Merkel cell carcinoma (MCC) is a rare aggressive primary neoplasm of neuroendocrine origin, typically presenting as dermal tumor; uncommonly, it can present as a neoplasm limited to the epidermis (intraepidermal Merkel cell carcinoma [MCCIS] ).1 Most MCCIS arise in association with other tumors (i.e. collision).1, 2 Reported cases in the literature mainly described dermatopathologic features and lacked detailed clinical and reflectance confocal microscopy (RCM) description.
Case report:
A Male in his 60s presented with a 3-year history of an asymptomatic, erythematous, scaly plaque on the right eyebrow (Figure 1A). Initial biopsy was suspicious for extramammary Paget disease (EMPD). Given clinical-histopathological disconnect, further evaluation was performed. Dotted vessels, pink structureless areas, shiny white lines, and scale were seen on dermoscopy (Figure 1B). RCM evaluation (VivaScope® 3000, Caliber Imaging and Diagnostics, Rochester, NY, USA) showed dark pagetoid cells in the epidermis and glandular nests at the dermoepidermal junction separated by septae (Figure 1C–D). A deeper biopsy was performed, showing an intraepithelial bowenoid and pagetoid proliferation of oblong hyperchromatic epitheloid cells with high mitotic rate, small nucleoli, and scant cytoplasm, all neuroendocrine features. Immunohistochemistry was positive for CK20, INSM1, and chromogranin; a subset of cells was positive for CK7 and EMA. Merkel cell polyomavirus (MCPyV) was negative. These findings were consistent with a neuroendocrine carcinoma confined to the epithelium (MCCIS). A PET-CT showed non-FDG avid pleural nodularity in the left lung. A 1-cm margin excision was performed with negative margins. Four sentinel lymph nodes and pleural biopsies were negative. The patient remains in close follow-up without evidence of local or regional recurrence.
Figure 1:
Intraepidermal Merkel cell carcinoma. A. Clinical features showing an erythematous scaly plaque on the right eyebrow (paper tape positioned at 5-mm from lesion for reflectance confocal microscopy mapping [RCM]). B. Dermoscopic features showing dotes vessels (white arrows), shiny white lines (blue arrows), and a pink homogeneous background (white asterisk) (polarized dermoscopy, original magnification 10X). C. RCM image showing dark, round, pagetoid cells at the level of the epidermis (750 × 750 μm; blue bar 200 μm). D. RCM image showing hyporeflective glandular nests (yellow arrows) separated by septae (white asterisk) at the dermoepidermal junction level (750 × 750 μm; blue bar 200 μm).
Discussion:
Herein, we described a case of MCCIS presenting as a scaly plaque on the right eyebrow. As with previously published cases of MCCIS, there was no association with MCPyV.1 Given the lack of prospective information, it is difficult to predict the prognosis of MCCIS compared to ‘classic’ MCC. In a series of 12 patients, only one patient had regional metastasis and local recurrence; none had positive sentinel lymph node.1 One out of 5 developed metastasis/recurrence with long-term follow-up available. This differs from traditional MCC in which up to 1 of 3 patients develop nodal metastasis.1
