Highlights
-
•
Probiotics and synbiotics reduced the risk of diarrhoea after pelvic radiotherapy.
-
•
Polyphenols, but not glutamine, may reduce the risk of diarrhoea.
-
•
Biotic supplements reduced the need for anti-diarrhoeal medication.
-
•
Large clinical trials are needed of biotics with modern radiotherapy techniques.
Keywords: Dietary supplements, Meta-analysis, Pelvic radiotherapy, Biotics, Gastrointestinal toxicity, Systematic review
Abstract
Background and purpose
Pelvic radiotherapy (RT) often results in gastrointestinal toxicity and clinical trials have demonstrated a potential benefit of dietary supplements in alleviating acute effects. However, no prophylactic agents have been approved to date for relief of gastrointestinal side-effects caused by pelvic radiation. In this systematic review, we evaluated the efficacy of dietary supplements in preventing or alleviating symptoms of gastrointestinal toxicity in patients undergoing pelvic RT.
Materials and methods
CENTRAL, MEDLINE, EMBASE, and ClinicalTrials.gov were searched up to June 2020 for randomised controlled trials. Interventions included four supplement categories: biotics, glutamine, poly-unsaturated fatty acids and polyphenols. Efficacy was determined with reference to outcomes based on symptoms of acute gastrointestinal toxicity, including diarrhoea, nausea, vomiting, flatulence/bloating, bowel movement frequency, tenesmus and rectal bleeding.
Results
Twenty-three randomised controlled trials (1919 patients) were identified in this review. Compared with placebo, probiotics (RR = 0.71; 95% CI: 0.52 to 0.99), synbiotics (RR = 0.45; 95% CI: 0.28 to 0.73) and polyphenols (RR = 0.30; 95% CI: 0.13 to 0.70) were significantly associated with a lower risk of diarrhoea. Biotic supplements also reduced the risk of moderate to severe diarrhoea (RR = 0.49; 95% CI: 0.36 to 0.67) and the need for anti-diarrhoeal medication (RR = 0.64; 95%CI: 0.44 to 0.92). In contrast, glutamine had no effect on acute symptoms (RR = 1.05; 95% CI: 0.86 to 1.29). There was a non-significant trend for reduction in nausea and mean bowel movements per day using dietary supplements.
Conclusions
Biotic supplements, especially probiotics and synbiotics, reduce acute symptoms of gastrointestinal toxicity in patients undergoing pelvic radiotherapy.
1. Introduction
Radiotherapy is a major cancer treatment modality, used to treat approximately 50% of patients [1]. Over 200,000 patients in the US are treated with pelvic or abdominal radiotherapy each year [2]. It is inevitable that normal gastrointestinal tissues are exposed to radiation during pelvic radiotherapy [3], with approximately 80% of patients developing acute symptoms of radiation-induced gastrointestinal toxicity [4]. However, despite their impact on patients’ quality of life, no prophylactic agents for the alleviation of gastrointestinal side-effects from pelvic radiation have been approved to date [5].
Acute symptoms usually develop during or immediately after RT, and typically improve within three months following RT [6]. The most common acute side effect is diarrhoea, affecting up to 80% of all patients [7]. Other symptoms, such as abnormal stool output, vomiting, rectal bleeding, tenesmus and gastrointestinal discomfort are also common. Late symptoms include GI bleeding, fistula, stricture and colostomy [8].
Use of a dietary supplement is aimed at boosting daily intake of specific nutrients, to much higher levels than obtained from the diet, to alleviate symptoms of gastrointestinal toxicity. Such dietary supplements include biotics, glutamine, poly-unsaturated fatty acids (PUFAs) and polyphenols. Probiotics, mainly of the Lactobacillus and Bifidobacteria genera, are live microorganisms thought to produce health benefits following passage to the intestine [9]. Prebiotics are soluble or non-soluble dietary fibres, that pass undigested through the upper gastrointestinal tract and are metabolised by bacteria in the colon, thus altering gut microbiota beneficial to the host’s health [10]. The use of synbiotics refers to administration of a combination of prebiotics and probiotics; the presence of the prebiotic enhances survival of the probiotics in the lower gastrointestinal tract. Administration of biotics can enhance production of key metabolites, particularly SCFAs, and butyrate reduces mucosal inflammation and promotes epithelial repair following injury [11]. Glutamine, poly-unsaturated acids (PUFAs) and polyphenolic compounds have also been employed in supplement intervention strategies in pelvic RT. Anti-inflammatory effects of the omega-6 PUFA conjugated linolenic acids are seen in inflammatory bowel disease [12]. Glutamine is the most abundant amino acid with important roles in support of mucosal growth and function. It can protect the oral and intestinal mucosa from radiation damage by improving nitrogen balance and detoxifying normal host tissue [13], [14], [15]. Polyphenolic compounds extracted from plants protect tissues against oxidative stress from ROS and RNS, both of which are products of radiotherapy [16].
