Abstract
This review provides an update on the neurocognitive phenotype of pediatric obstructive sleep apnea (OSA). Pediatric OSA is associated with neurocognitive deficits involving memory, learning, and executive functioning. Adenotonsillectomy (AT) is presently accepted as the first line surgical treatment for pediatric OSA, but the executive function deficits do not resolve post-surgery, and the timeline for recovery remains unknown. This finding suggests that pediatric OSA potentially causes irreversible damage to multiple areas of the brain. The focus of this review is the hippocampus, one of the 2 major sites of postnatal neurogenesis, where new neurons are formed and integrated into existing circuitry and the mammalian center of learning/memory functions. Here, we review the clinical phenotype of pediatric OSA, and then discuss existing studies of OSA on different cell types in the hippocampus during critical periods of development. This will set the stage for future study using preclinical models to understand the pathogenesis of persistent neurocognitive dysfunction in pediatric OSA.
Keywords: Hippocampus, Intermittent Hypoxia, Learning and Memory, Neurocognition, Pediatric Obstructive Sleep Apnea, Reactive Oxygen Species
Introduction
Obstructive sleep apnea (OSA) is characterized by repeated episodes of partial or complete airway obstruction, circadian dysrhythmias, and sleep fragmentation. Several types of neurocognitive deficits have been described based on the child’s age and disease severity 1,2. The pathogenesis of these changes, as well as why the neurocognitive changes in the pediatric population remain persistent despite treatment is a fundamental gap in our understanding of pediatric OSA 3,4. We briefly describe the neurocognitive phenotype followed the effect of intermittent hypoxia on individual cell types in the hippocampus.
Methods
We searched PubMed and Cochrane databases for English language studies with the following keywords: Pediatric OSA, intermittent hypoxia, hippocampus, neural progenitors, ANPs, neural stem cells (NSCs), immature and mature neurons published from 2010-2020 along with relevant references within those articles. Emphasis was given to randomized clinical trials, and original controlled research studies along with articles/journals that are frequently studied by clinicians and scientists.
Overview of OSA
OSA is characterized by cyclical hypoxia/normoxia that induces reactive oxygen species (ROS) and oxidative stress 5. Intermittent hypoxemia causes arousal from REM sleep with hypoxic hypercapnia being the most potent stimulus6. Sleep becomes fragmented, leading to daytime somnolence. Furthermore, the blood brain barrier (BBB), gray matter volume, and cerebral blood flow (CBF) are altered. The BBB becomes more permeable, gray matter volumes are decreased, and CBF fluctuates leading to a number of pathogenic changes 7 (Figure 1). Systemic inflammation8 and sympathetic excitation 9 are additional hallmark features.
Figure 1:
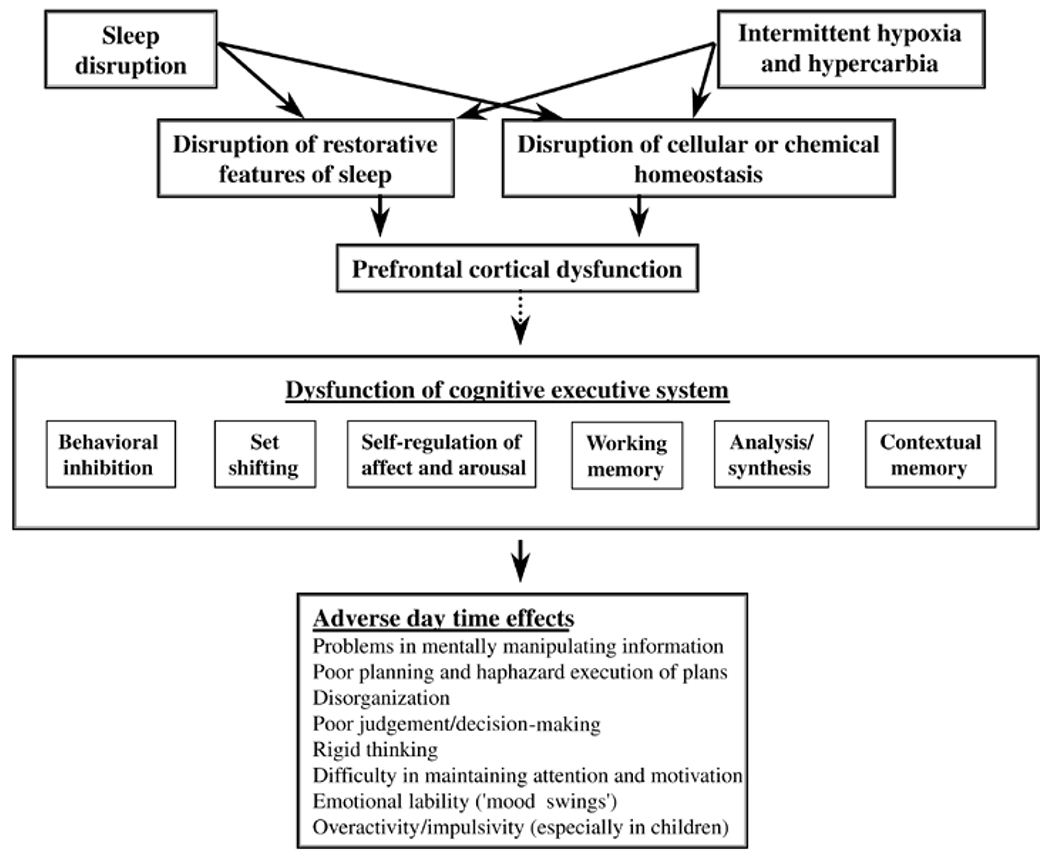
Demonstrates the multifactorial effects of arousal and somnolence on memory and learning. There are multiple pathological mechanisms that are affected, leading to multipathway effects on several organ systems, including the CNS. These effects lead to a variety of clinical presentations. The classical adult presentation of OSA is with daytime somnolence, whereas in pediatric OSA a number of children present with problems in the school environment. Reproduced with permission from: Beebe DW, Gozal D. J Sleep Res 2002;11(1):1-16.
While direct data in pediatric OSA is lacking, changes in the BBB during periods of chronic inflammation have been implicated in sleep and neurodegenerative disorders in the adult population10. There are several contrasts between pediatric and adult OSA with regards to the CNS features of the disease including symptomatology and areas of brain involved (Table 1).
