Abstract
Background
The benefit of endovascular thrombectomy for acute ischemic stroke with M2 segment middle cerebral artery occlusion remains controversial, with uncertainty and paucity of data specific to this population.
Objective
To compare outcomes between M1 and M2 occlusions in the Analysis of Revascularization in Ischemic Stroke with EmboTrap (ARISE II) trial.
Methods
We performed a prespecified analysis of the ARISE II trial with the primary outcome of 90-day modified Rankin Scale score of 0–2, which we termed good outcome. Secondary outcomes included reperfusion rates and major adverse events. The primary predictor was M2 occlusion, which we compared with M1 occlusion.
Results
We included 183 patients, of whom 126 (69%) had M1 occlusion and 57 (31%) had M2 occlusion. There was no difference in the reperfusion rates or adverse events between M2 and M1 occlusions. The rate of good outcome was not different in M2 versus M1 occlusions (70.2% vs 69.7%, p=0.946). In a logistic regression model adjusted for age, sex, and baseline National Institutes of Health Stroke Scale score, M2 occlusions did not have a significantly different odds of good outcome compared with M1 occlusions (OR 0.94, 95% CI 0.47 to 1.88, p=0.87).
Conclusion
In ARISE II, M2 occlusions achieved a 70.2% rate of good outcome at 90 days, which is above published rates for untreated M2 occlusions and superior to prior reports of M2 occlusions treated with endovascular thrombectomy. We also report similar rates of good outcome, successful reperfusion, death, and other adverse events when comparing the M1 and M2 occlusions.
Keywords: stroke, angiography, brain, thrombectomy
Introduction
The benefit of endovascular thrombectomy (EVT) for acute ischemic stroke (AIS) caused by M2 segment middle cerebral artery (MCA) occlusion has not been evaluated in a randomized controlled trial specific to that population. The natural history of an untreated M2 occlusion, although not as ominous as an untreated M1 segment or internal carotid artery occlusion, is nonetheless poor. Fewer than half of patients achieve functional independence by 90 days.1 A small number of patients with AIS with M2 occlusion were included in the landmark EVT trials, and multiple retrospective studies and meta-analyses have been published, all of which show benefit for EVT.2–6 A meta-analysis of the Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke (HERMES) trials database, which included pooled data from several of the landmark EVT trials, showed that patients with AIS with M2 occlusion who received EVT had significantly better 90-day outcomes than patients with an M2 occlusion who did not undergo EVT.7
The Analysis of Revascularization in Ischemic Stroke with EmboTrap (ARISE II) trial reported that the EmboTrap revascularization device achieved high rates of successful reperfusion and good functional outcome with minimal adverse events.8 A quarter of the patients enrolled in ARISE II had M2 occlusion, which was permissible in the trial because the 0.021” microcatheter compatibility of EmboTrap allowed entry into the M2 segment. Given the ongoing uncertainty about the efficacy of EVT for M2 occlusions and lack of data specific to EmboTrap in this population, we performed a subgroup analysis of ARISE II to compare rates of successful reperfusion, adverse events, and good outcome between M1 and M2 occlusions.
Methods
Cohort
We performed a prespecified analysis of the ARISE II trial, an open-label, single-arm, multicenter, prospective clinical study designed to evaluate the safety and efficacy of the EmboTrap device in patients with AIS compared with efficacy rates for two FDA-approved stent retriever devices.8 The data for this analysis were de-identified, which did not require institutional review board approval. All data relevant to the study are included in the article or uploaded as online supplemental information.
neurintsurg-2020-016427supp001.pdf (39.3KB, pdf)
The EmboTrap revascularization device (Neuravi/Cerenovus, Galway, Ireland) is a dual-layer stent retriever, engineered with articulating petals, and a distal capture zone for effectively trapping, retaining, and removing large vessel occlusive clots causing AIS. Key ARISE II inclusion criteria included pre-stroke modified Rankin Scale (mRS) score ≤1, baseline National Institutes of Health Stroke Scale (NIHSS) score ≥8 and ≤25, Alberta Stroke Program Early CT Score (ASPECTS) ≥6 or core infarct volume <50 mL on MRI or CT-based imaging (for anterior circulation strokes), and treatment with intravenous tissue-type plasminogen activator, if eligible, within 3 hours of stroke onset. For our analysis, we included ARISE II patients with angiographically confirmed M1 and M2 MCA occlusion and excluded those with posterior circulation or internal carotid artery occlusion.
