Abstract
Primary intraosseous oral squamous cell carcinoma (PIOSCC) is a rare malignant neoplasm that affects the jaws. Despite its aggressive biological behavior, there are no studies that evaluated the clinicopathological features of this tumor and parameters associated with its prognosis. The objective of the present study was to conduct a systematic review of the available data on oral and maxillofacial PIOSCC in order to determine its clinicopathological characteristics and biological behavior. We conducted a systematic review in May 2020 in multiple databases using a specific search strategy. Cases diagnosed as PIOSCC in the oral cavity and maxillofacial complex that had sufficient histopathological data, absence of ulceration in the oral mucosa, a negative result for a distant primary tumor, and radiographic evidence of an osteolytic lesion that was entirely or mostly surrounded by the jaw bones were included. A total of 109 published articles were included in our systematic review, corresponding to 257 cases. PIOSCC showed a male predilection (69.3%) and a preference for the mandible (7:1), with the posterior region being the most commonly affected site. The mean age at diagnosis was 57.3 years. Cortical expansion, pain, and lip/facial paresthesia were the most common clinical features. Regarding histopathological features, most PIOSCC were well-differentiated and the solid subtype was the most common. Statistical analysis showed that PIOSCC located in the mandible (p = 0.03) and recurrence (p < 0.01) were significantly associated with a higher mortality rate. PIOSCC has a poor prognosis, with high rates of mortality.
Electronic supplementary material
The online version of this article (10.1007/s12105-020-01234-z) contains supplementary material, which is available to authorized users.
Keywords: Malignant neoplasm, Primary intraosseous squamous cell carcinoma, Clinicopathological feature, Prognosis
Introduction
Primary intraosseous carcinoma of the jaws is a rare neoplasm that is characterized by different histological subtypes, distinct clinicopathological features, and a varied biological behavior [1]. Intraosseous carcinomas of the jaws can be classified according to their histological subtype into (i) salivary gland carcinomas, (ii) odontogenic carcinomas, and (iii) primary intraosseous squamous cell carcinomas (PIOSCC) [2].
First described by Loos in 1913, PIOSCC is a rare malignant neoplasm that affects the jaws [3]. The latest WHO classification defines PIOSCC as a central tumor that probably arises from the odontogenic epithelium and that can be associated with odontogenic cysts or other benign precursors [4]. Because of its rarity, it is difficult to obtain detailed knowledge about the clinical and pathological features of PIOSCC [4, 5]. Histologically, PIOSCC varies from well-differentiated tumors to poorly differentiated carcinomas [4, 6].
The criteria established for the diagnosis of PIOSCC include the absence of a communication with the adjacent mucosa, the absence of a preexisting primary tumor at diagnosis (ruling out the hypothesis of metastasis to the jaws), and histological evidence of squamous cell carcinoma [4, 7].
The overall prognosis of PIOSCC is poor and high rates of recurrence and metastasis, as well as low survival rates, have been reported [5, 8]. Despite its aggressive biological behavior, to our knowledge, no systematic review has evaluated the clinicopathological features and possible parameters associated with the prognosis of PIOSCC. The majority of previous studies are reports of single cases or case series. Therefore, the aim of this systematic review is to evaluate the clinicopathological features and prognosis of PIOSCC in the literature.
Materials and Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. The study was registered with PROSPERO under number CRD42020181439.
Data Sources and Search Strategies
A literature search was performed in May 2020 in the following electronic databases: PubMed/MEDLINE, Scopus, Science Direct, Embase, and Cochrane Collaboration Library. Manual searches were also conducted by cross-checking the reference lists of the included articles. Duplicates were removed upon identification. The search was carried out without time and language restrictions. Manuscripts that were not published in English were translated for further evaluation.
The following review question was developed according to the population, intervention, comparison, and outcome (PICO) model: “What are the clinicopathological characteristics and prognostic factors in PIOSCC of the jaws?” A keyword search was performed.
The search strategy was based on combinations of the following keywords: (“Intraosseous carcinoma“[tw] OR “Squamous Cell Carcinoma“[tw] OR “Primary Intraosseous Carcinoma“[tw]) AND (“Jaws“[tw] OR “Mandible“[tw] OR “Maxilla“[tw]) AND (“Prognosis“[tw] OR “Prognoses“[tw] OR “Prognostic Factors“[tw] OR “Factor, Prognostic“[tw] OR “Factors, Prognostic“[tw] OR “Prognostic Factor” OR “Survival“[tw]).