Table 1:
Brain areas, postulated mechanisms, and reversibility of neurocognitive dysfunction.
| Adult | Pediatric | |
|---|---|---|
| Areas of Brain involved | • Right Middle Frontal Lobe Gyrus11 • Posterior cingulate gyrus • Left inferior frontal gyrus12 • Bilateral hippocampi13 |
• Bilateral hippocampi, Right frontal cortex14 |
| Pathogenesis of neurocognitive dysfunction | • Small vessel disease15
• CBF changes • Systemic inflammation16 |
• Oxidative Stress • Sleep Spindle Changes 17 |
| Reversibility with therapy | • Improvement in most cognitive domains after CPAP | • Improvement in behavior after surgery, but persistence in executive function deficits3,18 |
Hypercarbia
Arterial PCO2 rises during the apneic/hypopneic episodes in adult OSA19. Unfortunately, CO2 levels are often not measured or are not reported in many studies on pediatric OSA20. From sparse data available, higher baseline PCO2 levels in pediatric OSA correlated with persistent OSA following adenotonsillectomy 21. But even with very severe pediatric OSA, the elevation in CO2 is small and remains there for short periods of time in the context of total sleep time21. Therefore, we focus on chronic intermittent hypoxia rather than the hypercarbia, which in the context of current data appears to attenuate or has no effect on the nature of CNS injury.
Clinical Neurocognitive Phenotype of Pediatric OSA
Numerous studies in the pediatric OSA population have focused specifically on the CNS. Most of these studies are on school-aged children (5 years or older) with SDB/mild OSA22–24. Although changes secondary to OSA have been described in several areas of the brain, the hippocampal changes are the focus of this paper as it is the mammalian center of memory, learning, and early emotional behavior.25 Any changes early in the process of rapid neuronal growth have significant effects later in life26, partially explaining why even mild OSA in children can manifest with learning or cognitive deficits. Also, significant clinical deficits take time to come to the attention of parents or teachers as learning and memory integration is an ongoing process, 27. What mediates ongoing learning and memory deficits post-AT remains unclear28, and a preclinical model of the disease is urgently required. Given that early learning and memory are hippocampally mediated in the young29, the remainder of this article will focus on pre-clinical studies in the hippocampus, specifically the effects of hypoxia on the various cell types.
Adult disease affects several key brain areas through both local mechanisms and effect on vasculature. The effect of these factors on brain areas in the pediatric population remain unknown, however there are changes in sleep spindles. Neurocognitive morbidity is seen in both populations. For example, with dementia, as seen in the adult population, there is improvement in cognitive metrics after CPAP therapy. In children however, there are not notable increases in learning memory function after intervention.
Hippocampus
The hippocampus is one of two neurogenic niches for postnatal mammalian neurogenesis 30, a dynamic throughout the lifespan 31 and has numerous functions including the generation of new memories, learning, and pattern separation which are a function of newborn neurons 32. The neural stem cells (NSCs) reside in the sub granular zone (SGZ) of the dentate gyrus and give rise to amplifying neural progenitors (ANPs), which rapidly divide and eventually give rise to neuroblasts. Neuroblasts demonstrate high rates of apoptosis 33 and those that survive differentiate into immature neurons and subsequently mature neurons, which integrate into the hippocampal circuitry 34 (Figure 2).
Figure 2:
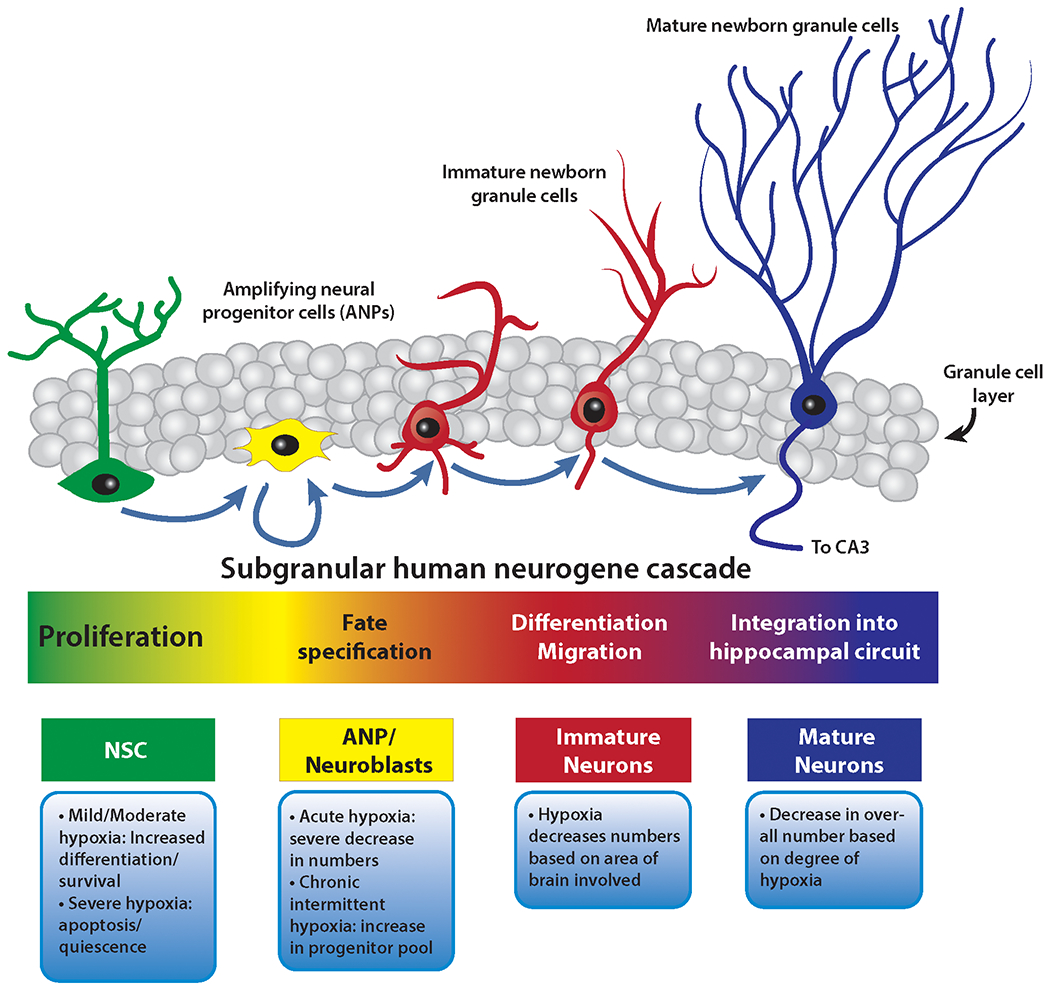
Various cell types and the effect of hypoxia on these cell types in the mammalian hippocampus. Hypoxia has different effects at different parts of the neurogenic cascade, and promotes apoptosis beyond the NSC stage. The morphology of each cell type under intermittent hypoxia has not been studied.Therefore, while varying levels of hypoxia may effect different areas of the cascade differently, the effects of intermittent hypoxia, with consequent ROS formation has not been elucidated. There could be differences in number, morphology, and function, all of which need to interrogated to properly understand how the mammalian hippocampus responds to intermittent hypoxia.