Dermoscopy showed dotted vessels, scale, and shiny white lines, similar to what has been described in EMPD.3 Other conditions that can mimic MCCIS on dermoscopy include Bowen’s disease (scale and glomerular vessels) and amelanotic melanoma (serpentine vessels, dotted vessels, or polymorphous vessels and absence of scale).4 The RCM appearance of this case showed dark pagetoid cells thorough the epidermis, ‘glandular nests’, and cord-like structures. These features have been previously described in EMPD. Other entities that can be included in the RCM differential diagnosis include amelanotic melanoma with amelanotic pagetoid cells and pagetoid Bowen’s disease. The RCM appearance seen in this case, however, differs from what has been described in classic MCC.5, 6 Longo et al. described the presence of aggregates of small cells surrounded by fibrotic septae.5 We hypothesize that MCC and MCCIS have a different RCM appearance given the different skin compartment from which they arise (i.e. dermis in MCC and epidermis in MCCIS). Recent data suggest that MCC and MCCIS might have a different cell origin: MCPyV-negative MCC emerges from epidermal keratinocytes and MCPyV-positive MCC from dermal fibroblast. This might explain the clinical, dermoscopy, and RCM differences.7, 8
Figure 2:
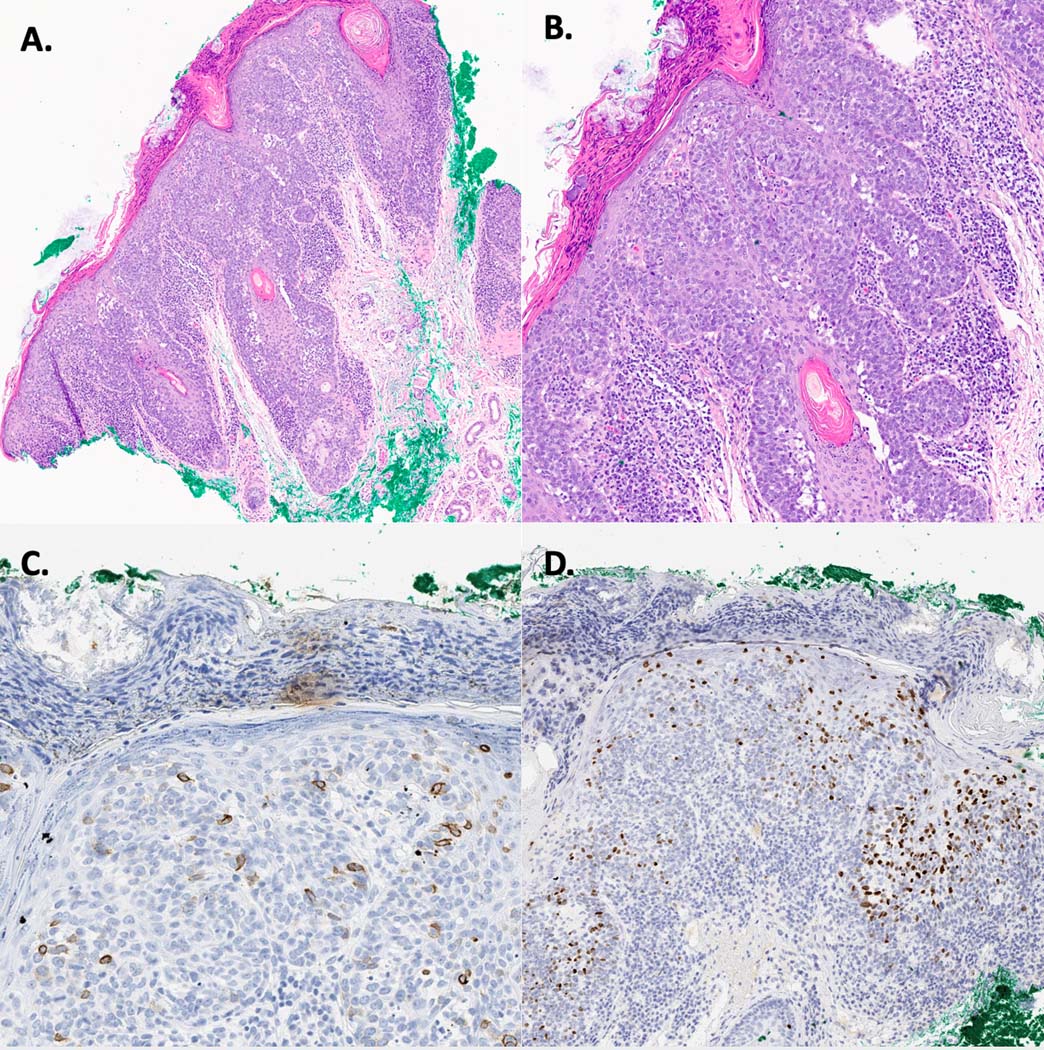
Intraepidermal Merkel cell carcinoma. A. Photomicrograph showing an intraepidermal proliferation of cells with widespread pagetosis (H&E, 10X). B. Photomicrograph showing an intraepidermal proliferation of cells with widespread pagetosis; the cells are made of hyperchromatic epitheloid cells with a high mitotic rate, small nucleoli, and scant cytoplasm, all neuroendocrine features (H&E, 20X). C. Cells were positive for CK20. D. Cells were positive for INSM1.
Acknowledgments
Founding source: This research is funded in part by a grant from the National Cancer Institute / National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center.
Footnotes
Conflict of interest: Anthony Rossi: Dr. Rossi has no relevant conflicts of interest related to this manuscript but has received grant funding from The Skin Cancer Foundation and the A.Ward Ford Memorial Grant for research related to this work. He also served on advisory board, as a consultant, or given educational presentations: for Allergan, Inc; Galderma Inc; Evolus Inc; Elekta; Biofrontera, Quantia; Merz Inc; Dynamed; Skinuvia, Perf-Action, and LAM therapeutics.
References:
- 1.Jour G, Aung PP, Rozas-Munoz E, et al. Intraepidermal Merkel cell carcinoma: A case series of a rare entity with clinical follow up. J Cutan Pathol. 2017; 44: 684–91. doi: 10.1111/cup.12966. [DOI] [PubMed] [Google Scholar]
- 2.Miraflor AP, LeBoit PE, Hirschman SA. Intraepidermal Merkel cell carcinoma with pagetoid Bowen’s disease. J Cutan Pathol. 2016; 43: 921–6. doi: 10.1111/cup.12813. [DOI] [PubMed] [Google Scholar]
- 3.Apalla Z, Errichetti E, Kyrgidis A, et al. Dermoscopic features of mammary Paget’s disease: a retrospective case-control study by the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2019. doi: 10.1111/jdv.15732 [DOI] [PubMed] [Google Scholar]
- 4.Mun JH, Kim SH, Jung DS, et al. Dermoscopic features of Bowen’s disease in Asians. J Eur Acad Dermatol Venereol. 2010; 24: 805–10. doi: 10.1111/j.1468-3083.2009.03529.x. [DOI] [PubMed] [Google Scholar]
- 5.Longo C, Benati E, Borsari S, et al. Merkel cell carcinoma: morphologic aspects on reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2017; 31: e480-e1. doi: 10.1111/jdv.14333. [DOI] [PubMed] [Google Scholar]
- 6.Cinotti E, Provvidenziale L, Habougit C, et al. Dermoscopic and reflectance microscopy features of primary and metastatic Merkel cell carcinoma: Ten cases. Skin Res Technol. 2019; 25: 407–9. doi: 10.1111/srt.12658. [DOI] [PubMed] [Google Scholar]
- 7.Yang A, Cordoba C, Cheung K, et al. Merkel cell carcinoma in situ: New insights into the cells of origin. Australas J Dermatol. 2019; 60: e311-e3. doi: 10.1111/srt.12658. [DOI] [PubMed] [Google Scholar]
- 8.Sunshine JC, Jahchan NS, Sage J, et al. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene. 2018; 37: 1409–16. doi: 10.1038/s41388-017-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]