Outcomes and predictors
The primary outcome of our analysis was good functional outcome defined as an mRS score of 0–2 at 90 days from stroke onset. The secondary outcomes included: (1) successful reperfusion, defined as modified thrombolysis in cerebral infarction (mTICI) score ≥2 b, ≥2c, and 3 after the first pass, last of up to three passes (ARISE II primary endpoint), and final pass; (2) the ordinal categories of the mRS; (3) good outcome defined as mRS score 0–1 at 90 days; and (4) major adverse events, including death within 90 days, symptomatic intracranial hemorrhage, and procedure-related serious adverse events. The imaging core laboratory defined mTICI for M2 occlusions based on the percentage reperfusion of the territory supplied by the occluded M2.
The primary predictor is M2 occlusion, which we compared with M1 occlusion because of its similarity in phenotype and procedural approach. We also analyzed our outcomes by the following imaging core laboratory adjudicated stratifications: dominant versus non-dominant M2 and proximal versus distal M2 origin. The following definitions were applied: dominant M2 (supplying ≥50% of the MCA territory), non-dominant M2 (supplying <50% of the MCA territory), proximal M2 (assessed on coronal images; origin proximal to the mid-sylvian point), and distal M2 (origin distal to the mid-sylvian point).7
Statistical analysis
Categorical data are presented as proportions, normally distributed continuous data as mean with SD, and ordinal data as median with IQR. Subject characteristics at enrollment were summarized by the full cohort and the subgroups of M1 versus M2 occlusion. We tested for intergroup differences in the M1/2 occlusion subgroups, M2 stratifications, and in our primary and secondary outcomes with the χ2 test or Fisher’s exact test (due to expected cell sizes <5) as appropriate for binary variables, the Wilcoxon rank-sum test for ordinal variables, and Student’s t-test or a Satterthwaite approximation in cases of unequal variances as appropriate for continuous variables. We fitted multivariate logistic regression models with covariates that were chosen from patient age (reference <75 years), sex (reference female), NIHSS score at admission, and time from symptom onset or last known well to arterial puncture using least absolute shrinkage and selection operator variable selection.
Results
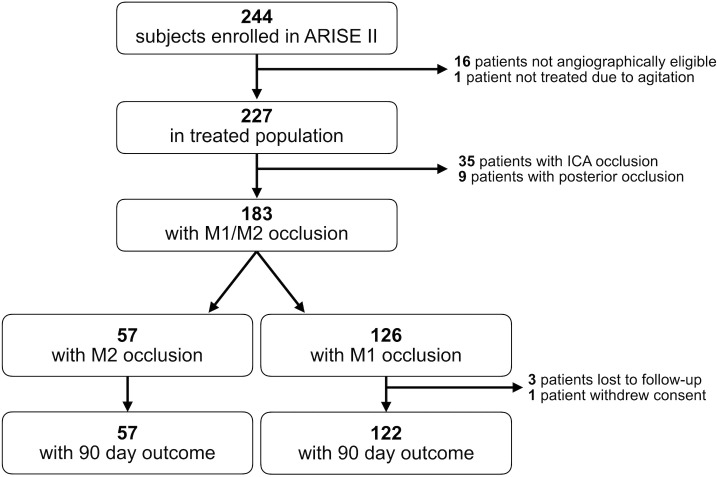
The ARISE II study enrolled 244 patients, of which we included 183 (see figure 1). In our cohort, 126 (69%) had M1 occlusion and 57 (31%) had M2 occlusion. Baseline demographics are shown in table 1. The patients with an M2 occlusion were significantly older than M1 occlusion patients (71.2 vs 66.9 years, p=0.033) and had a lower mean NIHSS score (14.4 vs 15.9, p=0.051).