Eligibility Criteria and Study Selection
Publications reporting cases of PIOSCC of the jaws were eligible. The studies needed to contain enough clinical, radiological and histological information to confirm the diagnosis. Randomized controlled clinical trials, cohort studies, case-control studies, cross-sectional studies, case series, and case reports were included. Exclusion criteria were: (1) immunohistochemical studies; (2) radiological studies; (3) cytological studies; (4) histomorphometric studies; (5) genetic expression studies; (6) histopathological studies; (7) in vitro studies, and (8) review papers, unless any of these publication categories reported cases with enough clinical, radiological and histological information.
The diagnostic criteria for inclusion in this systematic review were: (1) absence of ulceration in the oral mucosa, except when caused by other factors such as tooth extraction or trauma; (2) histopathological confirmation of squamous cell carcinoma; (3) a negative result for a distant primary tumor, and (4) radiographic evidence of an osteolytic lesion that was entirely or mostly surrounded by the jaw bones.
The titles and abstracts of all articles identified by the electronic searches were read independently by the authors. In a second round, the full text of studies that appeared to meet the inclusion criteria was read. Disagreements were resolved by discussion between the authors. The clinical and radiological features, as well as the histological/immunohistochemical description of the lesions reported in the studies, were thoroughly assessed by four of the authors in order to confirm the diagnosis of PIOSCC (E.F.M., H.D.D.M., H.G.F.M., and L.M.C.).
Data Extraction/Analysis
The review authors independently screened the articles for data extraction. Any disagreements were resolved by discussion. The following data were collected from each article: (1) authors’ name; (2) publication year; (3) number of cases reported; (4) patients’ age and sex, and (5) anatomic location. The following clinical information was obtained: (1) primary location of the tumor; (2) clinical features; (3) histopathological features; (4) subtype (solid, arising from odontogenic cysts and carcinomas associated with odontogenic tumors); (5) TNM classification; (6) treatment; (7) recurrence; (8) metastasis; (9) follow-up period; and (10) patient status at the time of publication.
The methodological quality of the articles was evaluated according to the CARE guidelines for case reports, a qualitative checklist for observational studies and case reports [10].
