Abstract
Childhood is an important time for the manifestation of psychopathology. Psychopathology is characterized by considerable comorbidity which is mirrored in the underlying neural correlates of psychopathology. Both common and dissociable variations in brain volume have been found across multiple mental disorders in adult and youth samples. However, the majority of these studies used samples with broad age ranges which may obscure developmental differences. The current study examines associations between regional gray matter volumes (GMV) and psychopathology in a large sample of children with a narrowly defined age range. We used data from 9607 children 9–10 years of age collected as part of the Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®). A bifactor model identified a general psychopathology factor that reflects common variance across disorders and specific factors representing internalizing symptoms, ADHD symptoms, and conduct problems. Brain volume was acquired using 3T MRI. After correction for multiple testing, structural equation modeling revealed nearly global inverse associations between regional GMVs and general psychopathology and conduct problems, with associations also found for ADHD symptoms (pfdr-values ≤ 0.048). Age, sex, and race were included as covariates. Sensitivity analyses including total GMV or intracranial volume (ICV) as covariates support this global association, as a large majority of region-specific results became nonsignificant. Sensitivity analyses including income, parental education, and medication use as additional covariates demonstrate largely convergent results. These findings suggest that globally smaller GMVs are a nonspecific risk factor for general psychopathology, and possibly for conduct problems and ADHD as well.
Subject terms: Human behaviour, Risk factors, Neuroscience
Introduction
Many forms of psychopathology first manifest during childhood [1] and the emergence of symptoms during development is a substantial risk factor for psychopathology during adulthood [2]. Childhood is characterized by extensive brain development [3] and neural plasticity [4]. As a result of enhanced neural plasticity, childhood is also a time in which individuals may be more susceptible to environmental influences that are likely to influence psychopathology development [4]. Identifying neural mechanisms underlying psychopathology in childhood is vital to the advancement of risk-identification, prevention, and intervention strategies.
Dimensions of psychopathology are continuous [5], highly correlated [6], and hierarchically organized [5–9]. Furthermore, research suggests that the comorbid nature of psychopathology is also manifested in the patterns of neural substrates of psychopathology, as similar neural mechanisms are shared across disorders [10]. For example, a meta-analysis demonstrated common gray matter volume (GMV) deficits in the dorsal anterior cingulate and bilateral insula across six different diagnoses: schizophrenia, bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety [11].
Nonetheless, the vast majority of research surrounding neural mechanisms of psychopathology has employed case-control designs in which healthy controls are compared to persons who meet criteria for categorically defined disorders. There are several disadvantages of this approach. First, case-control designs introduce biases in comparisons and need to be complemented by population-based studies [12]. Second, dimensional approaches to measuring psychopathology capture the continuous variation in symptoms more effectively than categorical diagnoses [13]. Third, dimensions of psychopathology are more reliable and valid than categories [14].
Researchers have proposed that psychopathology can be conceptualized as a hierarchy of correlated dimensional symptom domains [5, 8, 15, 16]. Using a variety of methods [17], this research suggests a hierarchical structure of psychopathology consisting of a general factor, which represents the shared variance of symptoms across all measured disorders, in addition to several factors that represent the variance in specific symptom domains (e.g., internalizing). Further, research has shown that a bifactor model in which all symptoms load onto both a general psychopathology factor and a single orthogonal specific factor is a useful model for testing hypotheses surrounding the distinct and shared features associated with dimensions of psychopathology, as it identifies uncorrelated phenotypes [8, 18]. Bifactor models have been applied and replicated in both adult [15, 19, 20] and youth samples [18, 21, 22]. Such models allow for the operationalization of general psychopathology and the examination of correlates and mechanisms associated with both general and specific factors of psychopathology.
Hierarchical symptom dimensions can be related to neurostructural measures to identify common and unique neural substrates of psychopathology [10]. Previous studies using this approach have shown inverse associations between psychopathology dimensions and GMV, i.e., greater loadings on psychopathology dimensions being related to smaller GMVs [23–26]. However, most of these studies have focused on adults or samples with broad age ranges, such as samples of 1394 youth 8–23 years of age [23], 254 children between 6 and 10 years [24], 1246 undergraduate students [25], and 1019 youth 11–21 years old [26]. Given that these dimensions may change over development, a large sample with a narrowly defined age range may be useful. The large size (11,875) and narrow age range (9–10 years) of the Adolescent Brain and Cognitive Development (ABCD) Study sample allows for greater power to detect differences and more reliability for generating latent psychopathology factors at a particular age. In addition, previous findings are in need of replication in order to further assess their validity and robustness [27].