This review tests the hypothesis that administration of oral dietary supplements for cancer patients receiving pelvic radiotherapy may trigger changes in the lower gastrointestinal tract which lead to a reduction in gastrointestinal toxicity.
2. Material and methods
2.1. Trial registration number
The study protocol was published on the PROSPERO international prospective register of systematic reviews (registration number CRD42020183304).
2.2. Search strategy and study selection
The following electronic databases were searched from inception to the search date (19/06/2020) for relevant literature: Cochrane CENTRAL, Ovid Medline, Ovid Embase, and ClinicalTrials.gov. The search strategies included both medical subject heading and free text terms to retrieve relevant RCTs and non-randomised studies regarding gastrointestinal side effects in cancer patients undergoing pelvic radiotherapy, limited to studies in humans only. The full set of search strategies is available in Appendix A to C, and protocol details are available in the PROSPERO registration [17]. Relevant articles were identified on PubMed. Handsearching of meta-analyses, systematic reviews and papers identified studies not indexed in the electronic databases used for this review. All titles and abstracts retrieved by electronic searches were downloaded and duplications removed using EndNote reference management software.
2.3. Data extraction
Systematic data collection from included studies was conducted using a data collection form designed specifically for this review. It included the following information (where available) for each dataset: publication year, study design, participants (number, age distribution, gender distribution, details of malignancy, details relevant to inclusion and exclusion criteria), current cancer treatment, intervention and measured outcomes.
2.4. Outcome assessment
Different measures of treatment effects were used for dichotomous and continuous outcomes, namely, risk ratio (RR) for dichotomous outcomes and the mean difference between the intervention and control arms for continuous outcomes. Standardised mean difference was used to compare results from studies that reported the same outcomes measured on different scales.
2.5. Study quality, assessment of heterogeneity, publication bias and quality assessment
Risk of bias assessment was carried out for all studies that met the inclusion criteria, using the Cochrane Risk of Bias 2 tool. To assess the heterogeneity, we used a chi-squared test and I2. P values less than 0.1 were considered as evidence of heterogeneity. Tau-squared is the estimated standard deviation of underlying effects across studies. Begg’s funnel plots were used to visually assess asymmetry potentially due to publication bias. Quality assessment was conducted using GRADEpro online software [18].
2.6. Data synthesis and statistical analysis
Meta-analyses were performed to measure the effect of dietary supplements on an outcome, in instances where there were three or more studies that reported the same outcome. All analyses were conducted using RevMan 5.4 and R version 4.0.2 with package ‘meta’. For dichotomous outcomes, RR were estimated and were meta-analysed using a random effects model using the Mantel-Haenszel method. For continuous outcomes, mean differences were estimated and were pooled using a random effects model with the inverse variance method. 95% confidence intervals (CI) for all estimates were calculated. Meta-regression was used to assess whether the effects on incidence of diarrhoea varied by study characteristics.
3. Results
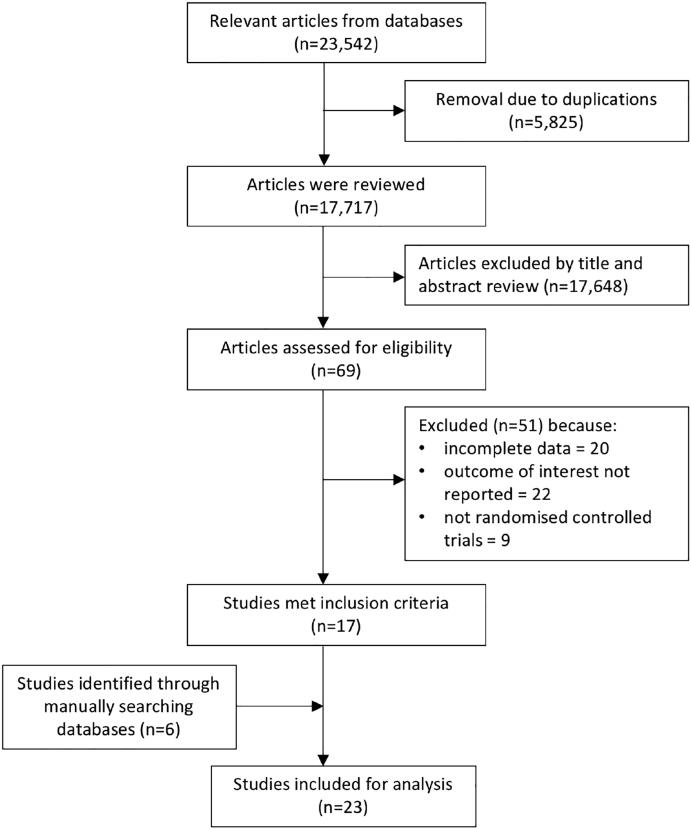
The search of the four primary databases identified 23,542 titles published between 1946 and June 2020 (search process summarised in Fig. 1). After 5825 duplications were removed, a total of 17,717 entries remained. These studies were manually reviewed by title and abstract and 17 met the inclusion criteria. Six further studies were identified from manual searches of the reference sections of research articles. Finally, 23 studies met the inclusion criteria and could be used for quantitative analysis. There was no evidence that the effects of interventions on incidence of diarrhoea varied by mean age (p = 0.552), proportion of male participants (p = 0.131), sample size (p = 0.131) or RT dose (p = 0.073) (Figure S1). Results of the overall and individual risk of bias assessments for each of the five domains are presented in Fig. 2.