The clinicopathological data were presented descriptively. The chi-square test was used to compare clinicopathological variables and patient status (alive or dead). Overall survival was determined by survival analysis using the Kaplan–Meier method. For the analysis of overall survival, cases were included only with a minimum follow-up of 5 years. All data were analyzed statistically using the Statistical Package for the Social Sciences (SPSS), version 23 (SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at P < 0.05.
Results
Study Selection
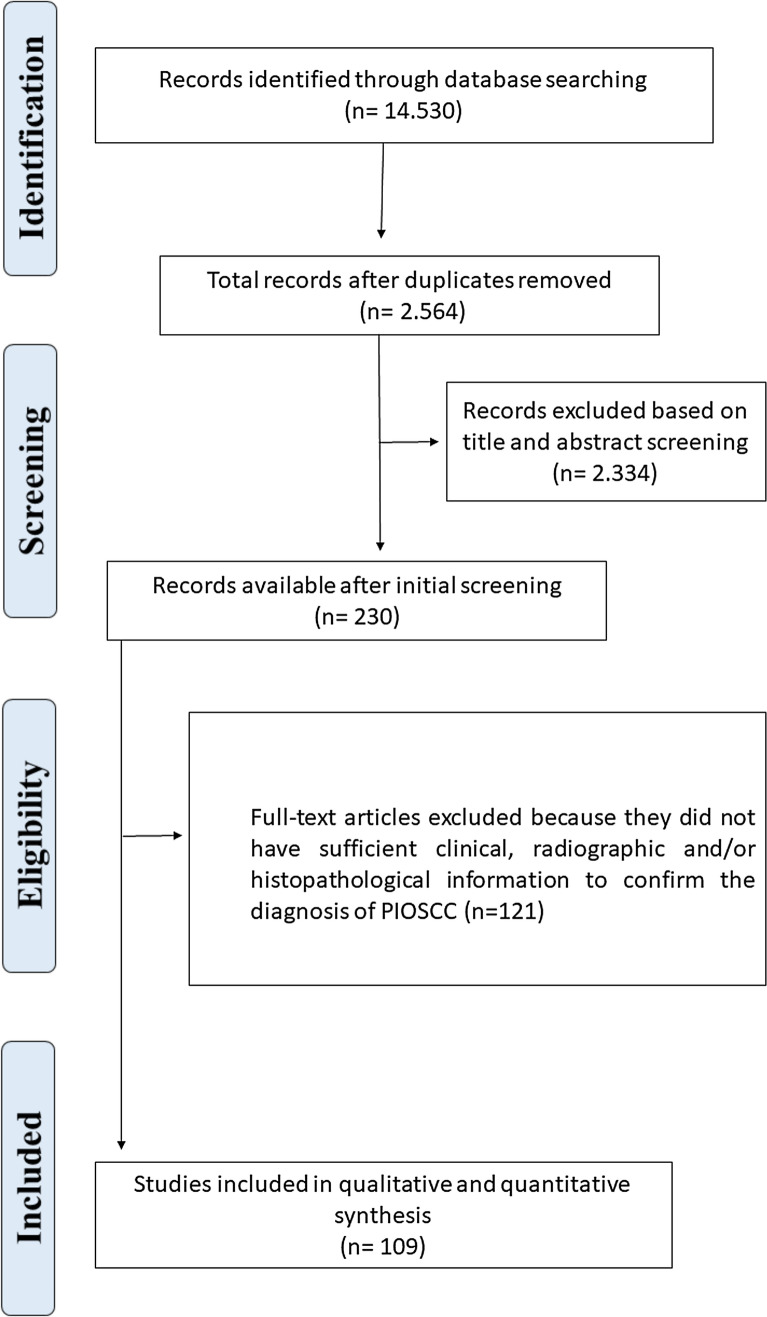
A broad survey was conducted in different databases and by manual search, which retrieved 14,530 articles. After reading the titles and abstract, 186 articles were considered potentially eligible and their full text was read by four evaluators (E.F.M., H.D.D.M., H.G.F.M., and L.M.G.).
After full-text reading of the pre-selected articles, 109 met all inclusion criteria and were selected for the present systematic review, totaling 257 cases of PIOSCC diagnosed in the jaws. The flow diagram of the article selection process is shown in Fig. 1. The publication languages of the selected articles were English (n = 104), Spanish (n = 3), Turkish (n = 1), and Korean (n = 1). The articles selected for the present systematic review are listed in Online Appendix I.
Fig. 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram of article selection for inclusion in the systematic review
Clinical and Histopathological Features
PIOSCC was most commonly diagnosed in male patients (69.3%). The mean age of the patients was 57.3 years, ranging from 5 [11] to 89 years [12]. Only two cases were diagnosed in patients younger than 18 years [11, 13]. The mandible was the most commonly affected site (mandible/maxilla ratio of 7:1), with most tumors occurring in the posterior region of the mandible (Fig. 2).
Fig. 2.
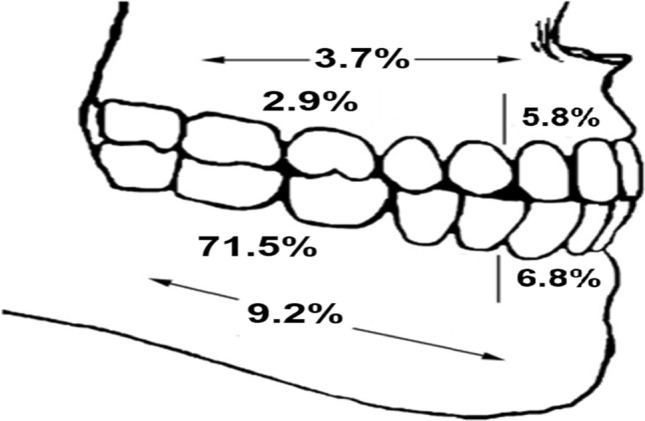
Anatomical distribution of cases diagnosed as primary intraosseous squamous cell carcinoma. The percentage of cases between arrows indicates PIOSCC located in the anteroposterior region of the mandible or maxilla
Clinically, cortical bone expansion was a common finding in the PIOSCC cases included in our analysis (60.3%). Pain (43.5%) and lip or facial paresthesia (28.4%) were frequently observed. Radiographically, PIOSCC appear as well-defined lesions (26%) or as irregular lesions with imprecise limits (31.1%). Less frequent findings are also observed. Cortical bone destruction was found in 33.5% of the included cases.
