Abstract
Background:
Adolescence has been proposed to be a period of heightened sensitivity to environmental influence. If true, adolescence may present a window of opportunity for recovery for children exposed to early-life adversity. Recent evidence supports adolescent recalibration of stress response systems following early-life adversity. However, it is unknown whether similar recovery occurs in other domains of functioning in adolescence.
Methods:
We use data from the Bucharest Early Intervention Project – a randomized controlled trial of foster care for children raised in psychosocially depriving institutions – to examine the associations of the caregiving environment with reward processing, executive functioning, and internalizing and externalizing psychopathology at ages 8, 12, and 16 years, and evaluate whether these associations change across development.
Results:
Higher quality caregiving in adolescence was associated with greater reward responsivity and lower levels of internalizing and externalizing symptoms, after covarying for the early-life caregiving environment. The associations of caregiving with executive function and internalizing and externalizing symptoms varied by age and were strongest at age 16 relative to ages 8 and 12 years. This heightened sensitivity to caregiving in adolescence was observed in both children with and without exposure to early psychosocial neglect.
Conclusions:
Adolescence may be a period of heightened sensitivity to the caregiving environment, at least for some domains of functioning. For children who experience early psychosocial deprivation, this developmental period may be a window of opportunity for recovery of some functions. Albeit correlational, these findings suggest that it may be possible to reverse or remediate some of the lasting effects of early-life adversity with interventions that target caregiving during adolescence.
Keywords: Institutionalization, reward, executive function, psychopathology, adolescence
Introduction
Recent evidence in both humans and animals suggests that the magnitude of changes in brain structure and function that occurs during adolescence is second only to those during the first five years of life (Fuhrmann, Knoll, & Blakemore, 2015). Emerging evidence from human and animal models suggests that adolescence may serve as a second period of heightened plasticity when neural systems may be particularly likely to be influenced by environmental experiences. Indeed, markers of the molecular mechanisms that govern periods of heightened plasticity in sensory cortex during early life are present in association cortex during adolescence (Larsen & Luna, 2018). This degree of rapid change in the brain and other neurobiological systems during adolescence and the possibility of heightened plasticity makes it a particularly important period to examine the influence of environmental factors – such as caregiving conditions – on behavioral and neurobiological development.
If adolescence is in fact a period of heightened sensitivity to environmental influences, it could provide a window of opportunity for recovery of children who have experienced early-life adversity. Given the high prevalence of exposure to early-life adversity and strong associations with the emergence of psychopathology and other negative developmental outcomes (Green, McLaughlin, & Berglund, 2010; McLaughlin et al., 2012), identifying points in development when children may be particularly likely to respond to interventions or positive environmental experiences has clear clinical implications. Here, we focus on recovery following early-life psychosocial deprivation related to institutional rearing, which is characterized by experiences of low-quality caregiving. Not surprisingly, children reared in these settings exhibit perturbed patterns of social, emotional, cognitive, and neurobiological development and often have difficulties across a variety of social, emotional, and cognitive domains that continue into adolescence and adulthood (Gunnar & Van Dulmen, 2007). The lasting effects of early institutionalization may likely persist over time when deprivation occurs during sensitive periods for systems underlying social, emotional, and cognitive development in infancy and early childhood (Nelson, Zeanah, & Fox, 2019; Opendak, Gould, & Sullivan, 2017).
Support for the idea of adolescence as a period of heightened plasticity that provides an opportunity for recovery following early-life adversity comes from studies situated within the pubertal stress recalibration hypothesis (DePasquale, Donzella, & Gunnar, 2018). This hypothesis suggests that if children exposed to early-life adversity are removed from the negative environment, puberty is a period during which the hypothalamic–pituitary–adrenal (HPA) axis can recalibrate – or recover – in response to the less harsh demands of the current environment. Evidence supporting this hypothesis has emerged from studies of previously institutionalized (PI) children adopted into supportive families in the United States across multiple metrics of cortisol regulation (DePasquale et al., 2018; Flannery, Gabard-Durnam, & Shapiro, 2017; Gunnar, Depasquale, Reid, & Donzella, 2019; Quevedo, Johnson, Loman, LaFavor, & Gunnar, 2012). For instance, PI children early in pubertal development exhibited significantly blunted cortisol responses to psychosocial stressors relative to their nonadopted peers, but PI adolescents later in pubertal development showed no difference in their cortisol levels relative to their nonadopted peers (DePasquale et al., 2018). This work was extended longitudinally, demonstrating that pubertal development was associated with increases in cortisol reactivity within individuals in PI adolescents, suggesting that the onset of puberty is associated with a recalibration of these systems for youth exposed to early-life adversity who are in positive environments during adolescence (Gunnar et al., 2019). These studies provide evidence for the idea that puberty may serve as a period of heightened plasticity when the effects of adversity on HPA axis function can be remediated for children in positive caregiving environments.
It has yet to be determined whether adolescence serves as a time when remodeling may occur in other domains beyond the HPA axis in ways that might reverse the effects of early-life adversity. In this study, we use data from the Bucharest Early Intervention Project (BEIP) to examine the possibility of adolescent recalibration of the detrimental effects of early-life institutionalization on three domains of functioning: reward processing, executive functioning, and symptoms of psychopathology. The BEIP began as a randomized controlled trial (RCT) examining the effects of high-quality foster care relative to institutional care on children’s development (Zeanah et al., 2003). Many of the children who were randomly assigned to the foster care group or care as usual group (i.e., more prolonged institutional care) underwent changes in their caregiving situation. For example, a number of children who were originally placed in foster care were reunited with their biological families. At age 16, only 45% of the group originally assigned to foster care remained in their original foster care placement, highlighting continued variability in caregiving environments into adolescence. Similarly, over half of the children randomized to the care as usual group were in some form of family care at age 16 years (Figure 1). Further, in these situations, it is not clear that reuniting with biological families always led to high-quality caregiving. Thus, rather than only examining initial randomization groups, we consider variation in the quality of care across all children who were ever institutionalized.
