Abstract
Background
Muscular fatigue and injury are frequently observed in critically ill COVID-19 patients. The aim of this study was to determine whether different muscle adipose tissue depots are associated with mortality and muscle damage in patients affected by COVID-19 admitted to the ICU.
Methods
CT images were obtained in 153 ICU patients with COVID-19 (121 males and 32 females). Height, weight, body mass index (BMI), C-reactive protein, Creatine PhosphoKinase (CPK), muscle density, and intermuscular adipose tissue (IMAT) were measured.
Results
Participants in the highest tertile of IMAT/muscle had the shorter 28-day survival from ICU admission as compared to subjects in the first tertile. Estimates derived from the Cox proportional hazard models, after adjustment for age, sex, and BMI, confirmed the results of the survival analysis (HR 3.94, 95% CI: 1.03–15.09). Participants in the lowest tertile of muscle density had the shorter survival at 28 days from ICU admission as compared to subjects in the highest tertile (HR 3.27, 95% CI: 1.18–4.61), but the relationship was no longer significant when age was included in the model. Subjects in the second muscle density tertile did not show an increased risk.
Participants in the highest tertile of IMAT/muscle and those in the lowest tertile of muscle density showed both significantly higher CPK adjusted for weight values as evaluated during the first 8 days of hospitalization.
Conclusion
Our data seem to suggest that higher levels of IMAT/muscle and low muscle density are both associated with higher risk of ICU mortality and muscle injury as evaluated with CPK level.
Keywords: SARS-CoV-2, COVID-19, obesity, intermuscular adipose tissue, intensive care unit
Introduction
Significant deficits in upper and lower limb strength have been observed in patients with COVID-19, resulting in slow functional recovery after the hospitalization period. At the muscular level, COVID-19 exaggerated inflammatory response can lead to the characteristic symptoms of the mild flu-like form of COVID-19, characterized by myalgia and muscular exhaustion, but it can also determine a picture of severe muscle mass and function loss, more markedly observed at the proximal muscular level.
COVID-19 associated myopathy with severe proximal and bulbar weakness is characterized by elevated levels of IL-6 and Creatine PhosphoKinase (CPK) and the histological features of COVID-19 are mostly anecdotic and only a few case reports have been recently published on this subject. In these patients, examined muscles show perivascular inflammatory infiltration with endomysial extension and regenerating fibers (Zhang et al., 2020).
Obesity is a recognized risk factor for infection, hospitalization, and worse clinical outcomes in subjects affected by COVID-19 (Hajifathalian et al., 2020; Simonnet et al., 2020).
Moreover, obesity is characterized by an increase of lipid fat deposition inside and between muscle fibers, the so-called intramuscular and intermuscular adipose tissue (IMAT), respectively. Intramuscular fat accumulation resulting in low muscle density is associated with aging, insulin resistance, and sedentary lifestyle, whilst IMAT has been observed in patients with excess total adiposity and metabolic syndrome. Both kinds of fat depots are metabolically active tissues, able to secrete inflammatory cytokines and contribute to muscle damage (Beasley et al., 2009; Zamboni et al., 2019).
The aim of the present study was to evaluate if muscle fat depots were associated with increased mortality and may play a different role on muscle injury, as evaluated with CPK plasma concentrations, in critically ill COVID-19 patients admitted to our ICUs.
Materials and Methods
Study Design and Participants
The main study population consisted of 338 COVID-19 patients from the REINSURE-ARDS registry (62.46 ± 11.81 years; with mean body mass index (BMI) 29.20 ± 3.49 kg/m2, 17 women), consecutively admitted to the ICU of the University Hospital Integrated Trust of Verona between March 8th, 2020 and January 31st, 2021. All subjects had microbiologic confirmation of COVID-19 diagnosis by sampling of oro/nasopharyngeal swab (World Health Organization, 2020).
This study was approved by the ethical board of the University of Verona (Prog1946CESC, Prot 72485, November 12, 2018). Patient identification remained anonymous and written informed consent was obtained for participants. The ICU admission criteria and treatment decisions, including the determination of the need for intubation and type of administered antibiotic and antiviral therapy, were not standardized and were made by the attending medical team.