They influence both regional physiology and the functional connectivity of the hippocampus with more distant brain regions, such as the prefrontal cortex, amygdala, and other structures within the limbic system.35 Pattern separation, which is a critical function of the hippocampus, facilitates temporal event discrimination, spatial processing, and short-term memory storage 36.
The role of Reactive oxygen species
Reactive Oxygen Species (ROS) are generated during oxidative metabolism in mitochondria and consist of, amongst others, hydrogen peroxide, radical and non-radical oxygen species. Excess ROS formation, which occurs in OSA37, leads to oxidative stress damage(Figure 3).
Figure 3:
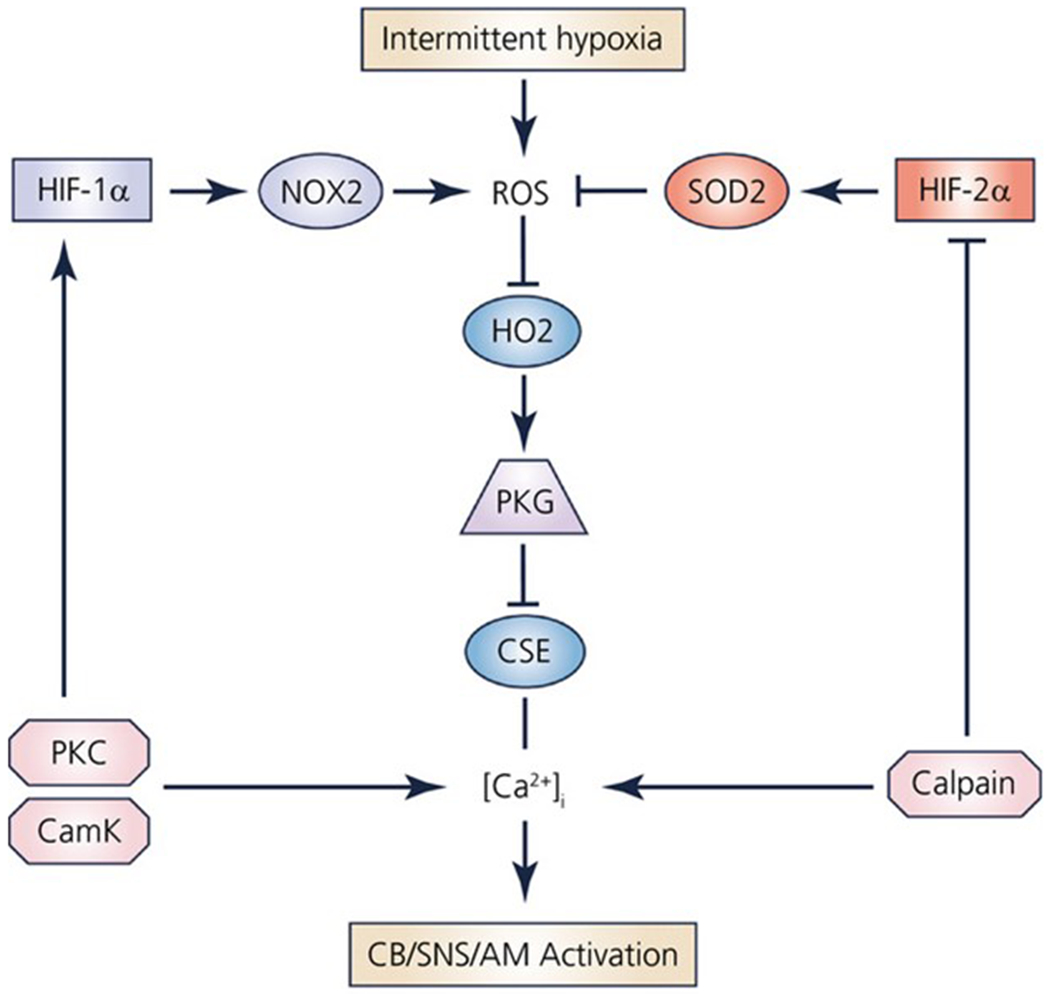
The interplay between ROS, HIF-α, Superoxide dismutase (SOD), haemoxygenase 2(HO2), and pathophysiological changes including sympathetic nervous system activation. HIF-α has downstream effects on several other pathways as well. The effects of ROS in the context of the pediatric OSA, specifically with regards to the intermittent hypoxia stimulus, remain unknown. There is a convergence on the end-point of both SNS/adrenal medulla activation, which leads to a higher level of circulating catecholamines in these patients. This has been one of the postulated mechanisms of hypertension in both pediatric and adult OSA. AM: Adrenal Medulla, SNS: sympathetic nervous system, Nox2 (NAPDH oxidase 2), PKG: Protein Kinase G, PKC: Protein Kinase C. With permission113
ROS have a dual role in that they are vital to cell repair and longevity 38, via both effects on transcription and synaptic activity. ROS effect transcription of several key gene mediators leading to several local and systemic effects. Acute intermittent hypoxia also does not lead to neuroinflammation 39. However, continuous exposure is detrimental to CA-1 neurons in the hippocampus, which are selectively vulnerable to hypoxia in adult rodent models of OSA40. CA-1 neurons show delayed injury pattern with impaired cellular metabolism41, from mitochondrial dysfunction42 as well as endoplasmic reticulum (ER) stress43,44.
Preclinical Rodent Models of Adult OSA
There have been two model types described for adult OSA. The first is the intermittent hypoxia model. This model demonstrated that after intermittent cyclical hypoxia for 14 days, adult rats demonstrated deficits in the Morris Water Maze Task (MWM)40. This test interrogates spatial memory which is a hippocampal function, and postmortem studies demonstrated CA-1 deficits. Follow up studies have revealed abnormalities in dentate gyrus (DG) function as well45. This exact model was replicated in younger rats46, utilizing a moderate-severe clinical phenotype, which are not the children in whom neurobehavioral deficits are typically seen. The second model is the tracheal balloon occlusion model, which utilizes a surgically implanted tracheal balloon with intermittent inflation47. This model results in hypoxia and hypercarbia as seen in OSA. The latter model has not been neurobehaviorally validated. To date, there is no preclinical model recapitulating the neurocognitive phenotype of pediatric OSA. This has at least partially been due to a lack of validated learning and memory paradigms in neonatal mice. For example, MWM is not technically feasible due to hypothermia risk, and object recognition is not validated for use in this age group48. Accordingly, to enable further study of pediatric OSA, an age-appropriate animal model is necessary.
Oxygen tension in the rodent neonatal hippocampus
Hippocampal O2 levels are far lower than the alveolar oxygen levels of 21% (room air/normoxia) 49 however remain exquisitely vulnerable to hypoxic injury 50. In the hippocampus normoxia is 8%, mild hypoxia is 5-8%, moderate hypoxia is oxygen at 1-5%, and less than 1% is severe hypoxia. Pimonidazole HCl labelling of hypoxic cells demonstrated that much of the SGZ lies within hypoxic zones which is believed to be less than 20 mm Hg51. Relative hypoxia in vitro (<5%) drives proliferation of neural progenitor and precursor cells51,52 (Figure 4).