Fig. 1.
PRISMA flow chart of studies evaluated in the systematic review.
Fig. 2.
Risk of bias summary for all studies that met the inclusion criteria.
3.1. Included studies and characteristics of included studies
In total 23 studies involving a total of 1919 patients met the inclusion criteria and for each outcome, they were grouped by intervention category. These studies were all randomised controlled trials and their characteristics are shown in the Table 1, Tables S1 and S2. In total, the trials included in the review reported ten different relevant symptoms, as shown in Table S3.
Table 1.
Characteristics of included studies.
| Study | Cancer type | Intervention | Sample size | Mean age (years) |
|---|---|---|---|---|
| Biotics | ||||
| Sasidharan, 2019 [47] | Cervical | Prebiotic: resistant starch | 100 | 48.0 |
| Linn, 2019 [48] | Cervical | Synbiotic: L. acidophilus, B. animalis andyoghurt | 54 | 54.8 |
| De Lorea-Rodriguez, 2018 [49] | Cervical | Synbiotic: L. acidophilus, B. lactis and inulin | 70 | 49.9 |
| Mansouri-Tehrani, 2016 [50] | Colorectal, prostate, endometrial, bladder, ovary, cervix, bone sarcoma | Synbiotic: S. thermophiles, Lactobacilli, Bifidobacter and honey | 46 | 62.0 |
| Garcia-Peris, 2016 [51] | Gynaecological - cervical, endometrial, vulval-vaginal, uterus | Prebiotic: inulin oligosaccharide and fructo-oligosaccharide | 38 | 60.3 |
| Itoh, 2015 [52] | Cervical | Prebiotic: hydrolysed rice bran | 20 | 49.3 |
| Demers, 2014 [53] | Gynaecological, rectal or prostate | Probiotic: L. acidophilus and B. longum | 148 | 61.2 |
| Chitapanarux, 2010 [54] | Cervical | Probiotic: L. acidphilus and B. bifidum | 63 | 47–52* |
| Castro, 2010 [35] | Cervical or endometrial | Probiotic: L. casei and B. breve | 40 | – |
| Giralt, 2008 [19] | Cervical or endometrial | Probiotic: L. casei, S. thermophiles and L. delbrueckii | 85 | 60.1 |
| Delia, 2007 [33] | Cervical or rectal | Probiotic: Four strains of lactobacilli, three strains of bifidobacteria and one strain of streptococcus | 482 | – |
| Urbancsek, 2001 [55] | Uterus, ovarian, prostate, rectal | Probiotic: L. rhamnosus | 205 | 59.5 |
| Murphy, 2000 [22] | Prostate, gynaecological | Prebiotic: psyllium | 60 | 64.5 |
| Salminen, 1988 [56] | Cervical, uterus | Synbiotics: L. acidophilus and lactulose | 21 | 40–75 |
| Glutamine | ||||
| Vidal-Casariego, 2014 [20] | Prostate, bladder, cervical, endometrium, rectal | Glutamine | 65 | 66.5 |
| Manir, 2014 [57] | Cervical, rectal, endometrium, prostate | Glutamine | 85 | 56.7 |
| Kucuktulu, 2012 [58] | Rectal, bladder, prostate or gynaecological, pelvic soft tissue sarcomas | Glutamine | 36 | 65.4 |
| Kozjek, 2011 [23] | Rectal cancer | Glutamine | 33 | 62.3 |
| Kozelsky, 2003 [24] | Gynaecological, rectal or prostate | Glutamine | 129 | 66.4 |
| PUFAs | ||||
| Aredes, 2019 [36] | Cervical | EPA and DHA | 42 | 44.5 |
| Faramarzi, 2017 [37] | Rectal | CLA | 26 | 60.2 |
| Polyphenols | ||||
| Emami, 2014 [59] | Pprostate, uterus, cervical, bladder, rectal and colon) | Green tea | 40 | 62.2 |
| Ahmad, 2010 [34] | Prostate | Soy isoflavones | 31 | 60–65* |
3.2. Efficacy of dietary supplements in preventing diarrhoea
The meta-analysis comprising 1625 patients showed that dietary supplements reduced the risk of diarrhoea (Fig. 3). The overall pooled analysis showed significant heterogeneity amongst the studies (I2 = 73%; P < 0.001). Meta-analyses were carried out for biotic, glutamine, poly-unsaturated fatty acid and polyphenol interventions. Although the funnel plot for this meta-analysis (Figure S2) was largely symmetrical, the distributions of subgroup studies tended to be less symmetrical, implying moderate publication bias in the references included. There was no evidence that heterogeneity was due to mean age or sex of participants or sample size of the studies.