The aim of the current study was to further delineate the neurostructural substrates of psychopathology through the investigation of associations between regional GMV and dimensions of psychopathology as defined by a bifactor model in a large sample of children with a narrowly defined age range. GMV was chosen based on prior work showing a relationship between global GMV deficits and a general psychopathology factor in a sample ranging from 8 to 23 years [23]. However, this study’s broad age range may obscure developmental changes in these brain–behavior relationships. Thus, it is imperative to examine these associations in a sample with a more homogeneous age range in order to map these associations more precisely.
The current study builds upon prior work by leveraging a large sample of children 9–10 years old from the ABCD Study. We examined associations between regional GMVs and four dimensions of psychopathology representing general psychopathology, internalizing symptoms, ADHD symptoms, and conduct problems. We controlled for demographic factors such as age, sex, and race/ethnicity in all analyses, and we performed sensitivity analyses with measures of socioeconomic status and medication use to test the robustness of these findings. Based on prior research [23], we hypothesized that general psychopathology would be associated with smaller regional GMVs throughout the brain. Given that a substantial portion of genetic variance in psychopathology is shared across disorders and genetics show nonspecific associations with the brain [9], we predicted a global rather than focal pattern for GMV and psychopathology. Such findings would provide important evidence that the relationship between smaller GMV and psychopathology is evident early in development. In addition, given that adolescence is a time of exponential increase in mental disorders [1], our study provides a baseline measure for future longitudinal analyses of the ABCD Study dataset. This will allow for a better understanding of how these brain–behavior relationships change throughout development, which is important for interpreting their clinical implications.
Materials and methods
Participants
The present analyses used data from Wave 1 (release 2.0.1) of the ABCD Study [28]. The institutional review board at Vanderbilt University approved the use of this dataset. As detailed previously [29], participants in the ABCD study were recruited at 21 sites across the United States. Wave 1 of the ABCD study includes data from 11,875 children between 9 and 10 years of age. After excluding cases with missing data or who failed to pass quality assurance measures (see Fig. S1), the final sample size included in analyses was N = 9607. Specifics regarding methods for imaging data exclusions can be found elsewhere [30]. In comparison to those excluded from analyses (N = 2268), the final sample had a higher proportion of females, a lower proportion of racial/ethnic minority status individuals, higher income, more parental education, and was older in age (p values ≤ 0.001). A summary of the demographic characteristics of the analyzed sample can be found in Table 1.
Table 1.
Summary of demographic characteristics of the sample included in analyses of associations between brain volume and psychopathology (N = 9607).
| Mean | SD | |
|---|---|---|
| Age (months) | 119.16 | 7.47 |
| N | % | |
| Gender | ||
| Female | 4686 | 48.78 |
| Male | 4921 | 51.22 |
| Race-Ethnicity | ||
| White | 5127 | 53.37 |
| Hispanic | 1961 | 20.41 |
| African American | 1365 | 14.21 |
| Other | 1154 | 12.01 |
| Household annual income | ||
| <$5000 | 320 | 3.33 |
| $5000–$11,999 | 323 | 3.36 |
| $12,000–$15,999 | 220 | 2.29 |
| $16,000–$24,999 | 411 | 4.28 |
| $25,000–$34,999 | 520 | 5.41 |
| $35,000–$49,999 | 735 | 7.65 |
| $50,000–$74,999 | 1219 | 12.69 |
| $75,000–$99,999 | 1301 | 13.54 |
| $100,000–$199,999 | 2753 | 28.66 |
| ≥$200,000 | 1003 | 10.44 |
| Missing | 802 | 8.35 |
| Parental education | ||
| No degree | 468 | 4.87 |
| Highschool degree/GED | 1147 | 11.94 |
| Some college | 1572 | 16.36 |
| Associate’s degree | 1228 | 12.78 |
| Bachelor’s degree | 2721 | 28.32 |
| Master’s degree | 1867 | 19.43 |
| Professional/Doctoral degree | 591 | 6.15 |
| Missing | 13 | 0.14 |
The “Other” Race-Ethnicity category includes those who were identified by their parent as American Indian/Native American, Alaska Native, Native Hawaiian, Guamanian, Samoan, Other Pacific Islander, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Other Asian, or Other Race.
Measure of psychopathology
Psychopathology was measured from parent reports on the Child Behavior Checklist (CBCL) for school-aged children [31]. The CBCL contains 119 items describing behaviors and emotions in children that may be of concern. Items were rated on a 3-point scale in which 0 = not true (as far as you know), 1 = somewhat or sometimes true, and 2 = very true or often true. Ratings were made by only one of the child’s parents or guardians. See the supplement for more details regarding CBCL item inclusions and exclusions. Internal consistency for the CBCL items included in analyses was excellent in the current sample (α = 0.94).
Image acquisition, processing, and quality assurance
Image acquisition, processing, and quality assurance procedures have been previously described [29, 30] and are detailed in the supplement.
Bifactor modeling of psychopathology dimensions
All analyses were completed using Mplus version 8.4 [32].