Figure 4:
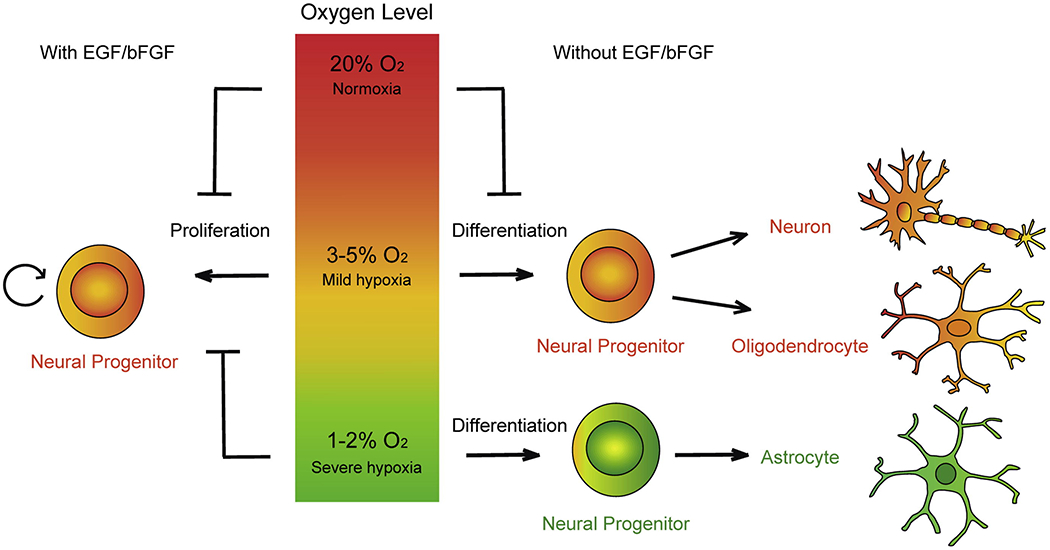
The effects of various levels of hypoxia on progenitor cell behavior. Severe continuous hypoxia seems to facilitate astrocytic transformation, whereas moderate hypoxia promotes precocious differentiation.Since self-renewal is important in the maintenance of the stem cell pool, both forms of hypoxia cause depletion of the progenitor pool leading to a net loss of available NSCs. The long term effect of progenitor pool depletion on lifelong learning and memory is not well understood. Xie Y, Lowery W. Methods 2018 with permission.
However, once these cells migrate away from hypoxic zones usually due to differentiation they seem to lose the hypoxic “protections” and are more susceptible to apoptosis 53–55. All the studies were performed using continuous hypoxic conditions; the effects of intermittent hypoxia on early stem cell growth remains unknown. These findings demonstrate that oxygen balance within the dentate gyrus is tightly regulated and a small change in either direction has a profound effect on NSC behavior 56. This balance is at least partially mediated by uncoupling protein 2(UP2), which effected mitochondrial-ER connections in the rodent cortex and hippocampus57, as well as by nitric oxide(NO) for perivascular cells58.
HIF-α is central to the cellular response to hypoxia59. HIF-α protects against hypoxic insult and allows for injury attenuation60. HIF-α inactivation causes impaired neurogenesis and learning deficits61 even in the absence of hypoxia. Under hypoxic conditions, HIF-α promotes NSC proliferation62. This is through HIF-α effect on numerous mechanisms/canonical pathways including p53, Notch, Wnt/beta-catenin, and Oct4 63–67.
Hypoxic Effect on Neural Progenitors
Santilli et al. 68demonstrated that severe hypoxia encouraged early ANP production from NSCs as compared to normoxic conditions. The same study showed that continuous in-vitro mild hypoxia drives self-renewal of NSCs. Similarly, deFillipis 69 demonstrated in vitro that mild hypoxia encouraged ANP production from NSCs whereas severe hypoxia facilitated apoptosis and quiescence. Chronic continuous hypoxia facilitates the survival and proliferation of progenitor cells [49]. In Notch knockout mice, the survival effect is not present, demonstrating that Notch1, at least in part, may mediate progenitor survival [85]. Furthermore, although hypoxic preconditioning increases differentiation of NSCs70 , the presence of co-existing hypertension had no added effect on the differentiation 71, suggesting that change in CBF/small vessel disease contributes minimally to the behavior of NSCs. Even perivascular placement, with greater access to blood supply did not seem to confer any survival advantage65.
ANPs have a precipitous drop in population after an acute hypoxic ischemic insult 72. However, self-renewal begins after a brief dip in the progenitor pool 73. Chronic severe hypoxia, however, appears to produce the opposite effect, decreasing the pool of ANPs, which in turn decreases the number of neuroblasts and immature/mature neurons as a downstream effect 74.
Intermittent hypoxia leads to an increase in progenitors; however, with a lower number of newborn neurons compared to normoxia in adult murine OSA models 45. Intermittent hypobaric hypoxia also increases the number of progenitors and newborn neurons in the adult rodent hippocampus 75, again suggesting that changes in oxygen tension and baricity (pressure) within a small range can cause large shifts in the progenitor pool. Given the sensitivity of the progenitor pool to minor O2 tension differences, work is required on the effect of IH on the progenitor pool in the young.
Hypoxic Effect on Neuroblasts
Neuroblasts are exquisitely vulnerable to hypoxic injury76. It has been demonstrated that immature but committed neuroblasts die after an acute hypoxic insult, followed by proliferation of ANPs that eventually replace the lost neuroblast pool 76 Because progenitors divide rapidly, a fast turnover of the neuroblasts can rescue the damage from the hypoxic injury 77. Therefore, despite the sensitivity of certain neuroblast populations to hypoxic injury, there are multiple mechanisms by which this population is able to repopulate78.
Hypoxic Effect on Immature Neurons
The mechanisms of injury and death of immature neurons is age-dependent and changes from apoptosis in young mammals to necrosis in older mammals 79. Both GABA and glycine have been noted to be critical mediators 80, as well as Notch81. Neuronal death can be attenuated with iron chelators and free radical scavengers 82, suggesting that ROSs are involved 83. The window of maximal damage is in postnatal days 2-5 in mice (corresponding to birth to 6 months age window in humans) 84,85. Mild hypothermia has been demonstrated to be somewhat protective after intermittent hypoxia at this stage 86.