Fig. 3.
Forest plot of effects of biotic, glutamine, PUFA and polyphenol supplements on incidence of diarrhoea.
3.2.1. Efficacy of biotics in preventing diarrhoea
Biotic interventions significantly reduced the risk of diarrhoea with a RR of 0.66 (95% CI: 0.51 to 0.86; P = 0.002) (Fig. 3). All studies, except Giralt et al[19], had a RR of less than 1, suggesting the protective role of biotics against diarrhoea. The heterogeneity, I2, among these studies was 76% (P < 0.001), so further analysis of the subclasses of probiotics and synbiotics was performed (Figure S3). The risk ratios were 0.45 (95% CI: 0.28 to 0.73) for synbiotics and 0.71 (95% CI: 0.52 to 0.99) for probiotics. Subgroup analysis was conducted by use of brachytherapy and chemotherapy (Figure S4 and S5). Patients not receiving brachytherapy (RR = 0.63; 95% CI: 0.54 to 0.73) or not receiving chemotherapy (RR = 0.62; 95% CI: 0.52 to 0.74) benefited from probiotics and synbiotics. With a smaller effect size, there was still a trend for those receiving brachytherapy (RR = 0.69; 95% CI: 0.41 to 1.15) or chemotherapy (RR = 0.72; 95% CI: 0.51 to 1.03).
3.2.2. Efficacy of glutamine in preventing diarrhoea
Glutamine interventions were not associated with risk of diarrhoea with a RR of 1.05 (95% CI = 0.86 to 1.29; P = 0.65, Fig. 3). We found that four studies had consistent results of RR which were close to 1, but only Vidal-Cassariego et al reported a high RR of 2.75. There was high heterogeneity among studies (I2 = 62%, P = 0.03) [20].
3.2.3. Efficacy of polyphenol in preventing diarrhoea
Two studies compared polyphenols and placebo among 64 patients (Fig. 3). Both showed that the intervention was associated with lower incidence of diarrhoea. The overall RR was 0.30 (95% CI = 0.13 to 0.70, P = 0.005). There was no evidence of heterogeneity between these two studies (I2 = 0%, P = 0.86).
3.3. Efficacy of dietary supplements in preventing moderate to severe diarrhoea
Efficacy of dietary supplements was assessed against moderate to severe diarrhoea, with this incidence defined as the incidence of grade 2 or higher diarrhoea, based on Common Terminology Criteria for Adverse Events (CTCAE) (older version: Common Toxicity Criteria; CTC)[21], except Murphy et al using the Murphy Diarrhoea Scale (MDS)[22] and Kozjek et al using their own criteria[23] (Fig. 4). The meta-analysis suggested that the association was mainly driven by biotic interventions for which the RR was 0.49 (95% CI: 0.36 to 0.67; P < 0.001), but not glutamine (RR = 1.05; 95% CI: 0.82 to 1.34; P = 0.70).
Fig. 4.
Forest plot of effect of dietary supplements on incidence of moderate to severe diarrhoea.
3.4. Efficacy of dietary supplements in preventing the use of anti-diarrhoeal medication
Anti-diarrhoeal medication, such as loperamide, is often employed for patients who experience diarrhoea during or after radiotherapy. Therefore, we measured the effect of dietary supplements against the incidence of anti-diarrhoeal medication use (Fig. 5), and found that biotic interventions were associated with lower risk of anti-diarrhoeal medication use in patients (RR = 0.64; 95% CI: 0.44 to 0.92; P = 0.02) and there was intermediate heterogeneity among studies (I2 = 45%; P = 0.11).
Fig. 5.
Forest plot of effect of dietary supplements on incidence of anti-diarrhoeal medication use.
3.5. Effects of dietary supplements on nausea, vomiting, flatulence/bloating, bowel movement frequency, tenesmus and blood in bowel movement
As shown in Table S4, dietary supplements tended to decrease the risk of nausea (RR = 0.74; 95% CI: 0.36 to 1.50; P = 0.40) and the mean number of bowel movements per day (mean difference = −3.88; 95% CI: −10.29 to 2.52; P = 0.23). The results also showed that the interventions had no effect on vomiting and flatulence/bloating with relative risks of 0.99 (95% CI: 0.79 to 1.25, P = 0.95) and 1.12 (95% CI: 0.59 to 2.12; P = 0.72) respectively. Only Kozelsky et al studied the outcomes of tenesmus and blood in bowel movements and found that glutamine had no effect on these symptoms [24].
4. Discussion
This review showed that dietary supplements are effective in reducing the risk of diarrhoea, experiencing moderate to severe diarrhoea and anti-diarrhoeal medication use, in the acute setting following radiotherapy. Subgroup analysis showed that biotic supplements and polyphenols were effective in reducing the risk of these outcomes, but glutamine was ineffective. Among the subclasses of biotic interventions, both probiotic and synbiotic supplements were shown to be effective in reducing the risk of diarrhoea, particularly among patients not receiving brachytherapy (p < 0.001) or chemotherapy (p < 0.001; Figure S4A and S5A). Although the fibre types of prebiotics included in this systematic review were heterogenous, the bacterial genera of probiotics and synbiotics were homogenous, as they contained Lactobacillus and Bifidobacteria only.