Regarding histopathological findings, in our review, most tumors were graded as well-differentiated, followed by moderately-differentiated and poorly-differentiated tumors (Table 1). The solid subtype was the most common (35%), 30.3% of the cases arose from odontogenic cysts, and only 0.3% from an odontogenic tumor (ameloblastoma) [14]. Table 1 shows the clinicopathological features of the PIOSCC cases.
Table 1.
Clinicopathological features of 257 PIOSCC cases published in the literature
| Clinicopathological variables | N | % |
|---|---|---|
| Sex | ||
| Male | 178 | 69.3 |
| Female | 79 | 30.7 |
| Age (mean age: 57.37 years) | ||
| Location of tumor | ||
| Maxilla | 32 | 12.4 |
| Mandible | 225 | 87.5 |
| Jaw swelling | ||
| Yes | 155 | 60.3 |
| No | 19 | 7.4 |
| NA | 83 | 32.3 |
| Pain | ||
| Yes | 112 | 43.5 |
| No | 38 | 14.7 |
| NA | 107 | 41.6 |
| Lip or facial numbness | ||
| Yes | 73 | 28.4 |
| No | 62 | 24.1 |
| NA | 122 | 47.4 |
| History of teeth extraction or mobility | ||
| Yes | 100 | 38.9 |
| No | 41 | 15.9 |
| NA | 116 | 45.1 |
| Radiographic features | ||
| Irregular osteolytic lesion | 80 | 31.1 |
| A well-delineated radiolucent lesion | 67 | 26.0 |
| Others | 110 | 42.8 |
| Destruction of bone cortex | ||
| Yes | 86 | 33.5 |
| No | 17 | 6.6 |
| NA | 154 | 59.9 |
| Size of tumor (cm) | ||
| ≤ 4 | 32 | 12.4 |
| > 4 | 30 | 11.7 |
| NA | 105 | 40.9 |
| Lymph nodes metastasis | ||
| Absent | 78 | 30.4 |
| Present | 33 | 12.8 |
| NA | 146 | 56.8 |
| Subtype | ||
| Solid | 90 | 35.0 |
| Arising from odontogenic cysts | 78 | 30.3 |
| Arising from odontogenic tumors | 1 | 0.3 |
| NA | 88 | 34.2 |
| Histological grading | ||
| Well-differentiated | 79 | 30.7 |
| Moderately-differentiated | 41 | 15.9 |
| Poorly-differentiated | 27 | 10.5 |
| NA | 110 | 42.8 |
| Treatment | ||
| Surgery alone OR surgery + ND | 102 | 39.6 |
| Surgery alone OR surgery + ND + RT | 89 | 34.6 |
| Surgery alone OR surgery + ND + RT + ChT | 20 | 7.7 |
| RT alone OR ChT alone | 1 | 0.3 |
| Others | 7 | 2.7 |
| NA | 38 | 14.7 |
| Recurrence | ||
| Yes | 57 | 22.1 |
| No | 102 | 39.7 |
| NA | 98 | 38.1 |
| Follow-up period (months) mean follow up: 31.1 months | ||
| ≤ 5.0 years | 106 | 41.2 |
| > 5.0 years | 14 | 5.5 |
| NA | 137 | 53.3 |
| Status | ||
| Alive | 103 | 40.0 |
| Death due to tumor | 40 | 15.5 |
| NA | 114 | 44.3 |
NA not available, ND neck dissection, RT radiotherapy, ChT chemotherapy
Treatment and Outcome
The most frequently employed treatment in the included studies was surgery (39.6%), followed by surgical treatment combined with adjuvant radiotherapy (34.6%). Lymph node metastasis was observed in 12.8% of the cases and local recurrence in 22.1%. Cases of PIOSCC located in the mandible (p = 0.03) and recurrence (p < 0.01) were associated with a higher mortality rate (Table 2).
Table 2.