Figure 1.
CONSORT flow diagram showing participants and placements over time
To evaluate whether sensitivity to positive caregiving environments is heightened during adolescence for systems other than the HPA axis, we examined three domains that have consistently been associated with early-life adversity and also demonstrate meaningful developmental variation during the adolescent period. First, reward processing encompasses processes reflecting motivation to pursue rewards and sensitivity to reward receipt (Olino, 2016). These reward-related behaviors are influenced by early-life adversity (Dennison et al., 2019; Sheridan et al., 2018), and exhibit meaningful developmental change during adolescence (Galvan, 2010). Second, children exposed to institutional rearing and other forms of early deprivation exhibit poorer executive function than peers raised in families (Bick, Zeanah, Fox, & Nelson, 2018; Tibu et al., 2016). Executive functions – including working memory, inhibitory control, and cognitive flexibility – continue to improve dramatically during adolescence (De Luca, Wood, & Anderson, 2003). Finally, adolescence is a period of heightened vulnerability for psychopathology (Lee et al., 2014), particularly for those who have experienced early-life adversity (McLaughlin et al., 2012). It is important to understand whether adolescent recalibration following early-life institutional rearing occurs only in the HPA axis or alternatively across multiple domains of functioning. If recovery occurs across multiple domains, this suggests that the mechanism through which it happens might be more general, supporting adolescence as a period of general heightened plasticity, than if recovery occurs only for the HPA axis.
We first explored whether the current caregiving environment, covarying for the early caregiving environment, was associated with reward processing, executive function, and psychopathology across childhood and adolescence. We then explored whether current caregiving was more strongly associated with these domains in adolescence than in childhood. To do so, we examined whether associations between caregiving quality and each of these domains varied across development, covarying for the early caregiving environment. If age-related variation was observed, we conducted follow-up analyses to explore the nature of the association among current caregiving quality and function at the 8-, 12-, and 16-year assessments. We predicted that higher quality caregiving would be associated with higher behavioral sensitivity to reward, better performance on tests of executive function, and lower symptoms of psychopathology across development, and that the associations between caregiving and each of our developmental domains would be stronger during adolescence (12 and 16-year assessments) than prior points in development.
We also explored whether the association between current caregiving and each of these domains varies as a function of the early caregiving environment. To do so, we estimated interactions of the current caregiving environment, the early caregiving environment, and age. The Pubertal Recalibration Hypothesis suggests this recalibration should be specific to individuals who have experienced severe adversity in early life (DePasquale et al., 2018; Gunnar et al., 2019), yet there is evidence to suggest that increased sensitivity to the environment may occur in adolescents regardless of their early caregiving experience (Blakemore & Mills, 2014; Fuhrmann et al., 2015; Larsen & Luna, 2018). Given this mixed evidence, we did not have specific hypotheses about whether adolescent sensitivity would be specific to children with exposure to early-life adversity.
Methods
Study design and participants
The original study was a randomized control trial of children living in government-run institutions in Bucharest, Romania (http://clinicaltrials.gov; NCT00747396). Approval for the study was received by the institutional review boards of the three principal investigators (N.A.F., C.A.N., and C.H.Z.) and by the local Commissions on Child Protection in Bucharest. The study was conducted in collaboration with the Institute of Maternal and Child Health of the Romanian Ministry of Health. For full details about the original sample and ethical implications of this work, see (Rid, 2012; Zeanah, Fox, & Nelson, 2012). Briefly, children were enrolled in the study between 6 months and 31 months (mean age = 22 months); half the children were randomly assigned to the care as usual group (CAUG), and the other half were assigned to the high-quality foster care group (FCG). An age- and sex-matched sample of never-institutionalized children from the community who were reared in their biological families were recruited as a comparison group (never-institutionalized group, NIG). Signed informed consent was obtained from the children’s legal guardian, and written or verbal assent was obtained from all children. Data reported in this manuscript were collected from the 8-year assessment (mean age = 8.08, SD = 0.65, range = 6.40–9.55), 12-year assessment (mean age = 12.53, SD = 0.55, range = 11.14–14.62), and most recent follow-up that began in January 2015 (mean age = 16.36 years of age, SD = 0.61 years, range = 15.18–19.37 years). See Table 1 for participant characteristics and Figure 1 for CONSORT diagram.
Table 1.