Several meta-analyses have been conducted regarding probiotic and synbiotic supplements over the last decade, but neither the category of prebiotics nor subgroups of patients receiving brachytherapy or chemotherapy have yet been studied. The previous meta-analyses investigating the effects of biotic supplements on acute symptoms of gastrointestinal toxicity are listed in Table S5 [25], [26], [27], [28], [29]. A Cochrane systematic review has investigated the efficacy of interventions, including radiotherapy techniques and pharmacological and non-pharmacological interventions, including dietary interventions, probiotics, glutamine, counselling, and protein supplements, on acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers [3]. In contrast, we took a more focused approach, and within our parameters showed that probiotics and synbiotics were the most beneficial interventions. Our search to June 2020 included three more recent studies of biotic supplements (224 patients) and one study focusing on PUFA supplements (40 patients), compared to the Cochrane study, whose search only extended to November 2017. Currently, there are no published meta-analyses that investigate the effect of PUFA or polyphenol supplements on acute symptoms of gastrointestinal toxicity, and two included studies of polyphenols suggested that they are beneficial in preventing diarrhoea.
Studies have shown that the risk factors for radiation enteritis include older age [4], dose of radiation used [30], combining internal (brachytherapy) and external RT [31] and the concomitant use of chemotherapy [32]. Figure S1 show that the effects of interventions on incidence of diarrhoea did not vary by mean age (A) or RT dose (D). Seventeen out of 23 studies used an RT dose of approximately 50 Gy, the exceptions being Delia et al [33], Murphy et al [22], Ahmad et al [34] which used differing higher doses, and 3 studies that did not specify the dosage, including Castro et al [35], Aredes et al [36] and Faramarzi et al [37], but details of techniques were limited. Few studies documented the use of more modern radiotherapy techniques, including intensity modulated radiotherapy. In the Cochrane systematic review [3], such modern techniques, including IMRT and 3D-conformal radiotherapy resulted in lower acute gastrointestinal toxicity than older techniques, and there was uncertain evidence for superiority of IMRT over 3D-conformal. However, high-dose IMRT can still perturb the gut microbiota by reducing its diversity [38]. Therefore, we hypothesise that, as probiotics and synbiotics can positively augment favourable gut microbiota colonization [39], their use will still have an impact in the modern radiotherapy era.
To measure the incidence of diarrhoea, 13 studies used the scale of CTC or CTCAE. It is noted that CTCAE assesses ‘diarrhoea’ by an increase in frequency and/or loose or watery bowel movements [40]. The other studies used either Bristol stool form scale (BSFS), WHO toxicity grading, Radiation Therapy Oncology Group (RTOG) toxicity scale, Murphy Diarrhea Scale (MDS), adapted NCI questionnaire, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire version 3.0 (EORTC QLQ-C30) or non-specified Quality of life (QOL) questionnaire. These have similar definitions of diarrhoea, which enabled us to combine these trials (Table S2). The heterogeneity found in the quality assessment (Table S6), may reduce the evidence certainty of this study. Therefore, in future, methodologically well‐designed, large-scale trials are needed to strengthen the evidence for the benefits of dietary supplements.
Preclinical studies have shown that prebiotics can enhance the efficacy of chemotherapy and radiotherapy [41], [42], in terms of tumour control. As most modern neoadjuvant/radical pelvic radiotherapy regimens (except prostate cancer) include chemotherapy, and with the current interest in combined brachy-EBRT dose-escalation in prostate cancer, the future clinical applicability of biotics in clinical practice should be rigorously evaluated in terms of both tumour control and sparing of normal tissue toxicities in these modern settings.
The underlying protective effects of dietary supplements against GI toxicities may be mediated as shown in Figure S6. A direct effect on the intestinal immune environment following intake of specific dietary agents may lead to anti-inflammatory changes that alleviate gastrointestinal toxicity. There may also be an indirect effect, whereby the above immunomodulatory actions are developed in response to changes in the gut microbiota and their metabolites, particularly SCFAs. A systematic review conducted by Tonneau et al emphasised the importance of probiotics for gastro-intestinal toxicities as radiotherapy can cause perturbation of gut microbiota [43].
Limitations of our study include the different methods of morbidity assessment used in different studies (see above), the difficulty in disentangling radiotherapy side effects from the occurrence of independent gastrointestinal symptoms, the lack of detailed radiotherapy dose parameters available, and the use of different pelvic malignancies requiring different target volumes within the pelvis, which may influence the severity of gastrointestinal side effects.
