To the Editor:
Kidney function is most commonly monitored using estimated glomerular filtration rate (eGFR) and albuminuria, which are markers of glomerular function.1 Tubular secretion is an important nonglomerular kidney function and is critical for toxin excretion and excretion of non-filtered endogenous metabolites.2,3 Creatinine is filtered by the glomerulus and secreted by the proximal tubule such that creatinine clearance (CLcr) overestimates GFR by 10% to 20%. Whether measuring secretory function provides additional insights into kidney tubule health is uncertain. The difference between measured CLcr (mCLcr) and measured GFR (mGFR) represents the clearance of creatinine due to tubular secretion (TScr). To assess whether TScr could be used to assess the impact of secretion on longterm clinical outcomes, we performed a post hoc analysis of the MDRD Study.
During the baseline period of the MDRD Study before randomization, mGFR was determined using 125I-iothalamate and mCLcr was determined using a 24-hour urine collection performed at 2 time points (Item S1).4 Of 840 participants with mGFR data, 838 completed a baseline urine collection. For participants with mCLcr and mGFR at both time points (n = 718), the average of the 2 was considered (Table S1). We evaluated the association of TScr with time to initiation of kidney replacement therapy (KRT) for kidney failure (primary outcome) and all-cause and cardiovascular disease (CVD) mortality (secondary outcomes) through December 31, 2010. We used Cox regression after adjusting for demographics, comorbid conditions, lifestyle factors, and laboratory parameters (including baseline proteinuria and mGFR). In sensitivity analyses, we calculated the slope of mGFR between the 2 prerandomization visits and adjusted for it instead of mGFR.
Mean age of 838 participants was 51.7 years and 60.5% were men. At baseline, mean mGFR, mCLcr, and TScr were 33.0, 42.4, and 9.5 ± 7.3 mL/min/1.73 m2, respectively. Compared with the highest TScr quartile, there were no statistically significant differences in age, sex, race, smoking status, or diabetes in participants with lower TScr, whereas BMI was higher across quartiles of TScr and prevalent CVD was lower (Table S2). At any level of mGFR, there was a wide distribution of TScr across participants, and TScr was positively and modestly correlated with mGFR (Fig 1).