Figure 1.
Study flow chart showing the derivation of our cohort.
Table 1.
Baseline demographic data for the full cohort (n=183), and after stratification by M1 (n=126) and M2 (n=57) middle cerebral artery (MCA) occlusions
| Demographic data | All subjects (n=183) |
MCA M1 (n=126) |
MCA M2 (n=57) |
P value |
| Age at enrollment, years | ||||
| Mean (SD) | 68.2 (12.9) | 66.9 (13.1) | 71.2 (12.0) | 0.0330 |
| Median | 70 | 69 | 75 | |
| Min to max | 20 to 86 | 23 to 85 | 20 to 86 | |
| ≥75, N (%) | 74 (40.4) | 45 (35.7) | 29 (50.9) | 0.0529 |
| <75, N (%) | 109 (59.6) | 81 (64.3) | 28 (49.1) | |
| Sex, N (%) | ||||
| Male | 87 (47.5) | 57 (45.2) | 30 (52.6) | 0.3537 |
| Female | 96 (52.5) | 69 (54.8) | 27 (47.4) | |
| NIHSS score at presentation | ||||
| Mean (SD) | 15.42 (4.42) | 15.90 (4.41) | 14.37 (4.31) | 0.0507 |
| Median | 16 | 16 | 14 | |
| Q1 to Q3 | 12 to 19 | 12 to 19 | 12 to 18 | |
| Min to max | 7 to 26 | 8 to 26 | 7 to 23 | |
| Baseline ASPECTS | ||||
| N | 168 | 117 | 51 | |
| Mean (SD) | 9.38 (1.16) | 9.38 (1.22) | 9.37 (1.00) | 0.3723 |
| Median | 10.00 | 10.00 | 10.00 | |
| Q1 to Q3 | 9.00 to 10.00 | 9.00 to 10.00 | 9.00 to 10.00 | |
| Min to max | 4.00 to 10.00 | 4.00 to 10.00 | 5.00 to 10.00 | |
| Pre-stroke mRS score, N (%) | ||||
| 0 | 139 (76.0) | 96 (76.2) | 43 (75.4) | 0.8983 |
| 1 | 43 (23.5) | 29 (23.0) | 14 (24.6) | |
| 2 | 1 (0.5) | 1 (0.8) | 0 | |
| Occlusion side, left, N (%) | 87 (47.5) | 57 (45.2) | 30 (52.6) | 0.3537 |
| Symptom onset or LKW to arterial puncture, min | ||||
| N | 140 | 95 | 45 | |
| Mean (SD) | 201.59 (77.28) | 198.72 (76.26) | 207.64 (79.93) | 0.5251 |
| Median | 195 | 187 | 214 | |
| Hypertension, N (%) | 127 (69.4) | 85 (67.5) | 42 (73.7) | 0.3975 |
| Diabetes mellitus, N (%) | 37 (20.2) | 25 (19.8) | 12 (21.1) | 0.8501 |
| Atrial fibrillation, N (%) | 72 (39.3) | 47 (37.3) | 25 (43.9) | 0.4003 |
| Hyperlipidemia, N (%) | 80 (43.7) | 50 (39.7) | 30 (52.6) | 0.1020 |
| Smoking, N (%) | 48 (26.2) | 36 (28.6) | 12 (21.1) | 0.2843 |
| Previous MI/CAD, N (%) | 39 (21.3) | 30 (23.8) | 9 (15.8) | 0.2199 |
| Previous stroke, N (%) | 33 (18.0) | 21 (16.7) | 12 (21.1) | 0.4748 |
| Intravenous tPA failure, N (%) | 123 (67.2) | 85 (67.5) | 38 (66.7) | 0.9157 |
For continuous variables, p values are generated using a t-test, with a Satterthwaite approximation in cases of unequal variances.
For ordinal variables (NIHSS score at presentation, baseline ASPECTS), p values are generated using the Wilcoxon rank-sum test.