Hypoxic Effect on Mature Neurons
Chatzi et al 53 used adult mice to test for the effects of hypoxia on newborn neurons. They demonstrated that these cells vacillate between continuous low-oxygen-tension environments, which promote early precursor proliferation, and continuous higher-oxygen-tension environments, which may be deleterious for newborn neurons. Taken together, these data suggest that oxygen tension is a critical component of newborn neuron fate and that the balance between hypoxia and normoxia is carefully maintained for newborn neurons 68,81 Given that integration into the hippocampal circuit is a competitive process, and not all newborn neurons are guaranteed survival benefit87, oxygen tension being a key mediator of survival. This further suggests that any disruption in oxygen tension may have effects not only on the neuronal differentiation process, but also their ability to contribute to hippocampal circuitry and function.
CA-3 neurons are more resistant to hypoxia vs CA-1 neurons, postulated to be due to Ca+2 mediation, 40 although the mechanisms are multifactorial41. Multiple mechanisms of hippocampal neuronal alterations have been suggested including c-AMP-protein kinase A signaling and CREB-mediated gene transcription [80]. Furthermore, glutamate mediates CA-1 neuronal apoptosis to hypoxia in guinea pig apnea models 88. Magnetic resonance spectroscopy (MRS) has also demonstrated that N-acetyl-aspartate (NAA, neuronal integrity biomarker) and choline (membrane turnover) were decreased in rodents exposed to intermittent hypoxia from P2-P12 [83]. This effect was validated in humans, where change in choline levels in the midbrain were correlated with an increased in excitotoxic biomarkers including glutamate89. Cofilin, an actin-binding protein which disassembles actin filaments, mediates dendritic spine loss and decreased hippocampal neuronal plasticity in CA-1 neurons 90. Elevated cofilin activity from sleep deprivation is caused by cAMP-degrading phosphodiesterase-4A5 (PDE4A5), which hampers cAMP-PKA-LIMK signaling. Inhibition of cofilin activity prevented spine loss and increased plasticity. Overall, there is a clear decline in the number of mature neurons after hypoxic insult, depending on degree and duration of hypoxemia 91. While adult OSA models have demonstrated disorganization of CA-1 neuron architecture after IH exposure85, the pediatric neuronal changes remain unknown.
Hypoxic Effect on Glial Cells
Glial cells (astrocytes, oligodendrocytes, and microglia) have scavenging and neuroprotective functions. Astrocytes have been found to be relatively more resistant to hypoxia than are microglia, through multiple mechanisms 92.
Astrocytes
Intermittent hypoxia in rats produces hippocampal astrocytosis with a high rate of neuronal cell death; however, chronic hypoxia increases both neuronal survival and expression of S100β (secreted by astrocytes or by injured neurons) 93 as compared to acute hypoxia. NADPH oxidase(Nox), a mammalian enzyme involved in both astrocytic and microglial phagocytic burst 94, has been demonstrated to be putative to several processes including hypoxia/reoxygenation injury, carbonylation, and pro-inflammatory states95. NADPH has been demonstrated to be involved in the pathogenesis of the neurocognitive deficit seen in adult OSA 96. A postulated mechanism is due to upregulation of CHOP (CCAAT enhancer binding homologous protein), which mediates HIF-α, Nox 2, oxidative and apoptosis markers97. Hypoxia also stimulates astrocyte expression of aquaporin 4 (AQ4), which leads to local edema 98. Aquaporin 1 is also increased in areas of high content of astrocytes, suggesting that cytotoxic edema is central to pathogenesis in OSA 99.
At least a part of astrocytes’ initial tolerance to hypoxia is conferred by upregulation of glucose transport and uptake in both astrocytes and mature neurons 100, postulated to be due to osmotic and electrochemical gradients101 and altered transport of other ions such as Na+ and neurotransmitters including glutamate across the cell junctions102. However, altering RAGE (receptor for advanced glycosylation end products) and nuclear factor kappa b (NF-kB), which are involved in converting astrocytes into a pro-inflammatory phenotype, reduced the neurocognitive deficit in adult male mice 103. Taken together, these suggest that astrocytic function is central to continued neuronal survival and health in OSA.
Oligodendrocytes
Oligodendrocytes myelinate axons and are found only in the CNS. After intermittent hypoxic exposure in neonatal rats, pathological findings include decreased oligodendrocyte markers 104. Several myelin proteins (MBP, PLP, MAG, and CNPase) were also down-regulated after exposure to intermittent hypoxia, suggesting arrest of oligodendrocyte maturation 105. Oligodendrocytes have also been demonstrated to be a key player in memory consolidation in mammals106. Therefore, any arrest or injury would not only affect motor function, but memory as well.
Microglia
Microglia mediate central nervous system ROS through NADPH oxidase, mitochondria, and excitatory neurotransmitters 107. Given the neurocognitive damage from OSA is at least partially mediated through ROS, there is a yet to be elucidated role for microglia in the pathogenesis of OSA induced neurocognitive dysfunction. Since microglia are also involved in synaptic pruning, perturbations in microglial activity have been postulated in several psychiatric disorders108. Suppression of microglia activation after sleep deprivation has been demonstrated to lead to improvement in adult neurogenesis and spatial memory109.The implication is that microglial activation is a key player in sleep deprivation based neurocognitive dysfunction.
Furthermore, TLR4, MAPK, transcription factors, and epigenetic factors all have been suggested to contribute to microglial activation 110. In animal models, the injection of LPS in stressed rats, but not unstressed rats, activate the pro-inflammatory microglial phenotype, leading directly to cell injury 111. This finding suggests that the interplay of oxidative stress and other environmental factors including ROS may be central to the pathogenesis of neurocognitive change, especially in the young 112. In summary, glial cells play critical roles in the post-hypoxic neuronal injury models; however, much remains to be elucidated about their function, especially in the pediatric population.
Conclusion:
Intermittent hypoxia in the young may have long lasting neurocognitive effects. These changes, while affecting multiple areas of the brain, are more pronounced in the hippocampus, which is the mammalian center for early learning and memory. These changes can be attributed to several constitutive changes in hippocampal cell types and function. Given the connections between the hippocampus and other areas of the brain, the changes can be more widespread. While the clinical reversibility of these changes is unknown, there is an urgent need for a preclinical model to further study this common disorder of childhood.
Acknowledgments
Financial Disclosures: Financial Disclosures: AC, ACA, and MT have no relevant financial disclosures. FK is supported by R01ES029442, I01CX0000104, and R01AI135803. RSR is supported by R01HL130249 and R21OD025327. SR is supported by R21EY027447 and R21EY028690. No other relevant non-financial disclosures.