The present analysis was performed in a subsample of 153 subjects for which thoracic CT images were available.
Anthropometric Measures
Patients’ height and weight were recorded at the beginning of hospitalization as previously reported (Rossi et al., 2016). BMI was calculated as the ratio between weight and height squared (kg/m2).
Biochemical Measures
Repeated venous blood samples for C-reactive protein (CRP) and creatine phosphokinase (CPK) levels were obtained after overnight fasting as reported elsewhere (Rossi et al., 2021).
For the measurement of CRP, a kit was used for the quantitative determination of CRP in serum (CRP ROCHE applied on ROCHE/HITACHI COBAS 702, Roche Diagnostics GmbH, Mannheim, Germany). Measurement uncertainty declared 5.8%. The coefficient of variation (CV) of annual CQI to 6 mg/L is 6%.
Creatine phosphokinase concentration was determined on a fully automated analyzer (ANALIZER ROCHE/HITACHI COBAS 702, Roche Diagnostics GmbH, Mannheim, Germany). A multicenter evaluation of the within-run precision of the Advia 2120 system showed CV always lower than 0.7% for CPK.
CT Analysis
Two trained operators measured muscle density and IMAT in a single image at the L3–L4 level with a dedicated workstation using Sliceomatic software (version 4.2; Tomovision, Montreal) (Ross et al., 1992). Density value ranges between −30 and −190 Hounsfield units (HU) and between 0 and +100 HU were considered for adipose tissue and muscle, respectively (Goodpaster et al., 2000).
The IMAT measurements were then normalized for the psoas muscle area, obtaining the psoas fat to muscle area ratio as reported elsewhere (Ahmadi et al., 2015). The mean psoas muscle density in HU, related with intramuscular adipose tissue, and the IMAT area to muscle area ratio (IMAT/muscle) were also calculated.
Study Outcomes
Our primary outcome was to assess if IMAT area to muscle area ratio and mean psoas muscle density are associated with mortality. Our secondary outcome was to assess if IMAT area to muscle area ratio and mean psoas muscle density can influence the trend of critically ill induced myopathy as evaluated with CPK.
Statistical Analysis
For the demographic and clinical characteristics of the patients, differences between groups were assessed using the Chi-squared test and Fisher’s exact test for categorical variables and the Student’s t-test or Mann–Whitney U test for continuous variables. Differences in mortality rates across IMAT/muscle tertiles and muscle density tertiles were preliminarily evaluated fitting Kaplan–Meier survival curves. Cox proportional hazard models and logistic regression models were used to assess the risk of death. Hazard ratio (HR) and 95% confidence intervals (95% CI) were estimated to investigate the association of the three tertiles groups for both variables, IMAT/muscle area ratio and mean muscle density, with the risk of mortality. Three models were fitted for each outcome: unadjusted, age-, and gender-adjusted and adjusted for BMI. Students t-test for unpaired data was used to compare the trend of CPK between tertiles of IMAT/muscle and mean muscle density. A p-value of 0.05 or less was considered to be statistically significant. Data analysis was conducted using SPSS 22.0 software.
Results
Table 1 shows the main characteristics of the study population (mean ± SD) at baseline, divided into subjects who survived and who died. 153 subjects, 121 men and 32 women (20.9%) were included in the study. Subjects included in the present analysis were not different in age, sex distribution, BMI; all studied subjects required invasive mechanical ventilation.
TABLE 1.
Characteristics of the study population according to mortality status.