ASPECTS, baseline Alberta Stroke Program Early CT score; CAD, coronary artery disease; IA, intra-arterial; IV, intravenous; LKW, last known well; MCA, middle cerebral artery; MI, myocardial; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale/Score; tPA, tissue plasminogen activator.
For categorical variables, p values are generated using a χ2 test or a Fisher’s exact test (due to expected cell sizes <5), as appropriate.
There was no difference in the imaging core laboratory adjudicated reperfusion rates between M1 and M2 occlusions (online supplemental table 1). The rates of mTICI score ≥2c at the first pass, last of up to three passes, and final pass were 38.6%, 64.9%, and 73.7% for M2 occlusions. These rates did not differ compared with M1 occlusions (all p values ≥0.7). We did observe a higher percentage of mTICI scores of 3 after the final pass for M2 occlusion than for M1 occlusion (56.1% vs 46.8%, p=0.243), but it did not reach statistical significance.
All serious procedure-related adverse events are shown in table 2. The rates of serious adverse events adjudicated by the ARISE-II Clinical Events Committee, including procedure-related complications, procedure-related mortality, and symptomatic intracranial hemorrhage within 24 hours of stroke onset, were not significantly different between M1 and M2 occlusions (table 2). Additional adverse events, including adjudication of relatedness to the study intervention, are shown in online supplemental table 2, including the Heidelberg Bleeding Classification of postprocedural hemorrhage.9 No serious adverse events adjudicated by the Clinical Events Committee were found to be related to the EmboTrap device.
Table 2.
Serious procedure-related adverse events as determined by the Clinical Events Committee
| Adverse events | MCA M1 (n=126) |
MCA M2 (n=57) |
P value* |
| Procedure-related serious adverse events | 4 (3.2%) | 5 (8.8%) | 0.1396 |
| Postprocedural subarachnoid hemorrhage | 2 (1.6%) | 2 (3.5%) | 0.5896 |
| Vessel perforation | 2 (1.6%) | 0 (0%) | 1.0000 |
| Carotid artery dissection | 0 (0%) | 1 (1.8%) | 0.3115 |
| Cerebral artery occlusion | 0 (0%) | 1 (1.8%) | 0.3115 |
| Ischemic stroke (iatrogenic) | 1 (0.8%) | 0 (0%) | 1.0000 |
| Neurological decompensation | 0 (0%) | 1 (1.8%) | 0.3115 |
| Vessel puncture site hemorrhage | 0 (0%) | 1 (1.8%) | 0.3115 |
| Vessel puncture site thrombosis | 0 (0%) | 1 (1.8%) | 0.3115 |
| Symptomatic intracranial hemorrhage within 24 hours | 6 (4.8%) | 2 (3.5%) | 1.0000 |
| Procedure-related mortality (at 7 days postprocedure) |
0 | 0 | – |
*For categorical variables, p values are generated using a χ2 test or a Fisher’s exact test (due to expected cell sizes <5), as appropriate.
MCA, middle cerebral artery.
A 90-day mRS score was available in 179/183 patients in our cohort. Good outcome (mRS score 0–2) was present in 125/179 (69.8%) of patients, and the rate was not different in M2 vs M1 occlusion (70.2% vs 69.7%, p=0.946). The percentage of patients in the individual categories of the 90 day mRS score was balanced (table 3), as was the percentage with a 90-day mRS of 0–1 (M2 vs M1 occlusion, 57.9% vs 54.9%, p=0.709).
Table 3.
Modified Rankin Scale (mRS) scores at 90 days, which were available in 122 patients with M1 and 57 patients with M2 middle cerebral artery (MCA) occlusions
| MCA M1 (n=122) |
MCA M2 (n=57) |
P value* | |
| Good outcome (90 day mRS score 0–2), n (%) | 85 (69.7) | 40 (70.2) | 0.9455 |
| 90-Day mRS score, n (%) | |||
| 0 | 37 (30.3) | 16 (28.1) | 0.9358 |
| 1 | 30 (24.6) | 17 (29.8) | |
| 2 | 18 (14.8) | 7 (12.3) | |
| 3 | 9 (7.4) | 4 (7.0) | |
| 4 | 15 (12.3) | 5 (8.8) | |
| 5 | 5 (4.1) | 2 (3.5) | |
| 6 | 8 (6.6) | 6 (10.5) | |
| 90-Day mRS score 0–1, n (%) | 67 (54.9) | 33 (57.9) | 0.7087 |
*For categorical variables, p-values are generated using a χ2 test or a Fisher’s exact test (due to expected cell sizes<5) as appropriate.