Participant characteristics
| CAUG | FCG | NIG | ||
|---|---|---|---|---|
| 8-year assessment | ||||
| Sample size (male, n) | 53 (27) | 51 (27) | 47 (19) | |
| Age, M (SD) | 8.66 (0.37) | 8.56 (0.32) | 8.46 (0.39) |
F(2,122) = 3.42, p < .05 CAUG > NIG |
| Caregiving quality, M (SD) | 3.21 (1.30) | 4.24 (0.97) | 4.79 (0.55) |
F(2,148) = 32.29, p < .01 NIG > FCG > CAUG |
| 12-year assessment | ||||
| Sample size (male, n) | 56 (29) | 52 (26) | 42 (18) | |
| Age, M (SD) | 12.76 (0.59) | 12.73 (0.51) | 12.84 (0.62) | F(2,133) = 22.41, p = .57 |
| Tanner stage, M (SD) | 2.60 (1.01) | 2.94 (1.04) | 3.15 (0.85) |
F(2,136) = 3.66, p = .03 NIG > CAUG |
| Caregiving quality, M (SD) | 3.21 (1.31) | 4.10 (1.20) | 4.79 (0.54) |
F(2,147) = 25.25, p < .01 NIG > FCG > CAUG |
| 16-year assessment | ||||
| Sample size (male, n) | 56 (29) | 53 (27) | 49 (19) | |
| Age, M (SD) | 16.71 (0.40) | 16.65 (0.64) | 17.03 (0.62) |
F(2,139) = 6.13, p < .01 NIG > CAUG/FCG |
| Tanner stage, M (SD) | 4.06 (0.70) | 4.04 (0.74) | 4.28 (0.74) | F(2,140) = 1.51, p = .22 |
| Caregiving quality, M (SD) | 3.17 (1.30) | 3.94 (1.19) | 4.79 (0.52) |
F(2,155) = 29.48, p < .01 NIG > FCG > CAUG |
| Per cent of life spent in institution, M% (SD) | 45 (33) | 15 (13) | 0 |
F(1,107) = 37.41, p < .01 CAUG > FCG |
CAUG, care as usual group; FCG, foster care group; NIG, never-institutionalized group.
Caregiving environments
Early caregiving quality.
We used the intent-to-treat groups (ITT; CAUG, FCG) and the NIG to examine variation in the early caregiving environment. The early caregiving environment was the lowest quality for the CAUG, who had more prolonged institutional care relative to the FCG (per cent of time spent in the institution as a per cent of the life span: CAUG: 45% vs. FCG: 15%; t(110) = 6.30, p < .01), and highest quality in the NIG given no history of institutionalization (Smyke, Zeanah, Fox, & Nelson, 2009).
Current caregiving quality.
In order to quantify caregiving quality in later childhood and adolescence, we obtained ratings of caregiving quality from study staff in Romania, most of whom have worked with the children for many years and some since the study’s inception. Two BEIP staff who were familiar with the child’s family independently made global ratings of the quality of caregiving the children received at 8, 12, and 16 years. Ratings were based on numerous hours of staff observations of interactions of the children and their caregivers, including observations made during and in between laboratory visits. The ratings were anchored as follows: (a) Dangerous (i.e., environment that constantly fails to meet at least one basic need [e.g., adequate shelter and food, constant care from at least one preferred caregiver]. Strong suspicion of maltreatment); (b) Unacceptable (i.e., physical needs may be met; however, caregivers consistently fail in providing emotional care for the child. Nonindividualized, instrumental, and/or regimented care may be present); (c) Marginal (i.e., physical needs are generally met. Emotional needs are not consistently met. A preferred caregiver is at least sometimes available for the child. Overall, the child does not lack basic protection and support, although significant deficiencies are notable); (d) Mixed (i.e., a reasonably good living environment is evident with regard to physical comfort provided to the child. Care is generally available at satisfactory levels, although the caregiver’s behaviors and relationship with the child are sometimes marked by significant problems [e.g., instances of harsh parenting, lack of support, nonconstructive ways of dealing with conflicts]); and (e) Acceptable (i.e., physical and emotional needs are met consistently. Child feels adequately safe, secure, and cared for. Good caregiver–child relationship with minimal discord and good strategies put in place by the caregiver for overcoming problems).
Independent raters demonstrated excellent inter-rater reliability (ICC = 0.93), and the scores from the two raters were averaged to form a caregiving quality rating that ranged from 1 to 5. There was a strong consistency in caregiving quality at ages 8, 12, and 16 years, although caregiving was most consistent for the NIG group (ICCCAUG = 0.77; ICCFCG = 0.78; ICCNIG = 0.95).
Reward processing
Participants completed a child-friendly version of the monetary incentive delay (MID) task called the Piñata task (Helfinstein et al., 2013). More details about the task are presented in Appendix S1.
Reward sensitivity was assessed by accuracy on the 4-point trials statistically covarying accuracy on the 0-point trials. The piñata task was completed by 111 participants at the 12-year follow-up and 138 participants at the 16-year follow-up. The piñata task was not administered at the 8-year follow-up.
Executive function
Participants completed the Cambridge Neuropsychological Test Automated Battery (CANTAB; http://www.cantab.com), a computerized set of tests assessing different domains of memory and executive function. As described in Wade, Zeanah, et al. (2019), four subtests of the CANTAB were administered at each follow-up and single outcomes from each task were selected and combined in order to estimate a global executive function using latent variable modeling. More details about this task are presented in Appendix S1. For this study, the executive function factor was saved and used as an outcome for 151 participants at the 8, 12, and 16-year follow-ups.
Psychopathology
Psychopathology was assessed using the MacArthur Health and Behavior Questionnaire (HBQ; Essex et al., 2002). More details about this measure are presented in Appendix S1. Teacher or caregiver report of internalizing and externalizing symptoms was available for 195 participants at the 8-year assessment, 151 participants at the 12-year assessment, and 149 participants at the 16-year assessment.