Figure 1.
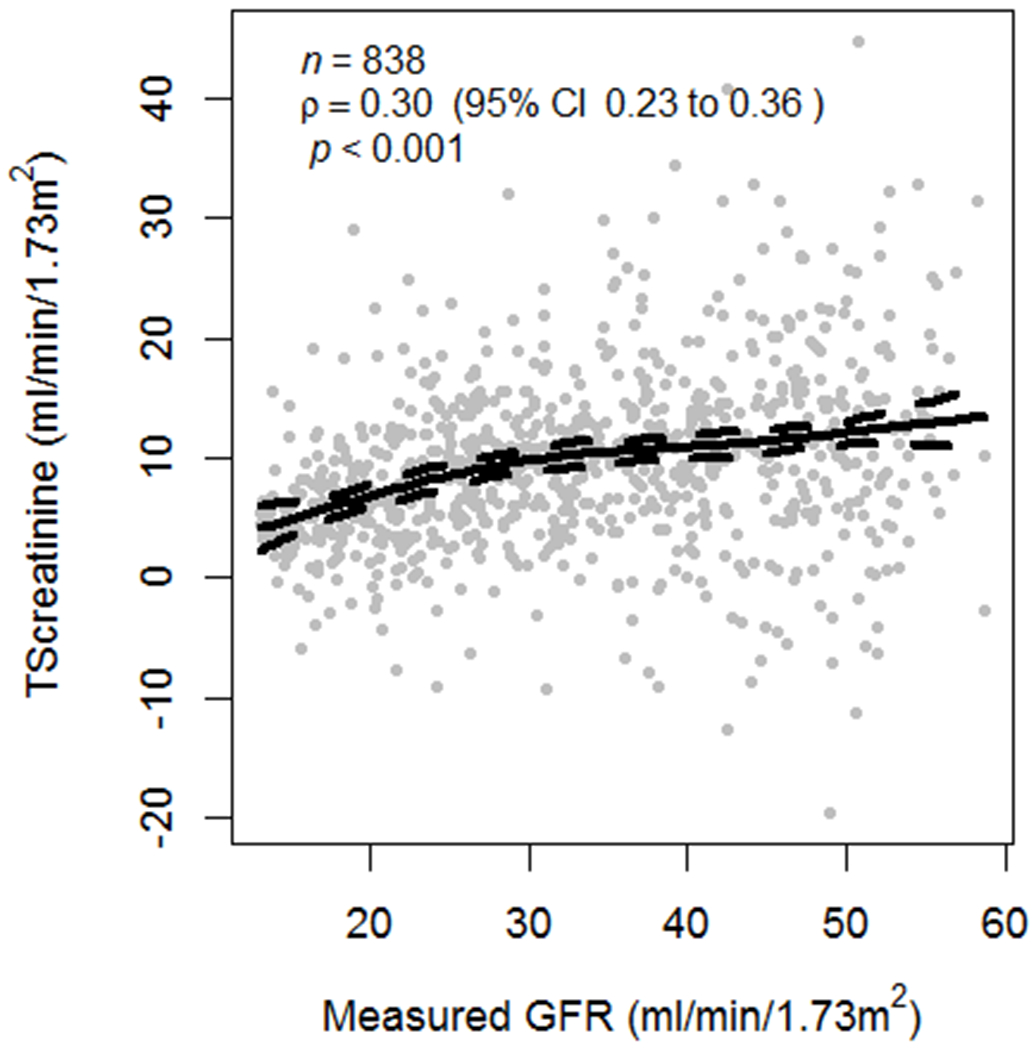
Scatter plot of creatinine secretion across the spectrum of mGFR at baseline among 838 MDRD Study participants, showing a correlation (Pearson correlation = 0.3) between creatinine secretion and mGFR (P for significance < 0.001).
There were 626 cases of incident KRT during a median follow-up of 6.0 (interquartile range, 3.5-11.6) years. Each 10-mL/min/1.73 m2 higher TScr was associated with 25% lower multivariable-adjusted risk for incident KRT; the lowest quartile of TScr had 1.6-fold higher risk for incident KRT versus the top quartile (P < 0.001; Table 1). Results of sensitivity analysis adjusting for mGFR slope instead of mGFR were similar, showing 27% lower risk for incident KRT (HR, 0.73 [95% CI, 0.65-0.82]) per 10-mL/min/1.73 m2 higher TScr. There was no interaction between diet randomization arm and TScr (P > 0.05). There were 444 cases of all-cause mortality and 202 CVD-related deaths during follow-up. Although the incidence rate of both outcomes was lower with higher quartiles of TSCr, after multivariable adjustment, TSCr was not statistically significantly associated with either outcome in multivariable-adjusted models (Tables S3 and S4).
Table 1.
Association of Tubular Secretion of Creatinine With Clinical Outcomes
| No. of Patients |
Incident KRT | All-Cause Mortality | CVD Mortality | ||||
|---|---|---|---|---|---|---|---|
| Events | HR (95% CI)a | Events | HR (95% CI) | Events | HR (95% CI) | ||
| TScr, per 10 mL/min/1.73 m2 greater | 838 | 626 | 0.75 (0.67-0.85) | 444 | 0.90 (0.78-1.04) | 202 | 0.83 (0.67-1.02) |
| TScr category | |||||||
| ≤4.9 mL/min/1.73 m2 | 209 | 168 | 1.64 (1.28-2.10) | 120 | 1.06 (0.79-1.42) | 58 | 1.16 (0.75-1.79) |
| >4.9-9.0 mL/min/1.73 m2 | 210 | 170 | 1.46 (1.15-1.87) | 114 | 1.22 (0.91-1.62) | 50 | 1.27 (0.82-1.97) |
| >9.0-13.5 mL/min/1.73 m2 | 210 | 150 | 1.16 (0.91-1.48) | 105 | 0.87 (0.65-1.15) | 51 | 0.99 (0.65-1.51) |
| >13.5 mL/min/1.73 m2 | 209 | 138 | 1.00 (reference) | 105 | 1.00 (reference) | 43 | 1.00 (reference) |
Abbreviation: HR, hazard ratio.
