantation, animals were placed in Med Associates (St. Albans, VT) conditioning chambers (30.5 × 24.1 × 29.2 cm) housed in individual sound attenuating chambers. Chambers were illuminated with an incandescent house light and exhaust fans provided a 65-dB background noise. A scent was presented by cleaning each chamber with ethanol immediately before the animal was placed inside. Following a six-minute baseline period, rats received 6 CS–UCS pairings. The CS was a 10-s 72 dB white noise stimulus played from a wall-mounted speaker mounted. The UCS was a 1-s / 1 mA footshock. A 20-s trace interval separated each CS and UCS. The ITI between these pairings was on average 240 s. Animals remained in the chamber for four minutes following the final footshock.
The next day, animals were given two behavioral tests (counterbalanced). Auditory trace fear retention rats was tested in a novel context (20.5 × 26.5 × 21 cm). This set of conditioning chambers in their own sound attenuating boxes were located in a separate room. These chambers were not illuminated and had smooth Plexiglas flooring. To create a distinctive scent, chambers were cleaned with a 5% acetic acid solution immediately before rats were brought to the lab. Following a two-minute baseline period, rats received eight 30-s CS presentations (60 s ITI) and remained in the chamber for one minute following the final CS presentation. Contextual fear retention was assessed by measuring freezing in the original conditioning chamber in the absence of any auditory stimuli or shocks for five minutes.
Freezing was defined as the cessation of all movement excluding respiration and was automatically scored in real-time with FreezeScan 1.0 detection software (Clever Sys, Inc.) calibrated to a trained human observer.
Light delivery
LEDs were controlled via TTL pulses from a computer running Med Associates software (Med Associates, St. Albans, VT). Rats were connected to a patch cord and placed in the chambers at the beginning of the training session. Continuous light activation (5 mW) began 1 s before each CS presentation and lasted until 1 s following the cessation of each UCS. In ITI control experiments, light exposure occurred for the same duration, but instead was presented in the period between CS presentations (the intertrial interval, ITI) and therefore not overlapping with training trials.
Histology
At the end of each experiment, rats were deeply anesthetized with an overdose of isoflurane and transcardially perfused with 0.1 M PBS followed by 10% phosphate-buffered formalin and the brain were placed in fixative overnight and were then transferred to a 30% sucrose solution (in 0.1 M phosphate buffer) for cryoprotection. For sagittal sectioning, brains were frozen and mounted on charged glasses slides. Slides were coverslipped with anti-fade UltraCruz mounting medium (Santa Cruz) and images of GFP-expressing cells were obtained with an Olympus FV1200 confocal microscope to verify the extent of virus expression.
Immunofluorescence
To verify the efficacy and specificity of the optogenetic manipulation, fifteen ArchT-expressing animals were treated the same as animals in each inactivation condition (aRSC or pRSC) but was sacrificed following conditioning to quantify immediate early gene zif268 expression associated with neural activity [36, 37]. Rats were deeply anesthetized with isoflurane 60 min following TFC with or without optogenetic inhibition of the region. Brains were immediately removed and stored at −80 °C until sliced in 20-micron coronal sections and mounted onto charged slides. Slides were rehydrated in wash buffer (PBS + 0.05% Tween-20) and permeabilized (PBS + 0.3% Triton X) for 15-min and incubated in blocking solution (PBS + 0.7% NGS). Slides were then incubated in zif268/EGR1 primary antibody (Cell Signaling, 1:500, #4153) solution (PBS + 0.3% Triton X + 5% NGS) overnight at 4 °C. The next day, slides were incubated in secondary antibody solution (Thermofisher: Alexa Fluor 594, 1:500) for 2 hours and rinsed with wash buffer, a DAPI counterstain was applied, and slides coverslipped. Images were captured on the Olympus Fluoview FV1200 confocal microscope using a 20x objective lens. Serial z-stack images covered a depth of 4.55 μm through five consecutive sections (0.91μm per section) and were acquired using Fluoview software (Olympus). Eight slides were collected to represent 1 mm of tissue along the A/P axis (e.g., −1, −2, etc.), covering the entire infusion area for both anterior and posterior groups. 12 images (6 from the left hemisphere, 6 from the right hemisphere) were taken for each millimeter for each animal (with exceptions being made for damaged tissue), giving a detailed representation of activity along the anterior/posterior axis. zif268 activity (total particle counts measured using the “Analyze Particles” plugin in ImageJ) was normalized as a proportion of total DAPI present on the same section. Representative images are depicted in Supplementary Figure 2.