Data analysis
Using a multilevel modeling approach (Woltman, Feldstain, Mackay, & Rocchi, 2012), all analyses were conducted using ‘lmer’ function in package ‘lme4’ and degrees of freedom and p-values were estimated using R package ‘lmerTest’ (Bates, Machler, Bolker, & Walker, 2015; Kuznetsova, Brockhoff, & Christensen, 2016). We followed a formal model building procedure (Chambers, 1992) to test whether the addition of parameters of interest (described below) improved model fit using a log-likelihood ratio test. The best-fit model was determined to be the model with the lowest Akaike information criterion (AIC; Akaike, 1974) and Bayesian information criterion (BIC) (Schwarz, 1978) values. The AIC and BIC values for all models are listed in Table S1. Similarly, the final model included sex only if it significantly improved model fit. The best-fitting model was then interpreted, and follow-up simple slopes analyses were conducted as necessary.
First, we explored the main effect of current caregiving with our outcomes, covarying for the early caregiving group (CAUG, FCG, and NIG; Model 1) across all assessment timepoints. Current caregiving quality and functioning across the three domains were nested within subject. Age at each of the three assessments was used as our estimate of time in these models, and all models included a random intercept of subject. Model 1: reward/executive function/psychopathologyij ~ β0 + β1(early caregiving groupi) + β2(current caregiving qualityij) + β3(ageij) + β4(current caregiving qualityij × ageij).
Next, we explored whether these associations varied by age. To do so, we tested whether adding a within-level interaction term between caregiving quality and age (covarying for early caregiving group) significantly improved model fit (Model 2). If this interaction term improved model fit, we examined the simple slopes of the association of current caregiving quality with the relevant outcome at each of the three developmental timepoints. The reward processing task was only conducted at the 12- and 16-year assessments; thus, only two timepoints were considered in these analyses.
Model 2: reward/executive function/psychopathologyij ~ β0 + β1(early caregiving groupi) + β2(current caregiving qualityij) + β3(ageij) + β4(current caregiving qualityij × ageij).
Finally, we conducted exploratory analyses to examine whether the association between current caregiving quality and age differed across the early caregiving groups. To do so, we test whether including a cross-level 3-way interaction among current caregiving quality, age, and early caregiving group significantly improved model fit (Model 3) using the same formal model-fitting procedure described above.
Model 3: reward/executive function/psychopathologyij ~ β0 + β1(early caregiving groupi) + β2(current caregiving qualityij) + β3(ageij) + β4(current caregiving qualityij × ageij × early caregiving group).
Results
Reward processing
The association of early caregiving group, current caregiving quality, age, and reward processing was best described by the model including only main effects of early caregiving group, current caregiving quality, and age (Model 1). Adding a main effect of sex to the model did not improve model fit (χ2(1) = 1.626, p = .202). Current caregiving quality was positively associated with reward processing across both timepoints: (B = 0.040, SE = 0.012, p < .001) (see Table S2; Figure 2). The association of caregiving quality with reward processing did not vary by age (the reward processing task was only conducted at the 12- and 16-year assessments): age 12: B = 0.225, SE = 0.112, p = .046; age 16: B = 0.322, SE = 0.103, p = .002. These results suggest that high-quality caregiving in adolescence is associated with greater behavioral sensitivity to reward value over and above the influence of early caregiving and in all three early caregiving groups.
Figure 2.
Association between caregiving quality and reward processing at the 12- and 16-year assessments
Executive function
The association of early caregiving group, current caregiving quality, age, and executive functioning was best described by the model including an interaction between current caregiving quality and age, covarying for the early caregiving group (Model 2). There was a significant interaction between current caregiving and age (B = 0.030, SE = 0.011, p = .006), suggesting that the association between current caregiving and executive function increased across development (Table S3; Figure 3).
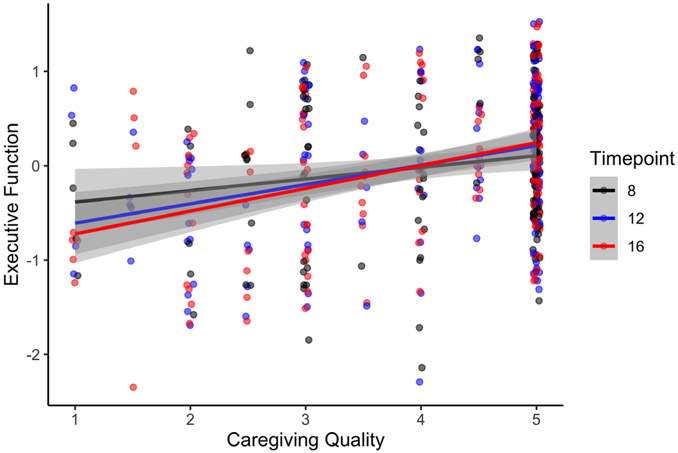
Figure 3.
Association between caregiving quality and executive function at the 8-, 12-, and 16-year assessments
Simple slopes analysis revealed that current caregiving quality was most strongly associated with executive function at age 16 (B = 0.454, SE = 0.078, p < .001) relative to age 8 (B = −0.266, SE = 0.090, p = .004) and age 12 (B = 0.374, SE = 0.085, p < .001). Adding a main effect of sex to the model did not improve model fit (χ2(1) = 0.083, p = .773) and was not included in the final model. These results suggest that the association between high-quality caregiving and executive function increases across development across all three early caregiving groups.
Psychopathology
Internalizing symptoms.