Abbreviations:
- ANP
(amplified nuclear progenitors)
- AT
(adenotonsillectomy)
- BBB
(blood brain barrier)
- CBF
(cerebral blood flow)
- CIHH
(chronic intermittent hypoxia and hypercarbia)
- CNS
(central nervous system)
- OSA
(obstructive sleep Apnea)
- ROS
(reactive oxygen species)
- SES
(socioeconomic status)
- REM
(rapid eye movement)
- NREM
(non-rapid eye movement)
- SGZ
(sub granular zone)
- NSC
(neural stem cells)
References
- 1.Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci 2006;9:388–99. [DOI] [PubMed] [Google Scholar]
- 2.Galland BC, Dawes PJ, Tripp EG, Taylor BJ. Changes in behavior and attentional capacity after adenotonsillectomy. Pediatr Res 2006;59:711–6. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, Paruthi S, Muzumdar H, Gozal D, Thomas NH, Ware J, Beebe D, Snyder K, Elden L, Sprecher RC, Willging P, Jones D, Bent JP, Hoban T, Chervin RD, Ellenberg SS, Redline S, Childhood Adenotonsillectomy T. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biggs SN, Vlahandonis A, Anderson V, Bourke R, Nixon GM, Davey MJ, Horne RS. Long-term changes in neurocognition and behavior following treatment of sleep disordered breathing in school-aged children. Sleep 2014;37:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie L Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev 2003;7:35–51. [DOI] [PubMed] [Google Scholar]
- 6.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol (1985) 1998;84:1926–36. [DOI] [PubMed] [Google Scholar]
- 7.Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002;166:1382–7. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep 2007;30:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Chen Y, Gua C, Wu B. Elevated Oxidative Stress and Inflammation in Hypothalamic Paraventricular Nucleus Are Associated With Sympathetic Excitation and Hypertension in Rats Exposed to Chronic Intermittent Hypoxia. Front Physiol 2018;9:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun 2017;60:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Dalmases M, Sole-Padulles C, Torres M, Embid C, Nunez MD, Martinez-Garcia MA, Farre R, Bargallo N, Bartres-Faz D, Montserrat JM. Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA: A Randomized Pilot Study. Chest 2015;148:1214–23. [DOI] [PubMed] [Google Scholar]
- 12.Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat 2015;11:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, Bozzali M, Fasano F, Giulietti G, Djonlagic I, Malhotra A, Marciani MG, Guttmann CR. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 2011;54:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halbower AC, Degaonkar M, Barker PB, Earley CJ, Marcus CL, Smith PL, Prahme MC, Mahone EM. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med 2006;3:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerner NA, Roose SP. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am J Geriatr Psychiatry 2016;24:496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin WC, Huang CC, Chen HL, Chou KH, Chen PC, Tsai NW, Chen MH, Friedman M, Lin HC, Lu CH. Longitudinal brain structural alterations and systemic inflammation in obstructive sleep apnea before and after surgical treatment. J Transl Med 2016;14:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockmann PE, Damiani F, Pincheira E, Daiber F, Ruiz S, Aboitiz F, Ferri R, Bruni O. Sleep spindle activity in children with obstructive sleep apnea as a marker of neurocognitive performance: A pilot study. Eur J Paediatr Neurol 2018;22:434–9. [DOI] [PubMed] [Google Scholar]
- 18.Waters KA, Chawla J, Harris MA, Heussler H, Black RJ, Cheng AT, Lushington K. Cognition After Early Tonsillectomy for Mild OSA. Pediatrics 2020;145. [DOI] [PubMed] [Google Scholar]
- 19.Earing C, Owen J, Griffith-Mcgeever C, McKeon D, Engeli S, Moore J, Kubis HP. An act of balance: interaction of central and peripheral chemosensitivity with inflammatory and anti-inflammatory factors in obstructive sleep apnoea. Respir Physiol Neurobiol 2019. [DOI] [PubMed] [Google Scholar]
- 20.Socarras MA, Landau BP, Durr ML. Diagnostic techniques and surgical outcomes for persistent pediatric obstructive sleep apnea after adenotonsillectomy: A systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol 2019;121:179–87. [DOI] [PubMed] [Google Scholar]
- 21.Isaiah A, Hamdan H, Johnson RF, Naqvi K, Mitchell RB. Very Severe Obstructive Sleep Apnea in Children: Outcomes of Adenotonsillectomy and Risk Factors for Persistence. Otolaryngol Head Neck Surg 2017;157:128–34. [DOI] [PubMed] [Google Scholar]
- 22.Hunter SJ, Gozal D, Smith DL, Philby MF, Kaylegian J, Kheirandish-Gozal L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am J Respir Crit Care Med 2016;194:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DL, Gozal D, Hunter SJ, Philby MF, Kaylegian J, Kheirandish-Gozal L. Impact of sleep disordered breathing on behaviour among elementary school-aged children: a cross-sectional analysis of a large community-based sample. Eur Respir J 2016;48:1631–9. [DOI] [PubMed] [Google Scholar]
- 24.Mietchen JJ, Bennett DP, Huff T, Hedges DW, Gale SD. Executive Function in Pediatric Sleep-Disordered Breathing: A Meta-analysis. J Int Neuropsychol Soc 2016;22:839–50. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev 2003;9:149–54. [DOI] [PubMed] [Google Scholar]
- 26.Takada SH, Motta-Teixeira LC, Machado-Nils AV, Lee VY, Sampaio CA, Polli RS, Malheiros JM, Takase LF, Kihara AH, Covolan L, Xavier GF, Nogueira MI. Impact of neonatal anoxia on adult rat hippocampal volume, neurogenesis and behavior. Behav Brain Res 2016;296:331–8. [DOI] [PubMed] [Google Scholar]
- 27.Hollands C, Tobin MK, Hsu M, Musaraca K, Yu TS, Mishra R, Kernie SG, Lazarov O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol Neurodegener 2017;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song SA, Tolisano AM, Cable BB, Camacho M. Neurocognitive outcomes after pediatric adenotonsillectomy for obstructive sleep apnea: A systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol 2016;83:205–10. [DOI] [PubMed] [Google Scholar]
- 29.Johnson EG, Prabhakar J, Mooney LN, Ghetti S. Neuroimaging the sleeping brain: Insight on memory functioning in infants and toddlers. Infant Behav Dev 2020;58:101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taupin P Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev 2006;2:213–9. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313–7. [DOI] [PubMed] [Google Scholar]
- 32.Kempermann G Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 2002;22:635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B, Sierra A, Deudero JJ, Semerci F, Laitman A, Kimmel M, Maletic-Savatic M. Multitype Bellman-Harris branching model provides biological predictors of early stages of adult hippocampal neurogenesis. BMC Syst Biol 2017;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 2007;10:727–34. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci 2006;26:12237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res 2007;163:567–76. [DOI] [PubMed] [Google Scholar]
- 37.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavie L Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev 2015;20:27–45. [DOI] [PubMed] [Google Scholar]