As detailed previously [18], exploratory factor analyses of the CBCL data from a random half of the ABCD Study sample (N = 5932) specified three correlated psychopathology dimensions: internalizing, ADHD, and conduct problems. A confirmatory bifactor analysis was completed with the second half (N = 5934) of the data, which defined a general psychopathology factor that reflects the shared symptoms across all participants, as well as the specific factors for internalizing, ADHD, and conduct problems (see Fig. 1). The four factors are orthogonal to each other. The psychometric properties of the factors met all standards for construct reliability and factor determinacy recommended for bifactor models [33], and each factor demonstrated adequate criterion validity. More specifically, Moore et al. [18] used additional variables from the ABCD Study, that held both clinical and theoretical relevance, as external criterion measures to investigate the bifactor model’s criterion validity. Factors yielded from the bifactor model had significant associations with external criterion measures. For additional details about the calculation processes and results of the bifactor modeling, as well as the validity and reliability of the psychopathology dimensions, see Moore et al. [18].
Fig. 1. Bifactor analyses delineate general and specific factors.
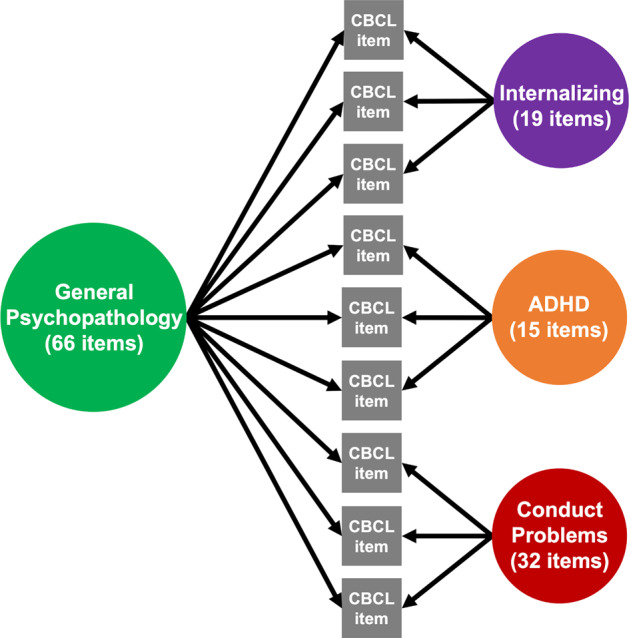
A confirmatory bifactor model of the CBCL data yielded four orthogonal factors of psychopathology: general psychopathology, which represents symptoms across all domains, as well as specific factors for internalizing symptoms, ADHD symptoms, and conduct problems.
Statistical analyses
Using the latent factors of psychopathology identified by Moore et al. [18], we examined associations between psychopathology and GMV. We performed analyses with the 68 cortical regions derived from the surface-based atlas procedure developed by Desikan et al. [34], as well as 19 subcortical regions derived by the automated labeling procedure developed by Fischl et al. [35]. In accordance with research that has demonstrated sex differences in brain volume [36], sex was included in the model as a covariate. Age and race/ethnicity were also included in the model as covariates. Finally, MRI scanner model was included as a covariate to account for scanner differences. For each brain region, we investigated associations between psychopathology and volume using the following formula: regional GMV = β × age + β × sex + β × race/ethnicity + β × MRI scanner model + β × general psychopathology + β × internalizing + β × ADHD + β × conduct problems. To control for multiple testing across regions, we controlled the false discovery rate (q < 0.05) using the stats package in R version 3.6.1 (http://www.r-project.org/). The orthogonality of the four psychopathology variables allows them to be included together in the same model without problems of multicollinearity. All analyses used post-stratification weights to account for the stratification in data collection sites [37]. In addition, the ABCD Study dataset includes some twins and siblings, so analyses accounted for clustering within families, with families being modeled with a random intercept.
Sensitivity analyses
Several iterations of sensitivity analyses were performed in order to test the robustness of the findings. First, intracranial volume (ICV) was added as an additional covariate to determine whether associations between regional GMVs and the psychopathology dimensions remained when controlling for cranial size. Second, total cortical GMV (for cortical regions) and total subcortical GMV (for subcortical regions) were added as additional covariates to determine whether associations between regional GMVs and the psychopathology dimensions remained when controlling for total cortical/subcortical GMV. Third, income and parent’s highest level of education were added as additional covariates in accordance with prior research showing associations between those factors and brain structure in children and adolescents [38]. Finally, medication (whether participants reported taking current medications or not) was added as an additional covariate to determine whether associations between regional GMVs and psychopathology shift when taking into account medication status.