The association of early caregiving group, current caregiving quality, age, and internalizing symptoms was best described by the model including an interaction between current caregiving quality and age, covarying for the early caregiving group (Model 2). There was a marginally significant interaction between current caregiving and age (B = −0.025, SE = 0.014, p = .076), suggesting an increasing negative association between current caregiving and internalizing symptoms across development (Table S4; Figure 4A).
Figure 4.
(A) Association between caregiving quality and internalizing symptoms at the at the 8-, 12-, and 16-year assessments. (B) Association between caregiving quality and externalizing symptoms at the 8-, 12-, and 16-year assessments
Simple slopes analysis revealed that current caregiving quality was not associated with internalizing symptoms at age 8 (B = −0.073, SE = 0.108, p = .501) or age 12 (B = 0.009, SE = 0.097 p = .924), but was significantly associated with internalizing symptoms at age 16 (B = −0.299, SE = 0.094, p = .002), such that higher quality caregiving was associated with lower internalizing symptoms. Adding a main effect of sex to the model did not improve model fit (χ2(1) = 1.281, p = .258), and was not included in the final model. These results suggest that the negative association between high-quality caregiving and internalizing symptoms increases across development across all three early caregiving groups.
Externalizing symptoms.
The association of early caregiving group, current caregiving quality, age, and externalizing symptoms was best described by the model including an interaction between current caregiving quality and age, covarying for the early caregiving group (Model 2). There was a significant interaction between current caregiving and age (B = −0.039, SE = 0.017, p = .019), suggesting an increasing negative association between current caregiving and externalizing symptoms across development (Table S5; Figure 4B). Adding a main effect of sex to the model improved model fit (χ2(1) = 7.17, p = .007), and was included in the final model.
Simple slopes analysis revealed that current caregiving was not significantly associated with externalizing symptoms at age 8 (B = −0.127, SE = 0.107, p = .235) and was marginally significantly associated with externalizing symptoms at age 12 (B = −0.167, SE = 0.865, p = .055). However, current caregiving was significantly associated with externalizing symptoms at age 16 (B = −0.556, SE = 0.079, p < .001), such that higher quality caregiving was associated with lower externalizing symptoms. These results suggest that the negative association between high-quality caregiving and externalizing symptoms increases across development across all three early caregiving groups.
Discussion
In the current study, we explored correlational associations among the early caregiving environment and later caregiving quality with three domains of functioning that exhibit rapid developmental change during adolescence β reward processing, executive function, and psychopathology – using cross-sectional data from the 8-, 12-, and 16-year assessments of the BEIP RCT. We observed consistent associations of adolescent caregiving quality with reward processing and executive function at ages 12 and 16, and internalizing/externalizing symptoms at age 16, such that adolescents who experienced more positive caregiving during adolescence had better reward processing/executive function and lower levels of externalizing symptoms over and above the effects of the early caregiving intervention. These findings highlight the ongoing importance of the caregiving environment, even for children exposed to extreme forms of early-life adversity. Critically, the strength of the association of the caregiving environment with executive function and internalizing and externalizing psychopathology increased significantly as participants aged from middle childhood into adolescence, with the strongest caregiving associations observed during mid (12-year assessment) to late adolescence (16-year assessment). These findings support the idea that adolescence reflects a period of heightened sensitivity to environmental influences, specifically caregiving. Positive caregiving experiences during adolescence may help to promote recovery for children exposed to adversity at earlier points in development.
Across all three domains of functioning, we found a consistent positive influence of adolescent caregiving quality that was stronger than that of the early caregiving environment. This finding is fairly striking given the intense nature of the early intervention in question, in which children were removed from institutions lacking in stable caregiving and placed in supportive family environments. Indeed, this intervention has been shown to have dramatic and positive effects on attachment security (Smyke, Zeanah, Fox, Nelson, & Guthrie, 2010), cognitive ability (Nelson et al., 2007), reward processing (Sheridan et al., 2018), and symptoms of psychopathology (Humphreys et al., 2015) at earlier ages of assessment. The strength of the associations among the caregiving environment and executive function and psychopathology increased as participants aged from middle childhood into adolescence, with the strongest caregiving associations observed during mid to late adolescence. After the formal RCT ended at age 54 months, children experienced numerous changes in caregiving such that fewer than half of those randomized to foster care remained in the families in which they were initially placed, and many children randomized to care as usual subsequently experienced family care. By adolescence, the proximal caregiving environment appears to have a more powerful influence on reward processing, executive functioning, and psychopathology than caregiving early in life. In adolescence, regardless of early environmental experiences, youth with more positive caregiving in adolescence exhibited higher behavioral sensitivity to reward, better executive functioning, and lower symptoms of internalizing and externalizing psychopathology. Together, these findings suggest a heightened sensitivity to environmental influences during adolescence, consistent with models of adolescent neuroplasticity (Larsen & Luna, 2018; Laube, van den Bos, & Fandakova, 2020), as well as the potential for recovery during this developmental period after exposure to early-life adversity.