This review aimed to investigate the effect of dietary supplements on acute and late symptoms, but no studies were available reporting on late side effects. Chronic symptoms of gastrointestinal toxicity typically emerge a few months to years following irradiation and occur in most of the intestinal compartments [44]. Evidence from clinical studies suggests that acute and chronic effects are linked, with the risk of developing late effects greater in patients that have developed acute effects (consequential late effects) [45], [46].
5. Conclusion
In conclusion, findings from our systematic review and meta-analysis suggest that biotic supplements, specifically probiotics and synbiotics, are effective in reducing the risk and severity of acute symptoms of gastrointestinal toxicity caused by pelvic radiotherapy. Our study highlights the need for large multi-centre clinical trials of biotic interventions in patients undergoing radiation and chemoradiation treatments, using modern radiotherapy techniques, with detailed dosimetry of external beam radiotherapy and brachytherapy and appropriate acute and late outcome measures.
Funding
This work was supported by Cancer Research UK Programme grant [C5255/A23755]. Chee Kin Then’s DPhil is funded by the Clarendon Fund, Balliol College and CRUK. The funding body had no role in the study design, collection, analysis, interpretation of data or in writing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.04.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Begg A.C., Stewart F.A., Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 2.Frazzoni L., La Marca M., Guido A., Morganti A.G., Bazzoli F., Fuccio L. Pelvic radiation disease: updates on treatment options. World J Clin Oncol. 2015;6(6):272–280. doi: 10.5306/wjco.v6.i6.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrie TA, Green JT, Beresford M, Wedlake L, Burden S, Davidson SE, et al. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev. 2018;1:CD012529. [DOI] [PMC free article] [PubMed]
- 4.Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut. 2005;54(8):1051–1054. doi: 10.1136/gut.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson R.J., Keefe D.M., Lalla R.V., Bateman E., Blijlevens N., Fijlstra M. Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer. 2013;21(1):313–326. doi: 10.1007/s00520-012-1644-z. [DOI] [PubMed] [Google Scholar]
- 6.Stacey R., Green J.T. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5(1):15–29. doi: 10.1177/2040622313510730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visich K.L., Yeo T.P. The prophylactic use of probiotics in the prevention of radiation therapy-induced diarrhea. Clin J Oncol Nurs. 2010;4(14):467–473. doi: 10.1188/10.CJON.467-473. [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Shen S., Moore D.F., Shih W., Lin Y., Li H. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60(5):908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11(5):4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 11.Hille A., Herrmann M.K., Kertesz T., Christiansen H., Hermann R.M., Pradier O. Sodium butyrate enemas in the treatment of acute radiation-induced proctitis in patients with prostate cancer and the impact on late proctitis. A prospective evaluation. Strahlenther Onkol. 2008;184(12):686–692. doi: 10.1007/s00066-008-1896-1. [DOI] [PubMed] [Google Scholar]
- 12.Bassaganya-Riera J., Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2010;13(5):569–573. doi: 10.1097/MCO.0b013e32833b648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson P.M., Lalla R.V. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. 2020;12(6) doi: 10.3390/nu12061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klimberg V.S., Souba W.W., Dolson D.J., Salloum R.M., Hautamaki R.D., Plumley D.A. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66(1):62–68. doi: 10.1002/1097-0142(19900701)66:1<62::aid-cncr2820660113>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Huang E.Y., Leung S.W., Wang C.J., Chen H.C., Sun L.M., Fang F.M. Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46(3):535–539. doi: 10.1016/s0360-3016(99)00402-2. [DOI] [PubMed] [Google Scholar]
- 16.Okunieff P., Swarts S., Keng P., Sun W., Wang W., Kim J. Antioxidants reduce consequences of radiation exposure. Adv Exp Med Biol. 2008;614:165–178. doi: 10.1007/978-0-387-74911-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiltie A, Bartsch B, Then CK, Kartsonaki C, Harriss E. Systematic review and meta-analysis of interventions with dietary supplements, including pre-, pro- and synbiotics, to reduce acute and late gastrointestinal side effects in patients undergoing pelvic radiotherapy. : PROSPERO; 2020 [Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=183304. [DOI] [PMC free article] [PubMed]
- 18.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.); [Available from: gradepro.org.
- 19.Giralt J., Regadera J.P., Verges R., Romero J., de la Fuente I., Biete A. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol. 2008;71(4):1213–1219. doi: 10.1016/j.ijrobp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Vidal-Casariego A., Calleja-Fernandez A., de Urbina-Gonzalez J.J., Cano-Rodriguez I., Cordido F., Ballesteros-Pomar M.D. Efficacy of glutamine in the prevention of acute radiation enteritis: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2014;38(2):205–213. doi: 10.1177/0148607113478191. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. In: SERVICES USDOHAH, editor.: National Cancer Institute, National Institutes of Health; 2017.