Multivariable adjusted (for age, sex, race, smoking status, MDRD [Modification of Diet in Renal Disease] Study A/B, blood pressure target, protein diet randomization, cause of kidney disease, history of CVD, proteinuria, transferrin level, mean arterial pressure, lower serum high-density lipoprotein cholesterol level, and mGFR).
Our findings suggest that lower secretion of creatinine may give insights about kidney health above and beyond GFR and proteinuria. Prior studies have shown that a number of small molecules are present in the plasma of dialysis patients and are excreted primarily through tubular secretion in healthy individuals.5 Low clearance of some of these markers has also been associated with mortality risk6 and kidney failure7 independent of eGFR in persons with advanced chronic kidney disease (CKD). However, measurement accuracy for these metabolites is yet to be established. Our results support the same concept but evaluate a precisely measured metabolite that is well known to be secreted by the proximal tubule (creatinine). Given that tubulointerstitial fibrosis is common in nearly all forms of kidney disease 8–10 and its severity is the most reliable feature on biopsy to predict progression to kidney failure,10 developing additional markers of tubule health is likely to refine our ability to detect and monitor global kidney health above and beyond eGFR and albuminuria.
Our study is limited by the use of only prerandomization TScr; potential measurement errors in variables used to calculate TScr; the lack of older participants and those with insulin-dependent diabetes, and the limited number of those of non-European ancestry; and the lack of detailed smoking history. Strengths include the large number of participants, long-term follow-up and large number of events, detailed ascertainment of potentially confounding variables, and concurrent availability of mGFR and mClcr at baseline (twice in most participants).
In conclusion, lower TScr is associated with risk for incident KRT independent of mGFR or proteinuria in a large cohort of persons with mild to moderate predominantly nondiabetic CKD. Future studies should further evaluate the role of tubule secretory markers, confirm their association with adverse kidney outcomes, and determine whether they may can be used for improving the dosing of drugs that are primarily excreted by secretion.
Supplementary Material
Acknowledgments
Support:
PSG is supported by NIDDK career development grant K23 DK114556. JHI is supported by grants from NIDDK (2R01DK098234 and K24DK110427) and the American Heart Association (14EIA18560026). The funders had no role in the design, analysis, interpretation, or reporting of these results.
Financial Disclosure:
PSG has received clinical trial support from Kadmon Inc and speaker fees from Otsuka. MJS serves on the steering committee for Akebia, served on an advisory board for Bayer, and is a consultant for Cardurian. JHI is principal investigator of an investigator-initiated research project from Baxter Int. The other authors declare that they have no relevant financial interests.
Footnotes
Contributor Information
Pranav S. Garimella, Division of Nephrology and Hypertension, Department of Medicine, University of California San Diego, San Diego, CA.
Hocine Tighiouart, The Institute for Clinical Research and Health Policy Studies, Tufts Medical Center; Tufts Clinical and Translational Science Institute, Tufts University.
Mark J. Sarnak, Division of Nephrology, Tufts Medical Center, Boston, MA.
Andrew S. Levey, Division of Nephrology, Tufts Medical Center, Boston, MA.
Joachim H. Ix, Division of Nephrology and Hypertension, Department of Medicine, University of California San Diego, San Diego, CA; Division of Preventive Medicine, Department of Family Medicine and Public Health, University of California San Diego, San Diego; Nephrology Section, Veterans Affairs San Diego Healthcare System, La Jolla, CA.
References
- 1.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol. 2015;10(11):2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K, Kestenbaum B. Proximal tubular secretory clearance: a neglected partner of kidney function. Clin J Am Soc Nephrol. 2018;13(8):1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–884. [DOI] [PubMed] [Google Scholar]
- 5.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int. 2013;84(3):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suchy-Dicey AM, Laha T, Hoofnagle A, et al. Tubular secretion in CKD. J Am Soc Nephrol. 2016;27(7):2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Zelnick LR, Wang K, et al. Kidney clearance of secretory solutes is associated with progression of CKD: the CRIC Study. J Am Soc Nephrol. 2020;31(4):817–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1):1–17. [DOI] [PubMed] [Google Scholar]
- 9.Ong AC, Fine LG. Loss of glomerular function and tubulointerstitial fibrosis: cause or effect? Kidney Int. 1994;45(2): 345–351. [DOI] [PubMed] [Google Scholar]
- 10.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