Adolescent caregiving was strongly associated with reward processing, over and above the influence of the early caregiving environment, and this association was stable across the 12- and 16-year assessments. Those in higher quality caregiving environments at ages 12 and 16 years demonstrated greater behavioral sensitivity to reward value, as indexed by greater accuracy to the high reward trials relative to low reward trials, even after adjusting for the early caregiving environment. This pattern supports adolescence as a period of sensitivity to environmental influences for the reward processing system. However, we cannot conclusively confirm that this sensitivity to environmental influences is specific to adolescence in this domain, given we only assessed this construct at the 12- and 16-year assessments. It is possible that if we had reward measures earlier in development, we may have seen that the strength of the association between caregiving and reward processing increases during adolescence, similar to the other domains examined here. Strong evidence exists for adolescent plasticity in reward processing, which may be due to the well-documented changes in neural circuitry underlying reward processing and learning that occur throughout adolescence (Braams, Van Duijvenvoorde, Peper, & Crone, 2015). This dramatic period of change in reward processing may create a window of plasticity with regard to environment influences on frontostriatal circuitry and reward processing. Future research should explore both the effects of the caregiving environment from childhood through adolescence, and the role of caregiving quality on development of the frontostriatal circuitry underlying reward processing in adolescence.
We found a pattern of increased adolescent sensitivity to environmental influences for executive functioning. This finding is particularly notable given that executive functioning is one of the few domains in which there was no effect of the early caregiving intervention in the BEIP study (Bick et al., 2018; Bos, 2009; Wade, Fox, Zeanah, & Nelson, 2019). In contrast to the persistent lack of effects of the early caregiving intervention, we demonstrate here that the adolescent caregiving quality is positively associated with a composite measure of executive function at all ages – the same composite for which no effects of the early caregiving intervention were observed (Wade, Zeanah, Fox, & Nelson, 2019) – such that higher quality caregiving was associated with higher levels of executive functioning. The strength of the association between caregiving environment and executive function increased as participants aged from middle childhood to adolescence, with the strongest caregiving effects in late adolescence (age 16). Adolescence is an important period for the development of higher-order cognition, such as executive function, potentially due to significant development of the prefrontal cortex across adolescence (Luna, Padmanabhan, & O’Hearn, 2010). Indeed, evidence exists for similar cellular mechanisms promoting plasticity in the prefrontal cortex in adolescence as those that promote plasticity in sensory systems in early life (Larsen & Luna, 2018). Our finding suggests that positive caregiving environments, particularly in adolescence, are associated with improved executive function, and may help to mitigate otherwise persistent effects of early-life deprivation on executive functioning. More broadly, these findings are broadly consistent with the notion that adolescence represents a second period of heightened plasticity for the development of cognitive function (Larsen & Luna, 2018).
We also observed an association of the adolescent caregiving environment with both internalizing and externalizing symptoms at age 16 that was not evident at earlier points in childhood. The strength of the association between caregiving quality and both internalizing and externalizing symptoms increased as participants aged, with the strongest caregiving effects occurring during mid to late adolescence. Adolescence is a period of heightened vulnerability for the onset of psychopathology (Lee et al., 2014). This risk is particularly heightened in individuals who have experienced early-life adversity (McLaughlin et al., 2012). Our findings suggest that high-quality caregiving in adolescence may reduce the risk of psychopathology during this developmental window, even in youths exposed to severe early-life adversity. This may be due to the effects of positive parenting on promoting adaptive coping skills and reducing risk-taking behaviors such as dangerous driving and substance use in adolescence (Fritz, de Graaff, Caisley, van Harmelen, & Wilkinson, 2018).
We also tested whether the association between current caregiving quality and age varied as a function of the early caregiving environment. A significant 3-way interaction across current caregiving quality, the early caregiving group, and age would suggest that sensitivity to the environment in adolescence may be specific to children and adolescents who have experienced adversity. However, we found no evidence for such interaction in our data, which suggests that adolescent sensitivity is not specific to children who experienced early-life adversity. These results stand in contrast to earlier work on the Pubertal Recalibration Hypothesis, which showed adolescent-specific changes in HPA axis function only for youths with a history of early-life adversity (DePasquale et al., 2018; Gunnar et al., 2019). However, these results are in line with previous theories arguing that adolescence represents a developmental period of enhanced sensitivity (Blakemore & Mills, 2014; Fuhrmann et al., 2015; Larsen & Luna, 2018), regardless of early experience. Given the relatively small sample size studied here, future work should continue to explore the possibility that adolescent recalibration may be greater in those who have experienced early-life adversity.
Together, these patterns suggest that adolescence may be a period of plasticity generally, not limited to the HPA axis (DePasquale et al., 2018), but also in a range of other emotional and cognitive processes. Additionally, these results highlight adolescence as a period in which the detrimental effects of early adversity can recalibrate or recover in the context of a supportive and responsive family environment. Given strong links between the HPA and hypothalamic–pituitary–gonadal (HPG) axis across development (King, Graber, Colich, & Gotlib, 2020), pubertal development may mediate adolescent recalibration of the HPA axis. It is less clear whether adolescent sensitivity to the environment as it relates to reward processing, executive function, and psychopathology is likely to be driven by pubertal development, or reflect other aspects of adolescent development. At age 16, the majority of adolescents in the BEIP were in the later stages of pubertal development (with mean Tanner stage scores greater than 4). In order to truly understand the mechanism underlying this period of increased plasticity, future work should explore the interactions among the early caregiving environment, the adolescent caregiving environment, and pubertal hormones in a sample of adolescents that spans the entire pubertal period (Laube et al., 2020).