Results
Associations of psychopathology dimensions with regional brain volumes
After FDR correction for multiple comparisons, the general psychopathology, conduct problems, and ADHD factors were all inversely associated with brain volume across multiple regions (pfdr-values ≤ 0.048). In particular, for general psychopathology and conduct problems, the associations were relatively global. Out of the 68 cortical and 19 subcortical regions included in analyses, general psychopathology was inversely associated with GMV in 54 cortical regions (Table S1 and Fig. 2) and in all 19 of the subcortical regions after FDR correction (Table S2). Conduct problems were inversely associated with GMV in 52 cortical regions (Table S1 and Fig. 3) and 15 subcortical regions after FDR correction (Table S2). ADHD symptoms were inversely associated with 25 cortical regions (Table S1 and Fig. 4) and 8 subcortical regions after FDR correction (Table S2). There were no significant associations between GMVs and internalizing symptoms (pfdr-values ≥ 0.068; Tables S1 and S2). Effect sizes for these associations were expressed as standardized beta estimates. The range for each factor was as follows: general psychopathology (−0.03 to −0.08), conduct problems (−0.04 to −0.09), and ADHD (−0.04 to −0.08) (Tables S1 and S2).
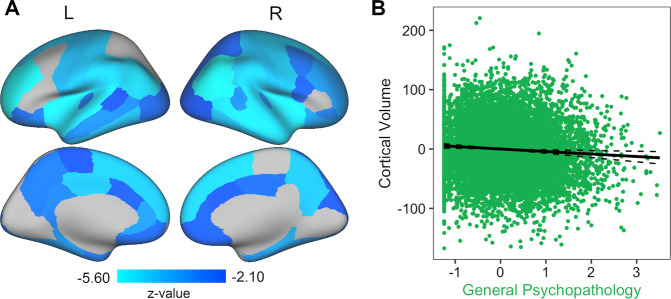
Fig. 2. General psychopathology shows smaller brain volumes nearly globally.
A Greater general psychopathology scores were associated with smaller cortical GMV in 54 out of 68 regions (FDR corrected). B As general psychopathology scores increase, cortical GMV decreases. Each dot in the plot symbolizes a participant. The full model R2 = 0.31.
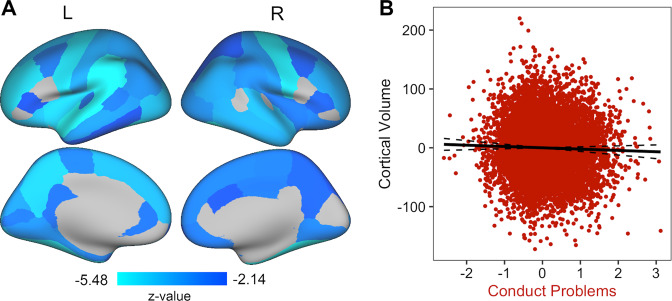
Fig. 3. Conduct problems show smaller global brain volumes.
A The specific conduct problems factor was associated with smaller cortical GMV in 52 out of 68 regions (FDR corrected). B An inverse relationship was apparent, with cortical GMV decreasing as conduct problems increase. Each dot in the plot symbolizes a participant. The full model R2 = 0.31.
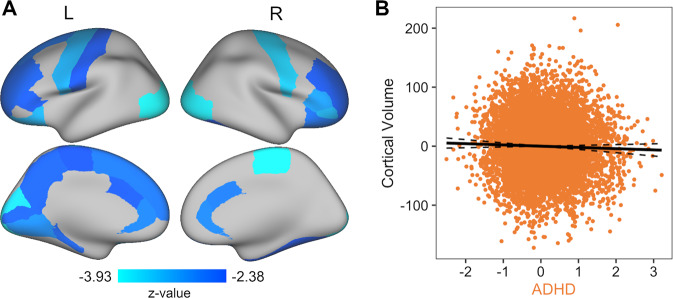
Fig. 4. ADHD symptoms are associated with smaller volumes in a number of regions.
A The specific ADHD factor was associated with smaller cortical GMV in 25 out of 68 regions (FDR corrected). B Greater ADHD scores were related to smaller cortical GMV. Each dot in the plot symbolizes a participant. The full model R2 = 0.31.