Statistical analysis
All results were analyzed using either one-way or repeated measures analyses of variance (ANOVAs) or t-tests using SPSS 25 (Statistical Package for Social Sciences; IBM) software, with alpha set to .05. One rat was excluded in the pRSC ArchT condition for insufficient virus spread (see Supplementary Figure 3 for depiction of virus spread, as well as Figs. 2 and 3 for representative images).
Fig. 2. Behavioral results following optogenetic inhibition of the aRSC during TFC (Mean/SEM).
A–D Inhibition of the aRSC during the CS–UCS pairings. E–H aRSC inhibition during ITI of training. Design schematic depicted directly above figures. Green overlay in schematic indicates temporal placement of optogenetic inhibition. No group differences were demonstrated during training. UCS deliveries are marked by dashed lines (A, E). When inhibition of the aRSC occurred in the CS of acquisition but not the ITI, ArchT animals froze less during the CS during the CS test (B, F). No differences were seen when tested in the acquisition context (C, G), indicating that inhibition of the aRSC during acquisition selectively impacts CS freezing. D, H Representative images of virus expression along the sagittal plane (×4 magnification). Asterisks indicate p < 0.05.
Fig. 3. Behavioral results following optogenetic inhibition of the pRSC during TFC (Mean/SEM).
A–D aRSC inhibition during CS–UCS pairings. E–H Inhibition during ITI of training. Experimental design schematic depicted directly above figures. Green overlay in schematic indicates temporal placement of optogenetic inhibition. UCS deliveries are marked by dashed lines (A, E). No group differences were demonstrated during training (A, E) or during the CS test (B, F). Animals froze less to the acquisition context when their pRSC was inhibited during the training CS (C) but not when it was inhibited during the training ITI (G). Thus, pRSC inhibition during acquisition selectively impacts context freezing. D, H Representative images of virus expression along the sagittal plane (×4 magnification). See text for more details. Asterisks indicate p < 0.05.
Results
Learned fear responses to both the CS and the context depend on pairing the CS with a UCS during acquisition
In order to ensure that behavioral changes in the present experiments were produced by pairing the CS with the UCS, a subset of animals was conditioned as described above and compared to animals who received equivalent exposure to the context and the CS but no footshocks. All animals received tests for freezing to the conditioning context as well as CS-elicited freezing (Supplementary Figure 1). A 2 (Group: TFC, CS Only) x 33 (Minute) ANOVA conducted to assess responding throughout acquisition found a main effect of minute, F(32, 416) = 29.87, p < 0.001, hp2 = 0.70, group, F(1, 13) = 37.22, p < 0.001, hp2 = 0.74, and an interaction between the two, F(32, 416) = 6.67, p < 0.001, hp2 = 0.34, suggesting that while both groups decreased their movement throughout the session, this was more pronounced in the TFC group. A 2 (Group) x 3 (Condition: Baseline, CS, ITI) ANOVA that assessed responding during the CS test found a main effect of condition, F(2, 28) = 83.77, p < 0.001, hp2 = 0.86, group, F(1, 14) = 40.42, p < 0.001, hp2 = 0.74, and an interaction between the two, F(2, 28) = 45.06, p < 0.001, hp2 = 0.76. Planned comparisons found that while no differences were found in baseline responding, p = 0.86, CS only animals froze less during both the CS, p < 0.001, and ITI, p = 0.001. TFC resulted in a similar increase in freezing to the conditioning context relative to the CS only group, t(14) = 12.05, p < 0.001. These results suggest that increases in freezing observed during testing are reflective of learning a CS–UCS association rather than habituation to the context.