| Total (SD) (n = 153) | Min-max | Survivors (SD) (n = 126) | Min-max | Deaths (SD) (n = 27) | Min-max | p | |
| Age (years) | 64.19 (9.98) | 29–85 | 63.32 (10.50) | 29–85 | 68.26 (5.55) | 55–87 | 0.019 |
| Sex (M) n (%) | 121 (79.1%) | 100 (79.4%) | 21 (77.8%) | 0.800 | |||
| Height (m) | 1.72 (0.07) | 1.40–1.96 | 1.72 (0.07) | 1.40–1.96 | 1.70 (0.07) | 1.50–1.86 | 0.8 |
| Weight (Kg) | 87.24 (15.64) | 46–157 | 83.54 (14.71) | 52–150 | 88.37 (19.58) | 46–157 | 0.234 |
| BMI (Kg/m2) | 29.30 (4.58) | 20.45–45.38 | 28.25 (4.43) | 21.37–41.90 | 30.58 (5.29) | 20.45–45.38 | 0.039 |
| Psoas area (cm2) | 16.66 (9.37) | 2.47–55.07 | 16.46 (9.40) | 2.47–55.07 | 17.59 (9.34) | 4.56–34.93 | 0.573 |
| Psoas IMAT (cm2) | 4.36 (3.77) | 0.47–22.33 | 3.68 (2.53) | 0.47–13.98 | 7.52 (6.31) | 0.98–22.33 | <0.001 |
| Psoas muscle density (HU) | 37.79 (8.55) | 14.70–67.91 | 38.38 (8.58) | 14.70–67.91 | 35.05 (8.03) | 19.42–49.42 | 0.066 |
| CRP (mg/dL) | 122.02 (79.64) | 4–410 | 115.95 (79.71) | 4–410 | 150.33 (75.01) | 2–281 | 0.042 |
| CPK adjusted per weight (U/L/kg) | 3.34 (6.42) | 0.16–51.19 | 2.67 (4.76) | 0.16–45.39 | 6.41 (10.95) | 0.18–51.19 | 0.006 |
| Hypertension | 88 (57.5%) | 68 (54%) | 20 (74.1%) | 0.085 | |||
| Hearth failure | 4 (2.6%) | 2 (1.6%) | 2 (7.4%) | 0.144 | |||
| Ischemic cardiopathy | 13 (8.5%) | 11 (8.7%) | 2 (7.4%) | 1.000 | |||
| Neurological disorder | 10 (6.5%) | 9 (7.1%) | 1 (3.7%) | 1.000 | |||
| Type 2 diabetes | 29 (19%) | 24 (19%) | 5 (18.5%) | 1.000 | |||
| Thyroid | 17 (11.1%) | 12 (9.5%) | 5 (18.5%) | 0.185 | |||
| Dyslipidemia | 25 (16.3%) | 19 (15.1%) | 6 (22.2%) | 0.392 | |||
| Immunodepression | 11 (7.2%) | 7 (5.6%) | 4 (14.8%) | 0.105 | |||
| Smoke habit | 25 (16.3%) | 22 (17.5%) | 3 (11.1%) | 0.571 | |||
| Chronic renal failure | 14 (9.2%) | 9 (7.1%) | 5 (18.5%) | 0.075 | |||
| Cancer | 15 (9.8%) | 8 (6.3%) | 7 (25.9%) | 0.006 |
SD, standard deviation; BMI, body mass index; IMAT, intermuscular adipose tissue; HU, Hounsfield units; CRP, C reactive protein; CPK, creatine phosphokinase.
Survivors showed lower age, BMI, IMAT area, higher CRP, and CPK levels as compared with death subjects. CRP level was significantly higher in subjects in the highest IMAT/muscle tertile as compared with subjects in the lowest tertile, while subjects with low muscle density did not (data not shown in Table 1).
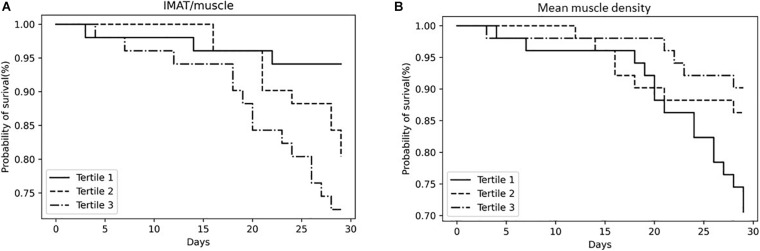
Figure 1A shows that participants in the highest tertile of IMAT/muscle had a shorter survival at 28 days from ICU admission as compared to subjects in the first tertile. Estimates derived from the Cox proportional hazard models, unadjusted confirmed the results of the survival analysis (HR 5.22, 95% CI: 1.50–18.18). After adjustment for age and sex, this association was still significant (HR 3.91, 95% CI: 1.06–14.42), even when BMI was included in the model (HR 3.94, 95% CI: 1.03–15.09).