Sensitivity results
Sensitivity analyses were conducted to ensure that the primary results were robust to possible confounds. Results of sensitivity analyses controlling for family income and parent education were largely convergent with the primary results (Tables S3 and S4 for cortical and subcortical results, respectively). Specifically, GMV was inversely associated with psychopathology in many regions for general psychopathology (37 cortical and 18 subcortical regions) and conduct problems (29 cortical and 3 subcortical regions), with a weaker inverse association demonstrated for ADHD symptoms (14 cortical and 0 subcortical) (pfdr-values ≤ 0.049). There were no significant associations between GMVs and internalizing symptoms (pfdr-values ≥ 0.133). Results of sensitivity analyses controlling for medication use were also convergent with the primary results (Tables S5 and S6 for cortical and subcortical results, respectively). GMV was inversely associated with psychopathology in many regions for general psychopathology (51 cortical and 19 subcortical regions) and conduct problems (50 cortical and 16 subcortical regions), with a weaker inverse association demonstrated for ADHD symptoms (23 cortical and 8 subcortical) (pfdr-values ≤ 0.049). There were no significant associations between GMVs and internalizing symptoms (pfdr-values ≥0.068). Results of sensitivity analyses controlling for ICV or total cortical/subcortical GMV revealed that most region-specific results became not significant, further supporting the importance of globally smaller GMV in association with psychopathology. When controlling for ICV, the only relationship that remained significant was an inverse association between bilateral hippocampus GMV and general psychopathology (left hippocampus: β = −0.04, pfdr = 0.019 and right hippocampus: β = −0.03, pfdr = 0.019) (Table S7). When controlling for total subcortical GMV, there were significant inverse associations between general psychopathology and GMV of the bilateral hippocampus (left: β = −0.04, pfdr = 0.010; right: β = −0.04, pfdr = 0.010), bilateral accumbens area (left: β = −0.03, pfdr = 0.041; right: β = −0.03, pfdr = 0.038), and left amygdala (β = −0.03, pfdr = 0.038), as well as a positive association between general psychopathology and right cerebellum cortex GMV (β = 0.01, pfdr = 0.038) (Table S8). No other regions were significant (pfdr-values ≥ 0.054).
Discussion
Capitalizing on a large sample of 9–10-year-old children from across the United States, we found that GMVs in many regions were significantly associated with general and specific psychopathology dimensions after correction for multiple comparisons. Specifically, smaller GMVs throughout most regions were associated with higher levels of the general factor of psychopathology symptoms. The global nature of this association is demonstrated by the loss of most regional associations when controlling for total cortical/subcortical GMV or ICV. However, several regional associations did remain after controlling for indices of brain size. Specifically, when controlling for ICV or subcortical GMV, a significant inverse association between bilateral hippocampus GMV and general psychopathology remained. When controlling for total subcortical GMV, inverse associations also remained between general psychopathology and regional GMVs of the bilateral accumbens area and left amygdala, and there was a significant positive association between general psychopathology and right cerebellum cortex GMV. In addition, a large portion of regional GMVs were inversely associated with the conduct problems factor, and a smaller portion with the ADHD symptoms factor, with smaller volumes being associated with greater symptoms in these domains. Sensitivity analyses suggest that these results are robust even after controlling for income, parental education, and medication use. Overall, these results suggest that globally smaller GMV in childhood may be a nonspecific risk factor for psychopathology across many mental disorders. There is also evidence of specific associations for the conduct problems and ADHD dimensions.
The results of the current study are consistent with prior work showing smaller volumes in multiple regions associated with a general psychopathology factor in children, adolescents, and/or young adults [23–26] and with meta-analyses showing common deficits across disorders in terms of GMV [11] and functional activity [39]. However, the broad age ranges used in prior studies make it difficult to disentangle the impact of development on these associations. The current study expands on prior work by investigating the associations between GMV and psychopathology dimensions in a large sample of children with a narrowly defined age range. Our finding of an inverse association between GMV and general psychopathology suggests that the relationship between smaller brain volume and psychopathology is evident at this early point in development. These results also suggest that GMV deficits across multiple forms of psychopathology in childhood are widespread across the brain and nonspecific to particular symptom classes. However, while these associations may appear broad and nonspecific, it is possible that neurostructural deficits diverge into specific patterns over the course of development. Future longitudinal data collection in the ABCD Study will allow us to evaluate whether distinctive patterns of brain variation manifest as children mature and develop divergent patterns of psychopathology.
Prior studies have speculated on the potential influences that may contribute to the relationship between GMV and psychopathology [23], which are important in understanding the clinical implications of our findings. For instance, a failure to achieve normative levels of cortical expansion during development with either reduced peak GMV or accelerated GMV loss could contribute to the association between smaller GMVs and psychopathology [23]. In addition, some neurostructural abnormalities may be present from in-utero or early life and persist through development as a result of factors such as maternal infections or obstetric complications [23]. Given the detrimental impact of environmental factors on the developing fetus, the most effective clinical interventions in response to our finding of an association between smaller GMV and psychopathology will likely need to come at the prenatal stage such as better maternal nutrition, prenatal care, and quick treatment of infections.
It is also important to understand our findings in the context of cognitive and behavioral development and related clinical outcomes. The general psychopathology factor in childhood has been associated with diffuse problems such as deficits in self-control, emotional regulation, and executive functions [7]. Thus, the observation of nonspecific smaller GMV across regions involved in self-control, emotional processing, executive functioning, and other functions is compatible with the nonspecific cognitive and behavioral deficits that the general factor may represent in children. This could mean that smaller global GMVs in childhood are related to an elevated risk of negative clinical outcomes in behavioral and cognitive domains.