In a multivariate logistic regression model adjusted for age, sex, and NIHSS, M2 occlusion did not have an association with good outcome (OR 0.94, 95% CI 0.47 to 1.88, p=0.87). In a multivariate logistic regression model adjusted for age and NIHSS, M2 occlusion also did not have an association with mRS score 0–1 (OR 0.92, 95% CI 0.43 to 1.96, p=0.83) or all-cause mortality (OR 1.43, 95% CI 0.43 to 4.70, p=0.56) (online supplemental table 3).
There were 33/57 (57.9%) patients with a dominant M2 versus 24/57 (42.1%) with a non-dominant M2, and 27/57 (47.4%) with a proximal M2 origin versus 30/57 (52.6%) with a distal M2 origin. The angiographic and functional outcomes were not significantly different in these M2 stratifications (table 4).
Table 4.
Primary outcomes in stratifications of patients with M2 occlusions, including dominant versus non-dominant M2 and proximal versus distal M2 origin
| Outcome | Dominant (n=33) |
Non-Dominant (n=24) |
P value* | Proximal (n=27) |
Distal (n=30) |
P value* |
| Good outcome (90 day mRS score 0–2) | 22 (66.7%) | 18 (75.0%) | 0.4971 | 17 (63.0%) | 23 (76.7%) | 0.2588 |
| 90-Day mRS 0–1 | 20 (60.6%) | 13 (54.2%) | 0.6269 | 14 (51.9%) | 19 (63.3%) | 0.3807 |
| All-cause mortality at 90 days | 2 (6.1%) | 4 (16.7%) | 0.2275 | 3 (11.1%) | 3 (10.0%) | 1.0000 |
| Final pass mTICI ≥2b | 30 (90.9%) | 22 (91.7%) | 1.0000 | 25 (92.6%) | 27 (90.0%) | 1.0000 |
| Final pass mTICI ≥2c | 24 (72.7%) | 18 (75.0%) | 0.8474 | 18 (66.7%) | 24 (80.0%) | 0.2537 |
| Final pass mTICI 3 | 16 (48.5%) | 16 (66.7%) | 0.1720 | 16 (59.3%) | 16 (53.3%) | 0.6526 |
*For categorical variables, p values are generated using a χ2 test or a Fisher’s exact test (due to expected cell sizes<5) as appropriate.
mTICI, modified thrombolysis in cerebral infarction.
Discussion
The rate of good functional outcome, successful reperfusion, and adverse events did not differ significantly between M1 and M2 occlusions in patients with AIS treated with the EmboTrap device in ARISE II. Among the 833 patients randomized to EVT in the seven major EVT trials published since 2015 (MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME, REVASCAT, DAWN, DEFUSE 3), only 55 patients had an M2 occlusion.10–16 In ARISE II, we enrolled 57 patients with M2 occlusion, which represents a larger sample size. Accordingly, these results are important given the rigor of outcome adjudication in ARISE II and the lack of data specific to the EmboTrap device. Prior studies have established that the M2 segment is a common site of large vessel occlusion that can cause significant morbidity, and the expansion of EVT to this patient population could improve stroke outcomes.17
Compared with patients with AIS with untreated M2 occlusion, EVT with the EmboTrap device almost doubled the percentage of patients who achieve a good outcome (70.2%). In the HERMES database there were 62 patients with untreated M2 occlusion, derived from control arms of clinical trials.7 Those patients had a 39.7% rate of good outcome at 90 days. A multicenter retrospective study by Sarraj et al had 234 patients with untreated M2 occlusion, who had a 35.4% rate of good outcome at 90 days.3 In a final study of the RESUCE-Japan Registry 2 cohort, there were 188 untreated M2 occlusions, who had a higher 50.5% rate of good outcome at 90 days.18 Compared with previously reported data, the outcomes for patients with M2 occlusion with AIS treated with EVT in ARISE II were excellent. For example, in ARISE II, good outcome (mRS score 0–2) at 90 days was seen in 70.2% of M2 occlusions. In the HERMES, Sarraj, and RESCUE-Japan studies, M2 occlusion treated with EVT achieved a rate of good outcome of 58.2%, 62.8%, and 57.1%, respectively. In two meta-analyses comparing EVT in M1 versus M2 occlusions,5 6 the rate of good outcome for M2 occlusions was 59%. All these studies also reported the safety of EVT for M2 occlusions, similar to the data we report.