Optogenetic inhibition of either the aRSC or pRSC resulted in selective suppression of local neural activity
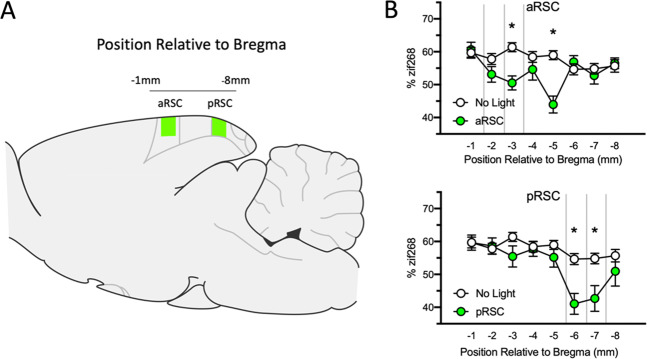
To ensure that inhibition of the aRSC or the pRSC selectively reduces activity within the targeted region without influencing activity throughout the RSC, immediate early gene (zif268) expression was measured following aRSC or pRSC inhibition. One-way ANOVAs comparing groups on each millimeter of tissue (Fig. 1) revealed significant differences at −3.00 mm posterior to bregma (F(2, 176) = 8.54, p < 0.001), −5.00 mm (F(2, 165) = 14.56, p < 0.001), −6.00 mm (F(2, 165) = 12.46, p < 0.001), and −7.00 mm posterior to bregma (F(2, 164) = 6.06, p < 0.01), but nowhere else (largest F = 1.72, p = 0.18). Bonferroni tests confirmed that at both −3.00 mm and −5.00 mm, animals that had their aRSC inhibited during acquisition showed less zif268 expression than No Light controls (ps < 0.001), and at −6.00 mm and −7.00 mm, animals that had their pRSC inhibited during conditioning showed less zif268 expression than controls (ps < 0.001 and .01, respectively). These results demonstrate that optogenetic inhibition of discrete regions of the RSC selectively decreases cellular activity within that region without significantly suppressing neural activity in adjacent regions.
Fig. 1. Optogenetic inhibition results in a selective decrease in zif268 expression.
A A sagittal view highlighting the RSC along the anterior/posterior axis. B Percent zif268 expression (expressed as a proportion of total DAPI staining; mean and SEM) across the retrosplenial cortex in animals that had their aRSC inhibited or pRSC inhibited. Asterisks indicate a significant difference from the no light control group (p < 0.05). Vertical lines represent target virus infusion sites along the A/P axis.
Inhibiting activity in the aRSC during acquisition of trace fear conditioning impairs event-related memory
We predicted that aRSC activity would be needed to encode event-related but not context-related memory. To test this, we optogenetically inhibited the aRSC during acquisition of TFC during the entire CS–UCS period (including the 20-s trace interval) on each training trial. Groups did not differ during training (Fig. 2A), confirmed by a 2 (Group: GFP, ArchT) x 33 (Minute) ANOVA conducted to assess freezing throughout the training session which found a main effect of minute, F(32, 416) = 45.46, p < 0.001, ηp2 = 0.78, but no main effect of group nor an interaction, Fs < 1.
A 2 (Group: GFP, ArchT) x 3 (Condition: Baseline, CS, and ITI) ANOVA conducted to assess freezing during the CS test (Fig. 2B) found no main effect of group, F(1, 13) = 3.10, p = 0.10. However, there was a main effect of condition, F(2, 26) = 127.12, p < 0.001, ηp2 = 0.91, and the interaction was significant, F(2, 26) = 4.32, p = 0.02, ηp2 = 0.25. Planned comparisons found that while there were no group differences during baseline, F < 1, groups differed in CS responding, F(1, 13) = 5.38, p < 0.05, ηp2 = 0.29. ITI freezing was not different between groups, F(1, 13) = 3.54, p = 0.08. An independent samples t-test conducted to assess freezing during the context test (Fig. 2C) found no differences, t(13) = 0.12, p = 0.91. Together, these results demonstrate that aRSC inhibition during the entire CS–UCS period of acquisition resulted in decreased freezing to the CS, but not to the context, despite having no impact on behavioral performance during acquisition.