- 39.Peters ME, Kimyon RS, Mitchell GS, Watters JJ. Repetitive acute intermittent hypoxia does not promote generalized inflammatory gene expression in the rat CNS. Respir Physiol Neurobiol 2015;218:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 2001;21:2442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartsch T, Dohring J, Reuter S, Finke C, Rohr A, Brauer H, Deuschl G, Jansen O. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab 2015;35:1836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medvedeva YV, Ji SG, Yin HZ, Weiss JH. Differential Vulnerability of CA1 versus CA3 Pyramidal Neurons After Ischemia: Possible Relationship to Sources of Zn2+ Accumulation and Its Entry into and Prolonged Effects on Mitochondria. J Neurosci 2017;37:726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu LH, Xie H, Shi ZH, Du LD, Wing YK, Li AM, Ke Y, Yung WH. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid Redox Signal 2015;23:695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandrakantan A, Adler AC. Pediatric Obstructive Sleep Apnea: Neurocognitive Consequences. Curr Anesthesiol Rep 2019;9:110–5. [Google Scholar]
- 45.Khuu MA, Pagan CM, Nallamothu T, Hevner RF, Hodge RD, Ramirez JM, Garcia AJ 3rd. Intermittent Hypoxia Disrupts Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. J Neurosci 2019;39:1320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 2002;52:449–53. [DOI] [PubMed] [Google Scholar]
- 47.Crossland RF, Durgan DJ, Lloyd EE, Phillips SC, Reddy AK, Marrelli SP, Bryan RM, Jr. A new rodent model for obstructive sleep apnea: effects on ATP-mediated dilations in cerebral arteries. Am J Physiol Regul Integr Comp Physiol 2013;305:R334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contreras MP, Born J, Inostroza M. The expression of allocentric object-place recognition memory during development. Behav Brain Res 2019;372:112013. [DOI] [PubMed] [Google Scholar]
- 49.Schneider J, Berndt N, Papageorgiou IE, Maurer J, Bulik S, Both M, Draguhn A, Holzhutter HG, Kann O. Local oxygen homeostasis during various neuronal network activity states in the mouse hippocampus. J Cereb Blood Flow Metab 2017:271678X17740091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt-Kastner R Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience 2015;309:259–79. [DOI] [PubMed] [Google Scholar]
- 51.Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 2001;128:263–76. [DOI] [PubMed] [Google Scholar]
- 52.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010;7:483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatzi C, Schnell E, Westbrook GL. Localized hypoxia within the subgranular zone determines the early survival of newborn hippocampal granule cells. Elife 2015;4:e08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platero-Luengo A, Gonzalez-Granero S, Duran R, Diaz-Castro B, Piruat JI, Garcia-Verdugo JM, Pardal R, Lopez-Barneo J. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell 2014;156:291–303. [DOI] [PubMed] [Google Scholar]
- 55.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011;9:298–310. [DOI] [PubMed] [Google Scholar]
- 56.Vieira HL, Alves PM, Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol 2011;93:444–55. [DOI] [PubMed] [Google Scholar]
- 57.Varela L, Schwartz ML, Horvath TL. Mitochondria controlled by UCP2 determine hypoxia-induced synaptic remodeling in the cortex and hippocampus. Neurobiol Dis 2016;90:68–74. [DOI] [PubMed] [Google Scholar]
- 58.Lourenco CF, Santos RM, Barbosa RM, Cadenas E, Radi R, Laranjinha J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic Biol Med 2014;73:421–9. [DOI] [PubMed] [Google Scholar]
- 59.Ashok BS, Ajith TA, Sivanesan S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin Exp Pharmacol Physiol 2017;44:327–34. [DOI] [PubMed] [Google Scholar]
- 60.Xing J, Lu J. HIF-1alpha Activation Attenuates IL-6 and TNF-alpha Pathways in Hippocampus of Rats Following Transient Global Ischemia. Cell Physiol Biochem 2016;39:511–20. [DOI] [PubMed] [Google Scholar]
- 61.Carrica L, Li L, Newville J, Kenton J, Gustus K, Brigman J, Cunningham LA. Genetic inactivation of hypoxia inducible factor 1-alpha (HIF-1alpha) in adult hippocampal progenitors impairs neurogenesis and pattern discrimination learning. Neurobiol Learn Mem 2019;157:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi C, Zhang J, Chen X, Wan J, Wang J, Zhang P, Liu Y. Hypoxia stimulates neural stem cell proliferation by increasing HIF1alpha expression and activating Wnt/beta-catenin signaling. Cell Mol Biol (Noisy-le-grand) 2017;63:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavali PL, Saini RK, Matsumoto Y, Agren H, Funa K. Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. J Biol Chem 2011;286:9393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui XP, Xing Y, Chen JM, Dong SW, Ying DJ, Yew DT. Wnt/beta-catenin is involved in the proliferation of hippocampal neural stem cells induced by hypoxia. Ir J Med Sci 2011;180:387–93. [DOI] [PubMed] [Google Scholar]
- 65.Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol 2010;12:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, D’Avella D, Panchision DM, Della Puppa A, Scienza R, Basso G. Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells 2010;28:1918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 2008;9:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One 2010;5:e8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Filippis L, Delia D. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci 2011;68:2831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motomura A, Shimizu M, Kato A, Motomura K, Yamamichi A, Koyama H, Ohka F, Nishikawa T, Nishimura Y, Hara M, Fukuda T, Bando Y, Nishimura T, Wakabayashi T, Natsume A. Remote ischemic preconditioning protects human neural stem cells from oxidative stress. Apoptosis 2017;22:1353–61. [DOI] [PubMed] [Google Scholar]
- 71.Pedroso D, Nunes AR, Diogo LN, Oudot C, Monteiro EC, Brenner C, Vieira HLA. Hippocampal neurogenesis response: What can we expect from two different models of hypertension? Brain Res 2016;1646:199–206. [DOI] [PubMed] [Google Scholar]
- 72.Lim DC, Brady DC, Po P, Chuang LP, Marcondes L, Kim EY, Keenan BT, Guo X, Maislin G, Galante RJ, Pack AI. Simulating obstructive sleep apnea patients’ oxygenation characteristics into a mouse model of cyclical intermittent hypoxia. J Appl Physiol (1985) 2015;118:544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daval JL, Pourie G, Grojean S, Lievre V, Strazielle C, Blaise S, Vert P. Neonatal hypoxia triggers transient apoptosis followed by neurogenesis in the rat CA1 hippocampus. Pediatr Res 2004;55:561–7. [DOI] [PubMed] [Google Scholar]
- 74.Chung E, Kong X, Goldberg MP, Stowe AM, Raman L. Erythropoietin-mediated neuroprotection in a pediatric mouse model of chronic hypoxia. Neurosci Lett 2015;597:54–9. [DOI] [PubMed] [Google Scholar]
- 75.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 2010;30:12653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus 2008;18:793–806. [DOI] [PubMed] [Google Scholar]