- 22.Murphy J., Stacey D., Crook J., Thompson B., Panetta D. Testing control of radiation-induced diarrhea with a psyllium bulking agent: a pilot study. Can Oncol Nurs J. 2000;10(3):96–100. doi: 10.5737/1181912x10396100. [DOI] [PubMed] [Google Scholar]
- 23.Kozjek N.R., Kompan L., Soeters P., Oblak I., Mastnak D.M., Mozina B. Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: a randomised double blinded, placebo controlled pilot study. Clin Nutr. 2011;30(5):567–570. doi: 10.1016/j.clnu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kozelsky T.F., Meyers G.E., Sloan J.A., Shanahan T.G., Dick S.J., Moore R.L. Phase III double-blind study of glutamine versus placebo for the prevention of acute diarrhea in patients receiving pelvic radiation therapy. J Clin Oncol. 2003;21(9):1669–1674. doi: 10.1200/JCO.2003.05.060. [DOI] [PubMed] [Google Scholar]
- 25.Qiu G., Yu Y., Wang Y., Wang X. The significance of probiotics in preventing radiotherapy-induced diarrhea in patients with cervical cancer: a systematic review and meta-analysis. Int J Surg. 2019;65:61–69. doi: 10.1016/j.ijsu.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Wei D, Heus P, van de Wetering FT, van Tienhoven G, Verleye L, Scholten RJ. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst Rev. 2018;8:CD008831. [DOI] [PMC free article] [PubMed]
- 27.Liu M.M., Li S.T., Shu Y., Zhan H.Q. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamad A., Fragkos K.C., Forbes A. A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32(3):353–360. doi: 10.1016/j.clnu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Fuccio L., Guido A., Eusebi L.H., Laterza L., Grilli D., Cennamo V. Effects of probiotics for the prevention and treatment of radiation-induced diarrhea. J Clin Gastroenterol. 2009;43(6):506–513. doi: 10.1097/MCG.0b013e3181a1f59c. [DOI] [PubMed] [Google Scholar]
- 30.Theis V.S., Sripadam R., Ramani V., Lal S. Chronic radiation enteritis. Clin Oncol (R Coll Radiol) 2010;22(1):70–83. doi: 10.1016/j.clon.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Coia L.R., Myerson R.J., Tepper J.E. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31(5):1213–1236. doi: 10.1016/0360-3016(94)00419-L. [DOI] [PubMed] [Google Scholar]
- 32.Ooi B.S., Tjandra J.J., Green M.D. Morbidities of adjuvant chemotherapy and radiotherapy for resectable rectal cancer: an overview. Dis Colon Rectum. 1999;42(3):403–418. doi: 10.1007/BF02236362. [DOI] [PubMed] [Google Scholar]
- 33.Delia P., Sansotta G., Donato V., Frosina P., Messina G., De Renzis C. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13(6):912–915. doi: 10.3748/wjg.v13.i6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad I.U., Forman J.D., Sarkar F.H., Hillman G.G., Heath E., Vaishampayan U. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62(7):996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro M.G., Sanchez P.X., Glasberg J., Horie L.M., Waitzberg D.L. Effects of probiotic in prevention of radiation-induced diarrhea. Clin Nutr Suppl. 2009;4(2):72–73. [Google Scholar]
- 36.Aredes MA, da Camara AO, de Paula NS, Fraga KYD, do Carmo M, Chaves GV. Efficacy of omega-3 supplementation on nutritional status, skeletal muscle, and chemoradiotherapy toxicity in cervical cancer patients: A randomized, triple-blind, clinical trial conducted in a middle-income country. Nutrition. 2019;67-68:110528. [DOI] [PubMed]
- 37.Faramarzi E., Mahdavi R., Mohammad-Zadeh M., Nasirimotlagh B., Sanaie S. Effect of conjugated linoleic acid supplementation on quality of life in rectal cancer patients undergoing preoperative Chemoradiotherapy. Pak J Med Sci. 2017;33(2):383–388. doi: 10.12669/pjms.332.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis Ferreira M., Andreyev H.J.N., Mohammed K., Truelove L., Gowan S.M., Li J. Microbiota- and radiotherapy-induced gastrointestinal side-effects (MARS) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin Cancer Res. 2019;25(21):6487–6500. doi: 10.1158/1078-0432.CCR-19-0960. [DOI] [PubMed] [Google Scholar]
- 39.Hemarajata P., Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0: National Institutes of Health, National Cancer Institute; November 2017 [07 February 2021]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- 41.Li Y., Elmen L., Segota I., Xian Y., Tinoco R., Feng Y. Prebiotic-induced anti-tumor immunity attenuates tumor growth. Cell Rep. 2020;30(6):1753–66 e6. doi: 10.1016/j.celrep.2020.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Then C.K., Paillas S., Wang X., Hampson A., Kiltie A.E. Association of Bacteroides acidifaciens relative abundance with high-fibre diet-associated radiosensitisation. BMC Biol. 2020;18(1):102. doi: 10.1186/s12915-020-00836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tonneau M., Elkrief A., Pasquier D., Paz Del Socorro T., Chamaillard M., Bahig H. The role of the gut microbiome on radiation therapy efficacy and gastrointestinal complications: a systematic review. Radiother Oncol. 2020;156:1–9. doi: 10.1016/j.radonc.2020.10.033. [DOI] [PubMed] [Google Scholar]