In order to examine if inhibition needed to overlap with training trials, the aRSC was inhibited during the ITI of trace fear acquisition. A 2 (Group: GFP, ArchT) x 33 (Minute) ANOVA conducted to assess freezing throughout the training session (Fig. 2E) found a main effect of minute, F(32, 320) = 30.28, p < 0.001, ηp2 = 0.75, but no main effect of group nor interaction, largest F = 1.21, demonstrating that groups did not differ during this period.
A 2 (Group: GFP, ArchT) x 3 (Condition: Baseline, CS, and ITI) ANOVA conducted to assess freezing during the CS test (Fig. 2F) found no main effect of group or interaction between group and condition, Fs < 1, but a main effect of condition, F(2, 20) = 29.03, p < 0.001, ηp2 = 0.74. An independent samples t-test conducted to assess freezing during the context test (Fig. 2G) found no differences, t(10) = 0.39, p = 0.70. aRSC inhibition during the ITI had no impact on later freezing to the CS or to the context.
Inhibiting activity in the pRSC during trace fear acquisition impairs memory for the conditioning context
We next inhibited the pRSC during acquisition and predicted that this inhibition would selectively reduce later memory for the context. Again, groups did not differ during training (Fig. 3A), as confirmed by A 2 (Group: GFP, ArchT) x 33 (Minute) ANOVA that found a main effect of minute, F(32, 384) = 39.89, p < 0.001, ηp2 = 0.77, but no main effect of group or interaction, Fs < 1.
A 2 (Group: GFP, ArchT) x 3 (Condition: Baseline, CS, and ITI) ANOVA conducted to assess freezing during the CS test (Fig. 3B) found no main effect of group, F(1, 12) = 1.53, p = 0.24, and no interaction, F < 1, but there was a main effect of condition, F(2, 24) = 70.25, p < 0.001, ηp2 = 0.85, indicating that freezing increased equally for all groups throughout training. Finally, when tested for freezing to the acquisition context (Fig. 3C), animals infused with the ArchT virus froze less than animals infused with the control virus, t(12) = 5.25, p < 0.001. Together, these results demonstrate that inhibiting activity in pRSC impaired freezing to the context but not CS-elicited freezing.
We next wanted to determine whether pRSC inhibition needed to coincide with CS–UCS pairings during acquisition or if inhibition of this region at any point during this session would be sufficient to reduce later freezing to the context. To test this, the pRSC was inhibited during the ITI when discrete stimuli were not presented. A 2 (Group: GFP, ArchT) x 33 (Minute) ANOVA that assessed freezing throughout the training session (Fig. 3E) found a main effect of minute, F(32, 320) = 29.44, p < 0.001, ηp2 = 0.75, but no main effect of group nor an interaction, Fs < 1, demonstrating that groups again did not differ.
A 2 (Group: GFP, ArchT) x 3 (Condition: Baseline, CS, and ITI) ANOVA conducted to assess freezing during the CS test (Fig. 3F) found no main effect of group or interaction, largest F = 1.14, but again a main effect of condition, F(2, 20) = 32.06, p < 0.001, ηp2 = 0.76. An independent samples t-test conducted to assess freezing during the context test (Fig. 3G) found no differences, t(10) = 1.55, p = 0.15. These results indicate that there is no impact on later memory performance when pRSC activity was inhibited during the ITI between acquisition trials.