Interestingly, analyses controlling for ICV or total subcortical GMV revealed focal results for the hippocampus, bilaterally. Analyses controlling for total subcortical GMV also revealed inverse associations between general psychopathology and GMV of the bilateral accumbens area and left amygdala, and a positive association between general psychopathology and right cerebellum cortex GMV. Thus, the results of the current study suggest both diffuse associations between regional GMV and general psychopathology across the brain, as well as potential focal associations with the hippocampus, amygdala, accumbens area, and cerebellar cortex. Notably, the hippocampus and amygdala are important for memory function, threat appraisal, and regulation of the hypothalamic-pituitary-adrenal axis, which is critical to responding to stress [40]. Smaller hippocampal volumes are also associated with childhood maltreatment, which is known to increase risk for psychopathology symptoms [41]. Our findings related to the hippocampus and amygdala specifically are consistent with prior research that demonstrated a significant negative correlation between overall mental illness and hippocampus and amygdala GMVs in a large sample of 11–21 years old [26]. In contrast to our finding of a positive association between general psychopathology and right cerebellum GMV, prior literature has yielded inverse associations between GMV of cerebellar lobules and general psychopathology [42, 43]. However, these results came from samples primarily made up of adolescents and young adults, so it is possible that this association manifests differently in children. Additional research is needed to characterize the specific relationship between general psychopathology and cerebellum GMV across development.
Of note, the specific internalizing factor showed no significant relationship with regional GMV in the current study. Given that each symptom assessed loads onto both the general psychopathology factor and one specific factor, the general factor may account for the majority of the variance explained, leaving little for the residual internalizing factor to explain. This finding could change as these children reach adolescence when the incidence of serious anxiety and mood disorders is greatest [1, 44]. Future waves of the longitudinal ABCD Study will allow us to further parse the heterogeneous trajectories of brain development associated with psychopathology [45]. Crucially, since the ABCD Study captures neurostructural development prior to the increase in anxiety and mood symptoms following puberty, this will offer a unique opportunity to examine changes in trajectories using a prospective approach, as only some children will go on to develop internalizing disorders. Identifying the risk factors for these children will be invaluable for advancing our knowledge about brain–behavior relationships during development.
Notably, the effect sizes found in our analyses of GMV and psychopathology were relatively small. Other studies with large samples have consistently observed small but reliable associations between brain structure and behavior [46]. One reason that this might be expected is the brief nature of many psychopathology measures used in large studies. These measures often have few items, which leads to modest validity and reliability. Furthermore, many studies rely on cross-sectional samples. This is particularly relevant in the context of data from Wave 1 of the ABCD Study, as childhood is a time of substantial change in both psychopathology [1] and brain development [3], yet both have been captured at only one timepoint. It is likely that the magnitudes of the associations between psychopathology and GMVs are underestimated here, as they are based on a single timepoint. This underscores why future waves of ABCD Study data will be vital to illuminating developmental trajectories of psychopathology and brain structure. While the current study is limited by a cross-sectional design, one strength of this study is the narrowly defined age range (in contrast to broad age ranges used in prior cross-sectional studies) that allows us to more precisely define the relationship between brain and behavior at a specific age.
Several additional limitations of the current study are important to note. First, the psychopathology factors are based on parent-report CBCL data. Thus, they reflect parent impressions of children’s behavior, and do not reflect child self-report, or formal clinical diagnoses. However, this type of symptom-level data, as compared to categorical diagnostic data, does aid in analyzing mechanisms of psychopathology from a dimensional perspective, which is likely to better reflect the spectrum of psychopathology symptoms. Second, as noted previously, the group of participants that was excluded from analyses due to missing data or failing to pass the various levels of quality assurance for imaging data was characterized by significantly lower income and parent education, higher age, and a higher proportion of females and of racial/ethnic minority status individuals. This non-randomness could be due to known associations between lower SES, higher psychopathology, and more motion in the scanner [47, 48]. Importantly, the non-random nature of missing data may decrease the generalizability of the current results and will likely underestimate the effects for psychopathology. However, these associations are difficult to address as motion is intertwined with SES and psychopathology, and excessive motion compromises the usability of the neuroimaging data. Finally, future work would benefit from using multivariate analytical approaches, such as those implemented with machine learning tools, to classify patients for predictive purposes or to identify subtypes of individuals based on neurobiological indices [10]. While beyond the scope of the current paper, such approaches would be invaluable next steps for understanding brain–psychopathology relationships in this age range.