- 77.Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci 2006;26:4359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costine BA, Missios S, Taylor SR, McGuone D, Smith CM, Dodge CP, Harris BT, Duhaime AC. The subventricular zone in the immature piglet brain: anatomy and exodus of neuroblasts into white matter after traumatic brain injury. Dev Neurosci 2015;37:115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu CL, Siesjo BK, Hu BR. Pathogenesis of hippocampal neuronal death after hypoxia-ischemia changes during brain development. Neuroscience 2004;127:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao P, Qian H, Xia Y. GABA and glycine are protective to mature but toxic to immature rat cortical neurons under hypoxia. Eur J Neurosci 2005;22:289–300. [DOI] [PubMed] [Google Scholar]
- 81.Zhang K, Zhao T, Huang X, Wu LY, Wu K, Zhu LL, Fan M. Notch1 mediates postnatal neurogenesis in hippocampus enhanced by intermittent hypoxia. Neurobiol Dis 2014;64:66–78. [DOI] [PubMed] [Google Scholar]
- 82.Almli LM, Hamrick SE, Koshy AA, Tauber MG, Ferriero DM. Multiple pathways of neuroprotection against oxidative stress and excitotoxic injury in immature primary hippocampal neurons. Brain Res Dev Brain Res 2001;132:121–9. [DOI] [PubMed] [Google Scholar]
- 83.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol 2006;575:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci 2016;152:244–8. [DOI] [PubMed] [Google Scholar]
- 85.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett 2001;305:197–201. [DOI] [PubMed] [Google Scholar]
- 86.Kwak M, Lim S, Kang E, Furmanski O, Song H, Ryu YK, Mintz CD. Effects of Neonatal Hypoxic-Ischemic Injury and Hypothermic Neuroprotection on Neural Progenitor Cells in the Mouse Hippocampus. Dev Neurosci 2015;37:428–39. [DOI] [PubMed] [Google Scholar]
- 87.Bergami M, Berninger B. A fight for survival: the challenges faced by a newborn neuron integrating in the adult hippocampus. Dev Neurobiol 2012;72:1016–31. [DOI] [PubMed] [Google Scholar]
- 88.Fung SJ, Xi MC, Zhang JH, Sampogna S, Yamuy J, Morales FR, Chase MH. Apnea promotes glutamate-induced excitotoxicity in hippocampal neurons. Brain Res 2007;1179:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macey PM, Sarma MK, Prasad JP, Ogren JA, Aysola R, Harper RM, Thomas MA. Obstructive sleep apnea is associated with altered midbrain chemical concentrations. Neuroscience 2017;363:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, Bruinenberg VM, Poplawski SG, Day JP, Aton SJ, Radwanska K, Meerlo P, Houslay MD, Baillie GS, Abel T. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.d’Anglemont de Tassigny X, Sirerol-Piquer MS, Gomez-Pinedo U, Pardal R, Bonilla S, Capilla-Gonzalez V, Lopez-Lopez I, De la Torre-Laviana FJ, Garcia-Verdugo JM, Lopez-Barneo J. Resistance of subventricular neural stem cells to chronic hypoxemia despite structural disorganization of the germinal center and impairment of neuronal and oligodendrocyte survival. Hypoxia (Auckl) 2015;3:15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo G, Bhat NR. Hypoxia/reoxygenation differentially modulates NF-kappaB activation and iNOS expression in astrocytes and microglia. Antioxid Redox Signal 2006;8:911–8. [DOI] [PubMed] [Google Scholar]
- 93.Aviles-Reyes RX, Angelo MF, Villarreal A, Rios H, Lazarowski A, Ramos AJ. Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: implications for sleep apnea. J Neurochem 2010;112:854–69. [DOI] [PubMed] [Google Scholar]
- 94.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 2004;76:760–81. [DOI] [PubMed] [Google Scholar]
- 95.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 2005;172:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 2011;6:e19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chou YT, Zhan G, Zhu Y, Fenik P, Panossian L, Li Y, Zhang J, Veasey S. C/EBP homologous binding protein (CHOP) underlies neural injury in sleep apnea model. Sleep 2013;36:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaur C, Sivakumar V, Zhang Y, Ling EA. Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia 2006;54:826–39. [DOI] [PubMed] [Google Scholar]
- 99.Baronio D, Martinez D, Fiori CZ, Bambini-Junior V, Forgiarini LF, Pase da Rosa D, Kim LJ, Cerski MR. Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respir Physiol Neurobiol 2013;185:217–21. [DOI] [PubMed] [Google Scholar]
- 100.Yu S, Zhao T, Guo M, Fang H, Ma J, Ding A, Wang F, Chan P, Fan M. Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res 2008;1211:22–9. [DOI] [PubMed] [Google Scholar]
- 101.Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci 1998;18:3554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orellana JA, Hernandez DE, Ezan P, Velarde V, Bennett MV, Giaume C, Saez JC. Hypoxia in high glucose followed by reoxygenation in normal glucose reduces the viability of cortical astrocytes through increased permeability of connexin 43 hemichannels. Glia 2010;58:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Angelo MF, Aguirre A, Aviles Reyes RX, Villarreal A, Lukin J, Melendez M, Vanasco V, Barker P, Alvarez S, Epstein A, Jerusalinsky D, Ramos AJ. The proinflammatory RAGE/NF-kappaB pathway is involved in neuronal damage and reactive gliosis in a model of sleep apnea by intermittent hypoxia. PLoS One 2014;9:e107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simonova Z, Sterbova K, Brozek G, Komarek V, Sykova E. Postnatal hypobaric hypoxia in rats impairs water maze learning and the morphology of neurones and macroglia in cortex and hippocampus. Behav Brain Res 2003;141:195–205. [DOI] [PubMed] [Google Scholar]
- 105.Cai J, Tuong CM, Gozal D. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir Physiol Neurobiol 2011;178:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW. Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020;105:150–64 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Q, Wang Y, Feng J, Cao J, Chen B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr Dis Treat 2013;9:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanger B, Couch A, Rajendran L, Srivastava DP, Vernon AC. Emerging Developments in Human Induced Pluripotent Stem Cell-Derived Microglia: Implications for Modelling Psychiatric Disorders With a Neurodevelopmental Origin. Front Psychiatry 2020;11:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, Kishore K, Kumar S, Panjwani U. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation 2017;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Kiernan EA, Smith SM, Mitchell GS, Watters JJ. Mechanisms of microglial activation in models of inflammation and hypoxia: Implications for chronic intermittent hypoxia. J Physiol 2016;594:1563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging 2011;32:85–102. [DOI] [PubMed] [Google Scholar]
- 112.Daulatzai MA. Role of sensory stimulation in amelioration of obstructive sleep apnea. Sleep Disord 2011;2011:596879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Semenza GL, Prabhakar NR. The role of hypoxia-inducible factors in carotid body (patho) physiology. J Physiol 2018;596:2977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