Both meta-analyses reported that EVT-treated patients with AIS with an M2 occlusion had better outcomes than patients with an M1 occlusion.5 6 In ARISE II, we found no difference in good outcome, reperfusion, or adverse events between patients with M1 and M2 occlusion, although patients in our cohort with M2 occlusion did have a lower mean NIHSS score and a trend towards a higher baseline ASPECT score. The primary results publication of ARISE II had a table in the online supplemental materials that compared its cohort to that of 11 other studies, and found that the ARISE II cohort had a shorter time from stroke onset or last known normal to EVT treatment.8 Time from stroke onset to reperfusion is a critical factor in the odds of achieving good outcome.19 20 The comparatively favorable outcomes for the M1 occlusions in ARISE II may reflect their earlier reperfusion. The EmboTrap also demonstrated higher than average rates of successful reperfusion, which may account for the better outcomes in this cohort.8 Finally, in a meta-analysis of the five randomized controlled trials from 2015 (MR CLEAN, ESCAPE, REVASCAT, SWIFT PRIME, and EXTEND IA), the median (IQR) ASPECT score of EVT-treated patients was 97–10 21 while in ARISE II it was 10 (9-10). This introduces the possibility that the better outcomes in ARISE II patients were due to smaller infarct volumes prior to EVT.
The present study has several limitations. The most important being that although this was a prespecified analysis, it is a subgroup of ARISE II with a limited number of M2 occlusions. We also present the results of a study where only one thrombectomy device was used, which limits the generalizability of our findings. Another limitation is that four patients were lost to follow-up in the M1 occlusion subgroup compared with none in the M2 occlusion subgroup, which might have introduced bias. Furthermore, the lack of a comparator or control group in ARISE II allows comparison only with published cohorts. A direct comparison would be necessary to draw conclusions about the relative efficacy of the EmboTrap for M2 occlusions. These limitations are offset by several strengths—in particular, the use of a clinical outcome and central imaging adjudication process for reperfusion and intracranial hemorrhage with at least two independent raters who were not involved in the trial. In addition, the imaging core laboratory adjudicated mTICI score after the first three and final endovascular thrombectomy passes, provided data on the full spectrum of mTICI scores (including 2c), and was blinded to clinical outcomes. Safety events were adjudicated by an independent Clinical Events Committee also blinded to clinical data.
Conclusion
In ARISE II, the EmboTrap device successfully treated M2 MCA occlusions in patients with AIS, achieving a 70.2% rate of good outcome at 90 days, which is well above published rates for untreated M2 occlusions and also superior to prior reports of EVT-treated M2 occlusions. When comparing the M1 and M2 occlusions in ARISE II, we report similar rates of good outcome, successful reperfusion, death, and other adverse events. The treatment of M2 occlusion in patients with AIS with the EmboTrap device appears safe and efficacious.