- 44.Shah V., Lyford G., Gores G., Farrugia G. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126(3):903–913. doi: 10.1053/j.gastro.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Bourne R.G., Kearsley J.H., Grove W.D., Roberts S.J. The relationship between early and late gastrointestinal complications of radiation-therapy for carcinoma of the cervix. Int J Radiat Oncol. 1983;9(10):1445–1450. doi: 10.1016/0360-3016(83)90316-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang C.J., Leung S.W., Chen H.C., Sun L.M., Fang F.M., Huang E.Y. The correlation of acute toxicity and late rectal injury in radiotherapy for cervical carcinoma: evidence suggestive of consequential late effect (CQLE) Int J Radiat Oncol. 1998;40(1):85–91. doi: 10.1016/s0360-3016(97)00560-9. [DOI] [PubMed] [Google Scholar]
- 47.Sasidharan B.K., Ramadass B., Viswanathan P.N., Samuel P., Gowri M., Pugazhendhi S. A phase 2 randomized controlled trial of oral resistant starch supplements in the prevention of acute radiation proctitis in patients treated for cervical cancer. J Cancer Res Ther. 2019;15(6):1383–1391. doi: 10.4103/jcrt.JCRT_152_19. [DOI] [PubMed] [Google Scholar]
- 48.Linn Y.H., Thu K.K., Win N.H.H. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicro. 2019;11(2):638–647. doi: 10.1007/s12602-018-9408-9. [DOI] [PubMed] [Google Scholar]
- 49.De Loera Rodriguez L.H., Ortiz G.G., Rivero Moragrega P., Velazquez Brizuela I.E., Santoscoy Gutierrez J.F., Rincon Sanchez A.R. Effect of symbiotic supplementation on fecal calprotectin levels and lactic acid bacteria, Bifidobacteria, Escherichia coli and Salmonella DNA in patients with cervical cancer. Nutr Hosp. 2018;35(6):1394–1400. doi: 10.20960/nh.1762. [DOI] [PubMed] [Google Scholar]
- 50.Mansouri-Tehrani H.S., Khorasgani M.R., Roayaei M. Effects of probiotics with or without honey on radiation-induced diarrhea. Int J Radiat Res. 2016;14(3):205–213. [Google Scholar]
- 51.Garcia-Peris P., Velasco C., Lozano M.A., Moreno Y., Paron L., de la Cuerda C. Effect of a mixture of inulin and fructo-oligosaccharide on lactobacillus and bifidobacterium intestinal microbiota of patients receiving radiotherapy; a randomised, double-blind, placebo-controlled trial. Nutr Hosp. 2012;27(6):1908–1915. doi: 10.3305/nh.2012.27.6.5992. [DOI] [PubMed] [Google Scholar]
- 52.Itoh Y., Mizuno M., Ikeda M., Nakahara R., Kubota S., Ito J. A randomized, double-blind pilot trial of hydrolyzed rice bran versus placebo for radioprotective effect on acute gastroenteritis secondary to chemoradiotherapy in patients with cervical cancer. Evid-Based Compl Alt. 2015 doi: 10.1155/2015/974390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demers M., Dagnault A., Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33(5):761–767. doi: 10.1016/j.clnu.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Chitapanarux I., Chitapanarux T., Traisathit P., Kudumpee S., Tharavichitkul E., Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010;5 doi: 10.1186/1748-717X-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbancsek H., Kazar T., Mezes I., Neumann K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus (R) in patients with radiation-induced diarrhoea. Eur J Gastroen Hepat. 2001;13(4):391–396. doi: 10.1097/00042737-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 56.Salminen E., Elomaa I., Minkkinen J., Vapaatalo H., Salminen S. Preservation of intestinal integrity during radiotherapy using live lactobacillus-acidophilus cultures. Clin Radiol. 1988;39(4):435–437. doi: 10.1016/s0009-9260(88)80296-4. [DOI] [PubMed] [Google Scholar]
- 57.Manir K.S., Kallol B., Gaurav K., Arnab A., Amitabha M., Shaymal S.K. Role of glutamine versus placebo in prevention of acute gastrointestinal toxicity in pelvic radiotherapy: a randomized control study. Clin Cancer Investig J. 2014;3(6):508–513. [Google Scholar]
- 58.Kucuktulu E., Guner A., Kahraman I., Topbas M., Kucuktulu U. The protective effects of glutamine on radiation-induced diarrhea. Support Care Cancer. 2013;21(4):1071–1075. doi: 10.1007/s00520-012-1627-0. [DOI] [PubMed] [Google Scholar]
- 59.Emami H., Nikoobin F., Roayaei M., Ziya H.R. Double-blinded, randomized, placebo-controlled study to evaluate the effectiveness of green tea in preventing acute gastrointestinal complications due to radiotherapy. J Res Med Sci. 2014;19(5):445–450. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.