FIGURE 1.
Kaplan–Meier survival curves for all-cause mortality according to IMAT/muscle tertiles (A) and mean muscle density tertiles (B). IMAT, intermuscular adipose tissue.
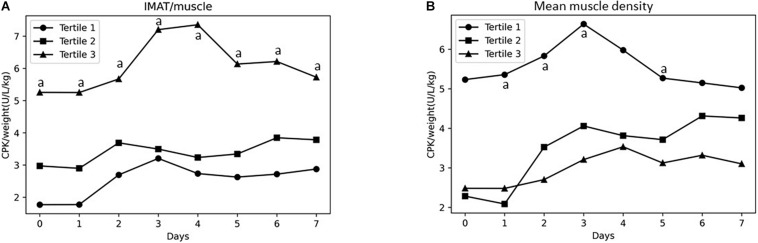
Survival of the study population stratified based on muscle density tertiles is shown in Figure 1B. Participants in the lowest tertile of muscle density had a shorter survival at 28 days from ICU admission as compared to subjects in the highest tertile. Estimates derived from the Cox proportional hazard models, unadjusted confirmed the results of the survival analysis (HR 3.27, 95% CI: 1.18–4.61), but the relationship was no longer significant when age was included in the model. Subjects in the second tertile of both, IMAT/muscle and muscle density, did not show an increased risk. Participants in the highest tertile of IMAT/muscle showed significantly higher CPK adjusted for weight values at baseline, day 1, 2, 3, 4, 5, 6, and 7, as compared to the first tertile (Figure 2A). Similarly, as shown in Figure 2B, subjects in the lowest muscle density tertile showed higher values of CPK adjusted values at baseline, day 1, 2, 3, and 5, as compared to the third tertile.
FIGURE 2.
Changes during the first 7 days from admission in ICU in CPK adjusted for weight level across IMAT/muscle tertiles (A) and mean muscle density tertiles (B). ap < 0.05.
Discussion
Our study shows that in COVID-19 intubated critically ill subjects with myosteatosis, as evaluated with CT, have higher ICU mortality risk. Moreover, we observed that both, subjects with higher IMAT/muscle ratio or low muscle density, experienced greater muscle injury, as evaluated with CPK levels, during the first 7 days of hospitalization in ICU.
This is in line with previous observations by Perkisas et al. (2018) who showed in a population of 302 geriatric patients that intramuscular adipose tissue, as evaluated with CT, was related to mortality during 4 years of follow-up.
Another prospective longitudinal study, including 1,652 African ancestry men, showed an independent association between myosteatosis and all-cause mortality during the 6 years follow-up (Zhao et al., 2016).
Our result that patients with higher IMAT are at higher risk of death than subjects with low muscle infiltration could be partially explained by the fact that IMAT is increased in obese subjects (Correa-de-Araujo et al., 2017). Indeed, the association remained significant after adjustment for age and sex, even when BMI was included in the model. On the contrary, after adjustment for age, the association between muscle density and mortality was no longer significant.
Subjects with higher IMAT/muscle showed higher CRP, while subjects with low muscle density did not.
Our data seems to suggest that the obese phenotype affected by severe COVID-19 with myosteatosis shows higher levels of inflammation and is at higher risk for unfavorable health outcomes.
COVID-19 is generally characterized by generalized weakness, myalgias, and elevated creatine kinase levels occurring in one-quarter of symptomatic patients (Guan et al., 2020; Guidon and Amato, 2020).
The pathophysiological mechanism associated with the elevation in creatine kinase level is still unclear.
Only a few reports characterized histologically muscle damage in COVID-19 subjects to investigate the relationship with CPK increase, but the oil-red O and perilipin staining were not quantified (Manzano et al., 2020; Zhang et al., 2020).
We observed an association between IMAT quantity and muscle damage, as evaluated with CPK level, in the first ICU days.
We can hypothesize that both high IMAT and low skeletal muscle density representation can be considered as a marker of muscle vulnerability.