In sum, consistent with the emerging notion that general psychopathology may relate to nonspecific variation in the brain, this study demonstrates that dimensions of general psychopathology, ADHD symptoms, and conduct problems are inversely associated with global GMV. This may suggest that genetic and environmental factors that globally influence the size of the brain are risk factors for general psychopathology. In addition, we found several focal relationships in the hippocampus, amygdala, accumbens, and cerebellum when these global associations are accounted for by including indices of brain size as covariates. Together, these results support and expand upon prior work on the relationship between neurostructural variations and psychopathology in childhood.
Funding and disclosure
This research was supported by grants UG3DA045251 (awarded to BBL) from the National Institute on Drug Abuse, R01MH098098 (BBL), R01MH117014 (TMM), and R00MH117274 (ANK) from the National Institute of Mental Health, UL1TR000430 (BBL) and UL1TR000445 (BBL) from the National Center for Advancing Translational Sciences, the NARSAD Young Investigator Award (ANK), the Sloan Research Fellowship (ANK), and the Lifespan Brain Institute of the University of Pennsylvania and the Children’s Hospital of Philadelphia (TMM). ELD, HJJ, TMM, RMD, CCI, ZC, FES, MGB, BBL, and ANK report no competing interests.
Supplementary information
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from RRID: SCR_015769, 10.15154/1519264.
Author contributions
ELD took the lead on analyzing the data, writing the manuscript and supplement, making the tables and figures, and making revisions, HJJ contributed to the writing of the manuscript, TMM wrote the Mplus code for the bifactor analyses and SEM models and provided statistical consultation, RMD adapted and further developed the R code to download and prepare the ABCD Study data for analysis and provided consultation for R and Mplus, CCI developed the initial R code to download and prepare the ABCD Study data for analysis, ZC developed the code for making the brain figures and provided consultation on the use of Connectome Workbench, FES contributed to the writing of the manuscript, MGB provided conceptual and statistical consultation and contributed to the writing of the manuscript, BBL provided conceptual and statistical consultation, contributed to data analyses, contributed to the writing of the manuscript, and contributed funding to support this project, ANK provided conceptual consultation, contributed to data analyses, contributed to the writing of the manuscript, and served as the primary mentor to ELD, HJJ, RMD, and FES. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Data availability
The ABCD Study data is available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd).
Code availability
For the code and a corresponding wiki describing the analytic procedures used in this study, see https://github.com/VU-BRAINS-lab/Durham_bifactor_volume.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00952-w).
References
- 1.Roza SJ, Hofstra MB, Van Der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. Am J Psychiatry. 2003;160:2116–21. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- 2.Reef J, Van Meurs I, Verhulst FC, Van Der Ende J. Children’s problems predict adults’ DSM-IV disorders across 24 years. J Am Acad Child Adolesc Psychiatry. 2010;49:1117–24. doi: 10.1016/j.jaac.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 4.Sale A, Berardi N, Maffei L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol Rev. 2014;94:189–234. doi: 10.1152/physrev.00036.2012. [DOI] [PubMed] [Google Scholar]
- 5.Kotov R, Waszczuk MA, Krueger RF, Forbes MK, Watson D, Clark LA, et al. The hierarchical taxonomy of psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126:454–77. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 6.Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, et al. A hierarchical taxonomy of psychopathology can transform mental health research. Perspect Psychol Sci. 2019;14:419–36. doi: 10.1177/1745691618810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175:831–44. doi: 10.1176/appi.ajp.2018.17121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull. 2017;143:142–86. doi: 10.1037/bul0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zald DH, Lahey BB. Implications of the hierarchical structure of psychopathology for psychiatric neuroimaging. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:310–7. doi: 10.1016/j.bpsc.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczkurkin AN, Moore TM, Sotiras A, Xia CH, Shinohara RT, Satterthwaite TD. Approaches to defining common and dissociable neurobiological deficits associated with psychopathology in youth. Biol Psychiatry. 2020;88:51–62. doi: 10.1016/j.biopsych.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental Illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W, Bindman J, Ford T, Glozier N, Moran P, Stewart R, et al. Bias in psychiatric case-control studies: Literature survey. Br J Psychiatry. 2007;190:204–9. doi: 10.1192/bjp.bp.106.027250. [DOI] [PubMed] [Google Scholar]