Several limitations of this study highlight key directions for future research. First, our study does not explore the direct impact of pubertal development on the associations between early-life institutionalization and adolescent caregiving. Future research should explore these associations to understand pubertal development and increases in adrenal and gonadal hormones as a potential mechanism underlying increasing sensitivity to proximal environmental influences across adolescence. Second, our analyses do not span the pubertal period, because not all measures examined here were administered at multiple timepoints spanning pre-to postpuberty. We do not have data regarding the pubertal stage at the 8-year assessment. By the 12- year assessment, participants were already in the peri-pubertal period (Johnson et al., 2018). Thus, we explored how age moderated the effect of current caregiving on function. In order to stringently test the idea of adolescent recalibration following adversity, and the role of puberty as a mechanism for this recalibration, future work should explore within-subject changes across the entire pubertal transition. Similarly, our research does not address pubertal recalibration of the HPA axis as a potential mediator for increased plasticity in reward processing, executive function, and psychopathology (given that cortisol was not collected at all timepoints). This is an important follow-up question that should be explored in future work. Third, our metric of caregiving, based on staff’s extensive interactions with the children and their families (some since infancy), was nonetheless subjective and retrospective. High inter-rater reliability gives us confidence that the caregiving context was rated similarly across independent observers. Future research exploring the impact of caregiving quality should focus on more objective metrics of the caregiving environment. Finally, it is important to note that analyses that set aside the original random assignment are associative, not causal. It is plausible that an alternative explanation of these results is that having a child with behavioral problems elicits parenting behaviors that can be perceived as lower quality (Burke, Pardini, & Loeber, 2008). Regardless of the directionality, these findings add to a body of work supporting the integral role of caregivers in interventions aimed at ameliorating adolescent psychopathology.
Conclusions
Our findings provide evidence that adolescence may be a period of heightened sensitivity to environmental influences and a period of opportunity for recovery following early-life adversity. These findings point to the possibility that the effects of early-life adversity might be remediated with interventions that target caregiving environments during adolescence. Increased resources should be geared toward improving parenting practices in adolescence (Dahl, Allen, Wilbrecht, & Suleiman, 2018). These results also highlight the continued plasticity of both cognitive and affective systems into adolescence. For instance, adolescence may be a particularly effective time to implement interventions targeting reward-related behaviors, such as behavioral activation. Our findings highlight continued plasticity of emotional and cognitive systems in adolescence; this plasticity appears to confer opportunities for recovery following early-life adversity.
Supplementary Material
Appendix S1. Supplemental materials.
Table S1. AIC and BIC values for all models tested to describe associations among early caregiving group, current caregiving quality and age.
Table S2. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and reward processing at the 12 and 16-year assessments.
Table S3. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and executive function at the 8, 12 and 16-year assessments.
Table S4. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and internalizing symptoms at the 8, 12 and 16-year assessments.
Table S5. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and externalizing symptoms at the 8, 12 and 16-year assessments.
Key points.
Adolescence may be a window of heightened plasticity and sensitivity to positive caregiving environments allowing for recovery following exposure to early-life adversity.
We investigate age-related variability in the associations of caregiving quality with reward processing, executive functioning, and internalizing and externalizing psychopathology in childhood and adolescence in a sample with exposure to early-life institutionalization.
Higher quality caregiving in adolescence was associated with greater reward responsivity, higher executive functioning, and lower internalizing and externalizing symptoms.
Associations of caregiving with executive functioning and psychopathology symptoms were strongest during adolescence.
It may be possible to remediate some of the lasting effects of early-life adversity during adolescence through interventions to romote positive caregiving environments.
Acknowledgements
This study received financial support from John D. and Catherine T. MacArthur Foundation, the Binder Family Foundation, the Jacobs Foundation, and NIMH (R01MH091363; F32MH114317). The authors thank the families and the children who participated in this study, as well as the research team and staff in Romania for their support and investment in this project. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Conflict of interest statement: No conflicts declared.
References
- Akaike H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723. [Google Scholar]
- Bates D, Machler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–51. [Google Scholar]
- Bick J, Zeanah CH, Fox NA, & Nelson CA (2018). Memory and executive functioning in 12-year-old children with a history of institutional rearing. Child Development, 89, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Bos KJ (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioural Neurosciences, 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, Van Duijvenvoorde ACK, Peper JS, & Crone EA (2015). Longitudinal changes in adolescent risk-taking: A comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. Journal ofNeuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD, Pardini DA, & Loeber R (2008). Reciprocal relationships between parenting behavior and disruptive psychopathology from childhood through adolescence. Journal of Abnormal Child Psychology, 36, 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JM (1992). Linear models. In Chambers JM & Hastie TJ (Eds.), Statistical models in S, Chapter 4. Pacific Grove, CA: Wadsworth & Brooks/Cole. [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, & Suleiman AB (2018). Importance of investing in adolescence from a developmental science perspective. Nature, 554, 441–450. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan J-A, Proffitt TM, Mahony K, & Pantelis C (2003). Normative data from the cantab. I: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology, 25, 242–254. [DOI] [PubMed] [Google Scholar]
- Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA, & McLaughlin KA (2019). Differential associations of distinct forms of childhood adversity with neurobehavioral measures of reward processing: A developmental pathway to depression. Child Development, 90, e96–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale CE, Donzella B, & Gunnar MR (2018). Pubertal recalibration of cortisol reactivity following early life stress: A cross-sectional analysis. Journal of Child Psychology and Psychiatry, 60, 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, & Kupfer DJ (2002). The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur health and behavior questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 588–603. [DOI] [PubMed] [Google Scholar]