Footnotes
Twitter: @marcriboj
Collaborators: ARISE II Collaborators: René Chapot, Ashutosh Jadhav, Jonathan A. Grossberg, Raul G. Nogueira, Tudor G. Jovin, Adnan H. Siddiqui, Mairsil Claffey, Steven W. Hetts, Werner Hacke, Brijesh P. Mehta, Lofti Hacein-Bey, Anthony W. Kim, Alex Abou-Chebl, Peter Shabe, Albert J. Yoo, Guilherme Dabus, Ryan A. Priest, Gary M. Nesbit, Wayne M. Clark, Masahiro Horikawa, David A. Hoak, Bryan D. Petersen, Noah C. Beadell, Kory S. Herrick, Corey R. White, Michelle T. Stacey, Sierra C. Ford, Jesse J. Liu, Alejandro Tomasello, Carlos A. Molina, David Rodriguez-Luna, Sandra Boned-Riera, Jorge Pagola, Marta Rubiera, Jesus M. Juega, Noelia Rodriguez-Villatoro, Hannes Nordmeyer, Michael Stauder, Christian P. Stracke, Markus Heddier, Denis Herbreteau, Richard Bibi, Oliver Francois, D. Pieters, Tom Dewaele, Paul Bourgeois, Frederik Vanhee, Patrick Vanderdouckt, Evelien Vancaester, Brian T. Jankowitz, Andrew F. Ducruet, Amin N. Aghaebrahim, Cynthia L. Kenmuir, Hazem M. Shoirah, Bradley J. Molyneaux, Prasanna K. Tadi, Gena M. Walker, Matthew T. Starr, Diogo C. Haussen, Michael R. Frankel, Nicolas A. Bianchi, Samir R. Belegaje, Nicole D. Mahdi, Sourabh Lahoti, Anna N. Katema, Melanie J. Winngingham, Aaron M. Anderson, Eugene Lin, Christian H. Riedel, Olav Jansen, Fritz Wodarg, Naomi Larsen, Andreas Binder, Daniel Wiesen, Kenneth V. Snyder, Elad I. Levy, Jason M. Davies, Ashish Sonig, Leonardo N. Rangel-Castilla, Ashkan Mowla, Hakeem J. Shakir, Vernard S. Fennel, Gursant S. Atwal, Sabareesh K. Natarajan, J. Beecher, John Thornton, Paul Brennan, Alan O’Hare, Hamed Asadi, Ronald F. Budzik, N. Voraco, Peter J. Pema, Thomas M. David, William J. Hicks, Jennifer D. Mejilla, Mohamed S. Teleb, Peter J. Sunenshine, Jacqueline M. Carter, Christian A. Taschner, Stephan Meckel, Samer Elsheik, Horst Urbach, Christoph A. Maurer, Karl Egger, Wolf D. Nieson, Blaise W. Baxter, Steven D. Quarfordt, Justin A. Calvert, Harris E. Hawk, Reza Malek, Arash M. Padidar, Ursula Kelly-Tolley, A. Gutierrez, Pasquale Mordasini, Rupashani Balasubramaniam, Jan Gralla, Urs Fischer, Felix Zibold, Eike I. Piechowiak, Reade A. DeLeacy, Johanna T. Fifi, Jay Mocco, Sidney Starkman, Viktor Szeder, Satoshi Tateshima, Gary R. Duckwiler, May Nour, Xian N. Tang, Jason D. Hinman, Anita Tipirneni, David S. Liebeskind, Dileep R. Yavagal, S. Suir, Justin M. Caplan, P. Kandewall, Eric C. Peterson, Robert M. Starke, Ajit S. Puri, David E. Rex, Francesco Massari, Ajay Wakhloo, Juan D. Lozano, Katyucia D. Rodrigues, Laurent Pierot, S. Sebastien, M. G. Emmoinoli
Contributors: AdH, APN, and OOZ conceived of the study, drafted the manuscript, and edited the manuscript. TA, MR, HPM, HB, JLS, and AA provided critical editing and guidance for the manuscript.
Funding: This study was the academic work of the authors. ARISE II was sponsored by Neuravi, now Cerenovus, part of Johnson & Johnson. AdH is supported by NIH-NINDS K23NS105924.