In the present dataset there was notable between-experiment variability in responding to the CS. While this between-experiment variability in responding is typical [38–41] we nonetheless wanted to ensure that between-experiment variability did not account for the pattern of results. First, we investigated if the dissociation observed between aRSC and pRSC inhibition during training trials was due to differences in variability in responding by each control group (GFP) to the CS and the context by conducting tests for homogeneity of variance. Variance was not different in freezing to the CS (F = 0.18, p = 0.68) or to the context (F = 0.19, p = 0.67). We then reanalyzed using each animal’s freezing to the CS and to the context during the test as a percentage of the control animals’ freezing for each experiment (Fig. 4). This eliminates between-experiment variability in absolute levels of freezing by placing each cohort on the same scale. When the aRSC was inhibited during the CS–UCS period during training (Fig. 4A), only freezing to the CS was affected (p = 0.037), but responding to the context was not (p = 0.91). No differences during CS (p = 0.60) or Context (p = 0.70) testing were found when the aRSC was inhibited during the ITI (Fig. 4B). When the pRSC was inhibited during the CS–UCS period of acquisition (Fig. 4C), freezing to the CS was unaffected (p = 0.23) but context freezing was reduced (p < 0.001). As before, pRSC inhibition during the ITI (Fig. 4D) had no impact on later freezing to the CS (p = 0.61) or context (p = 0.15). Thus, the pattern of results was maintained when accounting for between-experiment variability in responding.
Fig. 4. The main results from Figs. 2 and 3 were reanalyzed to express each animal’s responding as a percentage of the control animals responding in order to account for any between-experiment variability.
This analysis confirmed the results suggested by the raw data presented in Figs. 2 and 3. Inhibition of the aRSC during training trials reduced later freezing to the CS while leaving freezing to the context unaffected (A), but inhibition during the ITI had no impact (B). When the pRSC was inhibited during training trials (C), later freezing to the CS was not impacted but freezing to the training context was reduced. Inhibition of activity during the ITI did not impact later performance did not impact freezing to either the CS or the Context (D).
Discussion
We first demonstrated that optogenetic inhibition of either the aRSC or pRSC resulted in reduced activity in the targeted region without impacting activity throughout the RSC. We then found that aRSC inhibition during CS–UCS pairings in a trace conditioning paradigm impaired event-related memory, but context memory remained intact. When the pRSC was inhibited during CS–UCS pairings event-related memory was unaffected, but responding to the context was impaired. Importantly, inhibition of the aRSC or pRSC for the same duration during the ITI had no impact on responding to either the CS or the context, suggesting that activity in these regions specifically during CS–UCS pairings is crucial to distinct aspects of memory formation. Inhibition in these regions during the acquisition session is not sufficient to produce the observed effects; it instead needs to be applied to correspond with learning trials.
The current experiments add further support to findings suggesting separate roles for the aRSC and pRSC in encoding of event- and context-related memory [1, 12]. One likely explanation as to why the pRSC is preferentially involved in contextual learning is that it is highly interconnected with the DH, a brain region heavily involved in context-dependent learning and memory. The aRSC shares preferential connections with the ACC, a region critically important in CS-related memory [19, 20] but not context memory [42, 43].
Activity in the RSC is also important in retrieval of a trace fear memory [e.g., 30, 44]. While the present findings clearly demonstrate dissociable roles for the aRSC and pRSC during encoding of TFC, it has yet to be determined if a similar dissociation exists during memory retrieval. From the current results, there are two clear hypotheses as to how RSC subregions might contribute to retrieval of trace fear memory. The first is that a similar dissociation will exist at retrieval, with the aRSC being needed for recall of CS-related memory and pRSC being needed for recall of context-related memory. However, given the proposed role for the RSC in information integration, the RSC might more uniformly retrieve “what” (event-related) and “where” (context-related) information and inhibiting either aRSC or pRSC activity during retrieval would produce memory deficits.