- Flannery JE, Gabard-Durnam LJ, Shapiro M, Goff B, Caldera C, Louie J, … & Tottenham N (2017). Diurnal cortisol after early institutional care—Age matters. Developmental Cognitive Neuroscience, 25, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, de Graaff AM, Caisley H, van Harmelen A-L, & Wilkinson PO (2018). A systematic review of amenable resilience factors that moderate and / or mediate the relationship between childhood adversity and mental health in young people. Frontiers in Psychiatry, 9, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore S-J (2015). Adolescence as a sensitive period of brain development. Trends in Cognitive Sciences, 19, 558–566. [DOI] [PubMed] [Google Scholar]
- Galvan. (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Depasquale CE, Reid BM, & Donzella B (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proceedings of the National Academy of Sciences of the United States of America, 116, 23984–23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Van Dulmen MHM (2007). Behavior problems in postinstitutionalized internationally adopted children. Development and Psychopathology, 19, 129–148. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, Kirwan ML, Benson BE, Hardin MG, Pine DS, Ernst M, & Fox NA (2013). Validation of a child-friendly version of the monetary incentive delay task. Social Cognitive and Affective Neuroscience, 8, 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, McGoron L, Sheridan MA, McLaughlin KA, Fox NA, Nelson CA, & Zeanah CH (2015). High-quality foster care mitigates callous-unemotional traits following early deprivation in boys: A randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Tang A, Almas AN, Degnan KA, McLaughlin KA, Nelson CA, … & Drury SS (2018). Caregiving disruptions affect growth and pubertal development in early adolescence in institutionalized and fostered Romanian children: A randomized clinical trial. Journal of Pediatrics, 203, 345–353 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Graber MG, Colich NL, & Gotlib IH (2020). Coupling of waking cortisol with DHEA and testosterone across the pubertal transition: Associations with threat-related early life stress. Psychoneuroendocrinology, 115, 104651. 10.1101/691279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2016). Tests in linear mixed effects models. Journal of Statistical Software, 82, 1–26. [Google Scholar]
- Larsen B, & Luna B (2018). Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience and Biobehavioral Reviews, 94, 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube C, van den Bos W, & Fandakova Y (2020). The relationship between pubertal hormones and brain plasticity: Implications for cognitive training in adolescence. Developmental Cognitive Neuroscience, 42, 100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Estan N, Weinberger DR, & Casey BJ (2014). Adolescent mental health–Opportunity and obligation. Science, 346, 547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, & O’Hearn K (2010). What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition, 72, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69, 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, & Fox NA (2019). How early experience shapes human development: The case of psychosocial deprivation. Neural Plasticity, 2019, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, & Guthrie D (2007). Cognitive recovery in socially deprived young children: The bucharest early intervention project. Science, 318, 1937–1940. [DOI] [PubMed] [Google Scholar]
- Olino TM (2016). Future research directions in the positive valence systems: measurement, development, and implications for youth unipolar depression. Journal of Clinical Child & Adolescent Psychology, 45, 681–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, Gould E, & Sullivan R (2017). Early life adversity during the infant sensitive period for attachment: Programming of behavioral neurobiology of threat processing and social behavior. Developmental Cognitive Neuroscience, 25, 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, & Gunnar M (2012). The confluence of adverse early experience and puberty on the cortisol awakening response. International Journal of Behavioral Development, 36, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rid A (2012). When is research socially valuable? Lessons from the bucharest early intervention project: Commentary on a case study in the ethics of mental health research. The Journal of Nervous and Mental Disease, 200, 248–249. [DOI] [PubMed] [Google Scholar]
- Schwarz G (1978). Estimating the dimension of a model. Annals of Statistics, 6, 461–464. [Google Scholar]
- Sheridan MA, McLaughlin KA, Winter W, Fox N, Zeanah C, & Nelson CA (2018). Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nature Communications, 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Fox NA, & Nelson CA (2009). A new model of foster care for young children: The bucharest early intervention project. Child and Adolescent Psychiatric Clinics of North America, 18, 721–734. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Fox NA, Nelson CA, & Guthrie D (2010). Placement in foster care enhances quality of attachment among young institutionalized children. Child Development, 81, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibu F, Sheridan MA, McLaughlin KA, Nelson CA, Fox NA, & Zeanah CH (2016). Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychological Medicine, 46, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M, Fox NA, Zeanah CH, & Nelson CA (2019). Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proceedings of the National Academy of Sciences of the United States of America, 116, 1808–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M, Zeanah CH, Fox NA, & Nelson CA (2019). Global deficits in executive functioning are transdiagnostic mediators between severe childhood neglect and psychopathology in adolescence. Psychological Medicine, 50, 1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltman H, Feldstain A, Mackay JC, & Rocchi M (2012). An introduction to hierarchical linear modeling. Tutorials in Quantitative Methods for Psychology, 8, 52–69. [Google Scholar]
- Zeanah CH, Fox NA, & Nelson CA (2012). The bucharest early intervention project. The Journal of Nervous and Mental Disease, 200, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, & Koga S (2003). Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology, 15, 885–907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental materials.
Table S1. AIC and BIC values for all models tested to describe associations among early caregiving group, current caregiving quality and age.
Table S2. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and reward processing at the 12 and 16-year assessments.
Table S3. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and executive function at the 8, 12 and 16-year assessments.
Table S4. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and internalizing symptoms at the 8, 12 and 16-year assessments.
Table S5. Linear mixed effects model (LMER) results for associations among early caregiving quality, concurrent caregiving quality, age and externalizing symptoms at the 8, 12 and 16-year assessments.