Competing interests: AdH receives funding from AMAG and Regeneron pharmaceuticals for investigator initiated research. APN and AA have no personal, financial, or institutional interest to report. JLS is an employee of the University of California, which holds a patent on retriever devices for stroke; the University of California, Regents receives funding for Dr. Saver’s services as a scientific consultant regarding trial design and conduct to Covidien/Medtronic and Stryker; serves as a consultant for Modest, Abbott, Medtronic, Stryker, and Neuravi/Cerenovus; has contracted stock options for Modest and Rapid Medical. HB serves as a modest consultant for Neuravi/Cerenovus, and Stryker. HPM reports personal fees from Covidien/Medtronic, personal fees from Neuravi/Cerenovus, personal fees from Servier, and personal fees from Bayer outside the submitted work; served on the steering committees of the SWIFT PRIME and ARISE studies. MR is a shareholder in Anaconda Biomed, consultant for Neuravi/Cerenovus, Medtronic, Stryker, Apta Targets, and Vesalio. TA is a consultant for Neuravi/Cerenovus, Ablynx, Amnis Therapeutics, Medtronic, Rapid Medical, and Stryker. OOZ serves as a consultant for Neuravi/Cerenovus, Stryker, Penumbra, and Medtronic.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Hernández-Pérez M, Pérez de la Ossa N, Aleu A, et al. Natural history of acute stroke due to occlusion of the middle cerebral artery and intracranial internal carotid artery. J Neuroimaging 2014;24:354–8. 10.1111/jon.12062 [DOI] [PubMed] [Google Scholar]
- 2.Chen C-J, Wang C, Buell TJ, et al. Endovascular mechanical thrombectomy for acute middle cerebral artery M2 segment occlusion: a systematic review. World Neurosurg 2017;107:684–91. 10.1016/j.wneu.2017.08.108 [DOI] [PubMed] [Google Scholar]
- 3.Sarraj A, Sangha N, Hussain MS, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol 2016;73:1291–6. 10.1001/jamaneurol.2016.2773 [DOI] [PubMed] [Google Scholar]
- 4.Gory B, Lapergue B, Blanc R, et al. Contact aspiration versus stent retriever in patients with acute ischemic stroke with M2 occlusion in the ASTER randomized trial (contact aspiration versus stent retriever for successful revascularization). Stroke 2018;49:461–4. 10.1161/STROKEAHA.117.019598 [DOI] [PubMed] [Google Scholar]
- 5.Saber H, Narayanan S, Palla M, et al. Mechanical thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: a meta-analysis. J Neurointerv Surg 2018;10:620–4. 10.1136/neurintsurg-2017-013515 [DOI] [PubMed] [Google Scholar]
- 6.Li G, Huang R, Li W, et al. Mechanical thrombectomy with second-generation devices for acute cerebral middle artery M2 segment occlusion: a meta-analysis. Interv Neuroradiol 2020;26:187–94. 10.1177/1591019919886405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon BK, Hill MD, Davalos A, et al. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: meta-analysis of data from the Hermes collaboration. J Neurointerv Surg 2019;11:1065–9. 10.1136/neurintsurg-2018-014678 [DOI] [PubMed] [Google Scholar]
- 8.Zaidat OO, Bozorgchami H, Ribó M, et al. Primary results of the multicenter ARISE II study (analysis of revascularization in ischemic stroke with EmboTrap). Stroke 2018;49:1107–15. 10.1161/STROKEAHA.117.020125 [DOI] [PubMed] [Google Scholar]
- 9.von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015;46:2981–6. 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 10.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 12.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 13.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 15.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 16.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 17.Rai AT, Domico JR, Buseman C, et al. A population-based incidence of M2 strokes indicates potential expansion of large vessel occlusions amenable to endovascular therapy. J Neurointerv Surg 2018;10:510–5. 10.1136/neurintsurg-2017-013371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura M, Yoshimura S, Sakai N, et al. Endovascular therapy for middle cerebral artery M2 segment occlusion: subanalyses of RESCUE-Japan registry 2. J Neurointerv Surg 2019;11:964–9. 10.1136/neurintsurg-2018-014627 [DOI] [PubMed] [Google Scholar]
- 19.Jahan R, Saver JL, Schwamm LH, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA 2019;322:252–63. 10.1001/jama.2019.8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 21.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2020-016427supp001.pdf (39.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.