In fact, ectopic fat deposition out of the subcutaneous depot is associated with elevated circulating proinflammatory cytokines (Beasley et al., 2009). Previous in vitro studies have demonstrated that adipokines have an effect at the level of the muscle cell (Takegahara et al., 2014) and that they can act on muscle at a local level via paracrine signaling (Bryniarski and Meyer, 2019). Intriguingly, both IMAT and pro-inflammatory adipokines, TNFα and IL-6, were found to be locally increased in the paretic leg of stroke survivors (Hafer-Macko et al., 2005), suggesting that the inflammatory milieu could be locally conditioned by IMAT quantity. Only systemic CRP levels were available for our patients and they were increased in subjects with higher IMAT quantity and a higher level of inflammation could partially explain the association between IMAT and muscle damage observed in our study.
Moreover, IMAT infiltration is correlated with greater skeletal muscle myostatin and P-CDK2 (Tyr15) in sedentary individuals, and its training-induced change is accompanied by reductions in myostatin and P-CDK2 (Tyr15), which promote adipogenesis and inhibit myogenesis (Konopka et al., 2018).
We observed that not only IMAT interspersed between muscle bundles, but also intramyocellular lipids could play a role in muscle damage in critically ill patients affected by COVID-19.
In fact, the accumulation of “toxic” lipids such as ceramides and diacylglycerols in muscles promotes not only metabolic consequences, such as insulin resistance but also skeletal muscle dysfunction with reduction of muscle fiber number, fiber size, contractility, mitochondrial dysfunction, and apoptosis. Intermuscular lipids are associated with higher levels of myostatin, IL-6, and macrophages infiltration, observed in subjects with obesity (Bodell et al., 1985; Hittel et al., 2009).
We can therefore hypothesize that different factors, related to both intermuscular and intramyocellular adipose tissue deposition, can interfere with satellite cell activation and myoblast proliferation and differentiation, which represent necessary steps for muscle repair processes after COVID-19 induced muscle injury. The subsequent muscle damage induced by the exaggerated inflammatory response observed in COVID-19 patients is associated with significant strength deficit both of the upper and the lower limbs, a phenomenon even more relevant in people with obesity, leading to worse clinical outcomes, as higher mortality and longer hospitalization.
Some limitations should be acknowledged. First, muscle density is related to, but not a precise measure of intramuscular fat content (Hausman et al., 2014). Secondly, inflammatory cytokines levels, such as IL-6 and TNF-alpha were not available for the whole study population, and further insight into the relationship between systemic inflammation and muscle local consequences was not possible. Lastly, this is a cross-sectional study and further longitudinal imaging and/or histological studies are needed to better clarify the impact of COVID-19 induced inflammation on muscle damage in hospitalized subjects and the possible role of different muscle fat depots.
Conclusion
In conclusion, muscle quality measurements from thoracic imaging performed during the hospital stay of COVID-19 critically ill patients could provide valuable information on health outcomes, such as length of stay, in-hospital mortality, or post-COVID-19 functional recovery.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Verona University Ethical Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LG, EP, and KD designed the research. EP, AF, KD, RB, and GP conducted the research. AR and LG had primary responsibility for final content and analyzed the data or performed the statistical analysis. AR, PB, and GZ collected and analyzed the CT images. AR, LG, KD, EP, and MZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmadi H., Montie J. E., Weizer A. Z., Morgan T., Montgomery J. S., Lee C. T. (2015). Patient psoas muscle mass as a predictor of complications and survival after radical cystectomy. Curr. Urol. Rep. 16 79. 10.1007/s11934-015-0548-0 [DOI] [PubMed] [Google Scholar]