Some seemingly disparate findings do exist. Pre-training infusions of the protein synthesis inhibitor anisomycin into the aRSC weakened memory for the CS following trace fear conditioning and context memory following both delay and trace fear conditioning [45]. Related experiments have demonstrated that aRSC activity following acquisition is important for object memory, both when contextual information is needed and when it is not [1]. This might suggest that activity in the aRSC during CS–UCS encoding is not needed to learn context fear, but protein synthesis in this region following TFC is important, supporting the view that the RSC is a critical hub of information binding during memory consolidation [9]. A similar information-binding role has also been attributed to the DH [46]. Future work will need to determine the distinct role of the RSC subregions in this process.
Given the importance of contextual processing in memory encoding and maintenance, understanding neural processes that encode precise contextual information associated with fear conditioning will aid our understanding the neural and molecular contributions to this phenomenon. The current findings suggest that the RSC may be an important target for treating behavioral disorders that stem from disrupted contextual processing and dysfunction in this basic memory process has far-reaching implications for several neuropsychiatric disorders.
Funding and disclosures
This work was supported by NIH grants MH069558 (FJH) and F32MH120938 (ST). The authors declare no competing interests.
Supplementary information
Author contributions
Experiments were designed by ST, SEP, and FJH. Data were collected by ST, SEP, and NCF. Data were analyzed by ST. Manuscript was written by ST and FJH with input from SEP and NCF.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-00959-x.
References
- 1.de Landeta AB, Pereyra M, Medina JH, Katche C. Anterior retrosplenial cortex is required for long-term object recognition memory. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-60937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cain DP, Humpartzoomian R, Boon F. Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behav Brain Res. 2006;170:316–25. doi: 10.1016/j.bbr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Harker KT, Whishaw IQ. Impaired spatial performance in rats with retrosplenial lesions: Importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci. 2002;22:1155–64. doi: 10.1523/JNEUROSCI.22-03-01155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 2002;116:85–94. doi: 10.1037/0735-7044.116.1.85. [DOI] [PubMed] [Google Scholar]
- 5.Ino T, Doi T, Hirose S, Kimura T, Ito J, Fukuyama H. Directional disorientation following left retrosplenial hemorrhage: a case report with fMRI studies. Cortex. 2007;43:248–54. doi: 10.1016/S0010-9452(08)70479-9. [DOI] [PubMed] [Google Scholar]
- 6.Maguire E. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42:225–38. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- 7.Buckley MJ, Mitchell AS. Retrosplenial cortical contributions to anterograde and retrograde memory in the monkey. Cereb Cortex. 2016;26:2905–18. doi: 10.1093/cercor/bhw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 9.Todd TP, Bucci DJ. Retrosplenial cortex and long-term memory: molecules to behavior. Neural Plast. 2015. 10.1155/2015/414173. [DOI] [PMC free article] [PubMed]
- 10.Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. 10.1002/(SICI)1096-9861(19980824)398:23.0.CO;2-Y. [DOI] [PubMed]
- 11.Shibata H, Kondo S, Naito J. Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci Res. 2004;49:1–11. doi: 10.1016/j.neures.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Vann SD, Wilton LK, Muir JL, Aggleton JP. Testing the importance of the caudal retrosplenial cortex for spatial memory in rats. Behavioural Brain Res. 2003;140:107–18. doi: 10.1016/S0166-4328(02)00274-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Nie B, Duan S, Zhu H, Liu H, Shan B. Functionally brain network connected to the retrosplenial cortex of rats revealed by 7T fMRI. PloS one. 2016;11:e0146535. doi: 10.1371/journal.pone.0146535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamawaki N, Corcoran KA, Guedea AL, Shepherd GM, Radulovic J. Differential contributions of glutamatergic hippocampal→ retrosplenial cortical projections to the formation and persistence of context memories. Cereb Cortex. 2018;29:2728–36. doi: 10.1093/cercor/bhy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neave N, Lloyd S, Sahgal A, Aggleton JP. Lack of effect of lesions in the anterior cingulate cortex and retrosplenial cortex on certain tests of spatial memory in the rat. Behavioural Brain Res. 1994;65:89–101. doi: 10.1016/0166-4328(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 16.Vann SD, Aggleton JP. Testing the importance of the retrosplenial guidance system: effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behavioural Brain Res. 2004;155:97–108. doi: 10.1016/j.bbr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Opalka AN, Wang DV. Hippocampal efferents to retrosplenial cortex and lateral septum are required for memory acquisition. Learn Mem. 2020;27:310–8. doi: 10.1101/lm.051797.