- 13.Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, et al. Progress in achieving quantitative classification of psychopathology. World Psychiatry. 2018;17:282–93. doi: 10.1002/wps.20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychol Bull. 2011;137:856–79. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- 15.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2:119–37. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry. 2011;68:181–9. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahey BB, Moore TM, Kaczkurkin AN, Zald DH. Hierarchical models of psychopathology: empirical support, implications, and remaining issues. World Psychiatry. 2021;20:57–63. doi: 10.1002/wps.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore TM, Kaczkurkin AN, Durham EL, Jeong HJ, McDowell MG, Dupont RM, et al. Criterion validity and relationships between alternative hierarchical dimensional models of general and specific psychopathology. J Abnorm Psychol. 2020;129:677–88. doi: 10.1037/abn0000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is There a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121:971–7. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahey BB, Zald DH, Perkins SF, Villalta-Gil V, Werts KB, Van Hulle CA, et al. Measuring the hierarchical general factor model of psychopathology in young adults. Int J Methods Psychiatr Res. 2018;27:e1593. doi: 10.1002/mpr.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankin BL, Davis EP, Snyder H, Young JF, Glynn LM, Sandman CA. Temperament factors and dimensional, latent bifactor models of child psychopathology: Transdiagnostic and specific associations in two youth samples. Psychiatry Res. 2017;252:139–46. doi: 10.1016/j.psychres.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laceulle OM, Vollebergh WAM, Ormel J. The structure of psychopathology in adolescence. Clin Psychol Sci. 2015;3:850–60. [Google Scholar]
- 23.Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. Am J Psychiatry. 2019;17:1000–9. doi: 10.1176/appi.ajp.2019.18070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder HR, Hankin BL, Sandman CA, Head K, Davis EP. Distinct patterns of reduced prefrontal and limbic gray matter volume in childhood general and internalizing psychopathology. Clin Psychol Sci. 2017;5:1001–13. doi: 10.1177/2167702617714563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romer AL, Knodt AR, Houts R, Brigidi BD, Moffitt TE, Caspi A, et al. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol Psychiatry. 2017;23:1084–90. doi: 10.1038/mp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore TM, Calkins ME, Satterthwaite TD, Roalf DR, Rosen AFG, Gur RC, et al. Development of a computerized adaptive screening tool for overall psychopathology (“p”) J Psychiatr Res. 2019;116:26–33. doi: 10.1016/j.jpsychires.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achenbach TM. The Achenbach system of empirically based assessment (ASEBA): development, findings, theory, and applications. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2009. [Google Scholar]
- 32.Muthén LK, Muthén BO. Mplus user’s guide. 8th edn. Los Angeles, CA: Muthén & Muthén; 2017.
- 33.Bornovalova MA, Choate AM, Fatimah H, Petersen KJ, Wiernik BM. Appropriate use of bifactor analysis in psychopathology research: appreciating benefits and limitations. Biol Psychiatry. 2020;88:18–27. doi: 10.1016/j.biopsych.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 36.Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44:71–85. doi: 10.1038/s41386-018-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heeringa SG, Berglund PA. A guide for population-based analysis of the Adolescent Brain Cognitive Development (ABCD) study baseline data. 2020. https://www.biorxiv.org/content/10.1101/2020.02.10.942011v1.
- 38.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–8. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprooten E, Rasgon A, Goodman M, Carlin A, Leibu E, Lee WH, et al. Addressing reverse inference in psychiatric neuroimaging: meta-analyses of task-related brain activation in common mental disorders. Hum Brain Mapp. 2017;38:1846–64. doi: 10.1002/hbm.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mcewen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whittle S, Simmons JG, Hendriksma S, Vijayakumar N, Byrne ML, Dennison M, et al. Childhood maltreatment, psychopathology, and the development of hippocampal subregions during adolescence. Brain Behav. 2017;7:e00607. doi: 10.1002/brb3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moberget T, Alnæs D, Kaufmann T, Doan NT, Córdova-Palomera A, Norbom LB, et al. Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol Psychiatry. 2019;86:65–75. doi: 10.1016/j.biopsych.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Romer AL, Knodt AR, Houts R, Brigidi BD, Moffitt TE, Caspi A, et al. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol Psychiatry. 2018;23:1084–90. doi: 10.1038/mp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navarro-Pardo E, Meléndez Moral JC, Sales Galán A, Sancerni Beitia MD. Child and adolescent development: common mental disorders according to age and gender. Psicothema. 2012;24:377–83. [PubMed] [Google Scholar]
- 45.Becht AI, Mills KL. Modeling individual differences in brain development. Biol Psychiatry. 2020;88:63–69. doi: 10.1016/j.biopsych.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulus MP, Thompson WK. The challenges and opportunities of small effects: the new normal in academic psychiatry. JAMA Psychiatry. 2019;76:353–4. doi: 10.1001/jamapsychiatry.2018.4540. [DOI] [PubMed] [Google Scholar]
- 47.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peverill M, Dirks MA, Narvaja T, Herts KL, Comer JS, McLaughlin KA. Socioeconomic status and child psychopathology in the United States: a meta-analysis of population-based studies. Clin Psychol Rev. 2020;83:101933. doi: 10.1016/j.cpr.2020.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ABCD Study data is available through the National Institute of Mental Health Data Archive (https://nda.nih.gov/abcd).
For the code and a corresponding wiki describing the analytic procedures used in this study, see https://github.com/VU-BRAINS-lab/Durham_bifactor_volume.