- Beasley L. E., Koster A., Newman A. B., Javaid M. K., Ferrucci L., Kritchevsky S. B., et al. (2009). Inflammation race and gender differences in computer tomography-measured adipose depots. Obesity 17 1062–1069. 10.1038/oby.2008.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell P. W., Kodesh E., Haddad F., Zaldivar F. P., Cooper D. M., Adams G. R. (1985). Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J. Appl. Physiol. 2009 443–453. 10.1152/japplphysiol.90831.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryniarski A. R., Meyer G. A. (2019). Brown fat promotes muscle growth during regeneration. J. Orthop. Res. 37 1817–1826. 10.1002/jor.24324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-de-Araujo R., Harris-Love Michael O., Miljkovic I., Fragala M. S., Anthony B. W., Manini T. M. (2017). The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front. Physiol. 8:87. 10.3389/fphys.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster B. H., Thaete F. L., Kelley D. E. (2000). Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. J. Clin. Nutr. 71 885–892. 10.1093/ajcn/71.4.885 [DOI] [PubMed] [Google Scholar]
- Guan W. J., Ni Z. Y., Hu Y., Liang W. H., Ou C. Q., He J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidon A. C., Amato A. A. (2020). COVID-19 and neuromuscular disorders. Neurology 94 959–969. 10.1212/WNL.0000000000009566 [DOI] [PubMed] [Google Scholar]
- Hafer-Macko C. E., Yu S., Ryan A. S., Ivey F. M., Macko R. F. (2005). Elevated tumor necrosis factor-alpha in skeletal muscle after stroke. Stroke 36 2021–2023. 10.1161/01.STR.0000177878.33559.fe [DOI] [PubMed] [Google Scholar]
- Hajifathalian K., Kumar S., Newberry C., Shah S., Fortune B., Krisko T., et al. (2020). Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York city. Obesity 28 1606–1612. 10.1002/oby.22923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman G. J., Basu U., Du M., Fernyhough-Culver M., Dodson M. V. (2014). Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocyte 3 242–255. 10.4161/adip.28546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittel D. S., Berggren J. R., Shearer J., Boyle K., Houmard J. A. (2009). Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 58 30–38. 10.2337/db08-0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A. R., Wolff C. A., Suer M. K., Harber M. P. (2018). Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315 R461–R468. 10.1152/ajpregu.00030.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano G. S., Woods J. K., Amato A. A. (2020). Covid-19–associated myopathy caused by type I interferonopathy. N. Engl. J. Med. 383 2389–2390. 10.1056/NEJMc2031085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkisas S., De Cock A. M., Verhoeven V., Vandewoude M., Van Mieghem E., Degerickx R. (2018). The relation between mortality, intramuscular adipose tissue and sarcopenia in hospitalized geriatric patients. Eur. Geriatr. Med. 9 801–807. 10.3390/geriatrics2010011 [DOI] [PubMed] [Google Scholar]
- Ross R., Lèger L., Morris D., deGuise J., Gardo R. (1992). Quantification of adipose tissue by CT: relathionship with antropometric variables. J. Appl. Physiol. 72 787–795. 10.3109/14017431.2014.942872 [DOI] [PubMed] [Google Scholar]
- Rossi A. P., Gottin L., Donadello K., Schweiger V., Nocini R., Taiana M., et al. (2021). Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by COVID-19. Nutr. Metab. Cardiovasc. Dis. 10 762–768. 10.1016/j.numecd.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A. P., Zanandrea V., Zoico E., Zanardo M., Caliari C., Confente S., et al. (2016). Inflammation and nutritional status as predictors of physical performance and strength loss during hospitalization. Eur. J. Clin. Nutr. 70 1439–1442. 10.1038/ejcn.2016.159 [DOI] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. (2020). Lille intensive care COVID-19 and obesity study group. high prevalence of obesity in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 28 1195–1199. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegahara Y., Yamanouchi K., Nakamura K., Nakano S., Nishihara M. (2014). Myotube formation is affected by adipogenic lineage cells in a cell-to-cell contact-independent manner. Exp. Cell. Res. 324 105–114. 10.1016/j.yexcr.2014.03.021 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2020). Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases. Interim Guidance. ∗City pub. [Google Scholar]
- Zamboni M., Gattazzo S., Rossi A. P. (2019). Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur. Geriatr. Med. 10 5–6. 10.1007/s41999-018-0134-3 [DOI] [PubMed] [Google Scholar]
- Zhang, Charmchi Z., Seidman R. J., Anziska Y., Velayudhan V., Perk J. (2020). COVID-19 associated myositis with severe proximal and bulbar weakness. Muscle Nerve 62 E53–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Zmuda J. M., Kuipers A. L., Jonnalagadda P., Bunker C. H., Patrick A. L., et al. (2016). Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing 45 529–534. 10.1093/ageing/afw062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.