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugar J, Witter MP, van Strien N, Cappaert N. The retrosplenial cortex: Intrinsic connectivity and connections with the (para) hippocampal region in the rat. An interactive connectome. Front Neuroinformatics. 2011;5:7–20. doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci. 2003;100:13087–92. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain. 2005;1:1744–8069. doi: 10.1186/1744-8069-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jasnow AM, Cullen PK, Riccio DC. Remembering another aspect of forgetting. Front Psychol. 2012;3:175–83. doi: 10.3389/fpsyg.2012.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fanselow MS. Conditional and unconditional components of post-shock freezing. Pavlovian J Biol Sci. 1980;15:177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 23.Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–37. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 2008;122:1070–7. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- 25.Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 2008;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Robinson S, Poorman CE, Marder TJ, Bucci DJ. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. J Neurosci. 2012;32:12076–86. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd TP, DeAngeli NE, Jiang MY, Bucci DJ. Retrograde amnesia of contextual fear conditioning: Evidence for retrosplenial cortex involvement in configural processing. Behav Neurosci. 2017;131:46. doi: 10.1037/bne0000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marlin NA. Contextual associations in trace conditioning. Anim Learn Behav. 1981;9:519–23. doi: 10.3758/BF03209784. [DOI] [Google Scholar]
- 29.Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- 30.Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, Helmstetter FJ. Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiol Learn Mem. 2014;113:41–54. doi: 10.1016/j.nlm.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corcoran KA, Yamawaki N, Leaderbrand K, Radulovic J. Role of retrosplenial cortex in processing stress-related context memories. Behav Neurosci. 2018;132:388–95. doi: 10.1037/bne0000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowansage KK. Experience, memory, and the places they meet. Behav Neurosci. 2018;132:409. doi: 10.1037/bne0000280. [DOI] [PubMed] [Google Scholar]
- 33.Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behav Neurosci. 1983;97:675–96. doi: 10.1037/0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- 34.Robinson S, Adelman JS, Mogul AS, Ihle PC, Davino GM. Putting fear in context: elucidating the role of the retrosplenial cortex in context discrimination in rats. Neurobiol Learn Mem. 2018;148:50–9. doi: 10.1016/j.nlm.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–41. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrara, NC, Jarome, TJ, Cullen, PK, Orsi, SA, Kwapis, JL, Trask, S, et al. (2019). GluR2 endocytosis-dependent protein degradation in the amygdala mediates memory updating. Sci Rep. 2019; 9. 10.1038/s41598-019-41526-1. [DOI] [PMC free article] [PubMed]
- 37.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol Learn Mem. 2012;97:452–64. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YJ, Chiou RJ, Chang CH. The reuniens and rhomboid nuclei are required for acquisition of Pavlovian trace fear conditioning in rats. Eneuro. 2020;7:0106-20. doi: 10.1523/ENEURO.0106-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLOS One. 2011;6:e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem. 2007;87:464–75. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Cullen PK, Gilman TL, Winiecki P, Riccio DC, Jasnow AM. Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiol Learn Mem. 2015;124:19–27. doi: 10.1016/j.nlm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–3. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 44.Todd TP, Mehlman ML, Keene CS, DeAngeli NE, Bucci DJ. Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learn Mem. 2016;23:278–88. doi: 10.1101/lm.041822.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ. The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiol Learn Mem. 2015;123:110–6. doi: 10.1016/j.nlm.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai D, Aharoni D, Shuman T, Silva AJ. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–8. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.