Association analysis between the clinicopathological features of cases 257 PIOSCC of the jaws cases and the patients’ status at last follow-up
| Clinicopathological variables | Alive N (%) | Dead N (%) | p value |
|---|---|---|---|
| Sex | |||
| Male | 65 (72.2%) | 17 (65.3%) | 1.0 |
| Female | 25 (27.7%) | 9 (34.6%) | |
| Location of tumor | |||
| Maxilla | 19 (21.1%) | 2 (7.7%) | 0.03 |
| Mandible | 71 (78.8%) | 24 (92.3%) | |
| Radiographic features | |||
| Irregular osteolytic lesion | 27 (56.2%) | 8 (53.3%) | 0.79 |
| Well-delineated radiolucent lesion | 21 (43.7%) | 7 (46.6%) | |
| Destruction of bone cortex | |||
| Yes | 42 (87.5%) | 7 (100%) | 0.1 |
| No | 6 (12.5%) | 0 (0%) | |
| Lymph nodes metastasis | |||
| Absent | 56 (86.1%) | 13 (65%) | 0.12 |
| Present | 9 (13.8%) | 7 (35%) | |
| Subtype | |||
| Solid | 29 (53.7%) | 9 (56.2%) | 0.9 |
| Arising from odontogenic cysts OR tumors | 25 (46.3%) | 7 (43.7%) | |
| Histological grading | |||
| Well-differentiated | 27 (50.9%) | 7 (50%) | 1.0 |
| Poorly/moderately differentiated | 26 (49.0%) | 7 (50%) | |
| Treatment | |||
| Surgery alone OR surgery + ND | 29 (56.8%) | 5 (70.5%) | 0.7 |
| Surgery alone OR surgery + ND + adjuvant therapy | 22 (43.1%) | 12 (29.45) | |
| Recurrence | |||
| Yes | 7 (14.2%) | 17 (77.2%) | < 0.01 |
| No | 42 (85.7%) | 5 (22.7%) | |
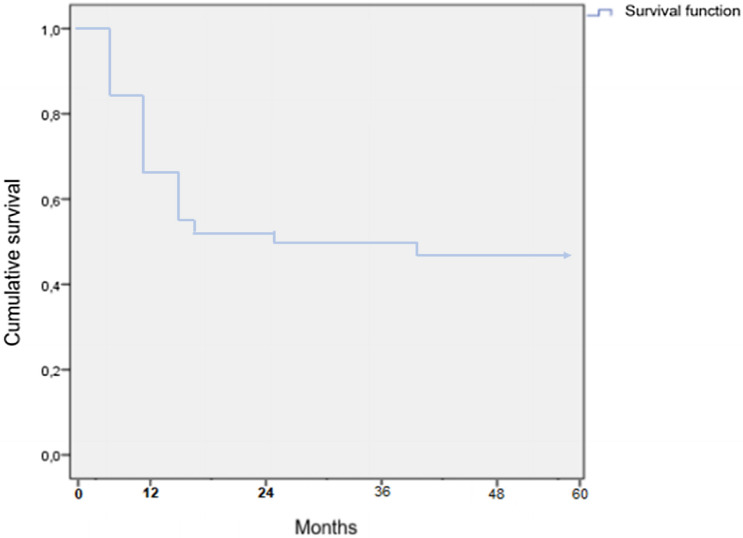
Taking into account all cases where the status of the patients was available, 72% of the cases diagnosed as PIOSCC were alive. However, considering only studies with at least 5 years follow-up, the overall survival rate of the patients included in this systematic review was 44.6%. Among the cases of death, most patients died before 2 years after initial diagnosis (Fig. 3).
Fig. 3.
Kaplan–Meier survival curve of intraosseous squamous cell carcinoma of the jaws
Quality Assessment
The results of the quality assessment of the selected studies are shown in appendix II. Few articles adequately reported the clinicopathological features and follow-up information of the patients analyzed.
Discussion
Primary intraosseous squamous cell carcinoma is a rare neoplasm that exclusively affects the jaws and that presumably arises from remnants of odontogenic epithelium [4, 8]. The estimated incidence of PIOSCC was projected to range from 1 to 2% of all oral cancers [3, 15]. Because of the rarity of PIOSCC, most available studies are case reports. We thus conducted this systematic review to obtain more in-depth knowledge of the clinicopathological features and prognostic factors of this tumor.
Squamous cell carcinomas originating from the jaws have a clinicopathological behavior and biological profile that differ from their counterparts affecting soft tissues of the oral mucosa. In a functional genomic analysis of a mandibular odontogenic carcinoma, Alevizos et al. [16] observed that PIOSCC possesses a different profile of oncogenes and tumor suppressors compared to soft tissue squamous cell carcinoma.
Like its counterpart in the oral mucosa, PIOSCC has a male predilection and more commonly affects patients in their fifth to seventh decade of life [3]. The mandible is significantly more involved than the maxilla [8]. PIOSCC has a poor prognosis, with low survival rates and recurrences being common. Previous studies reported a 2-year survival rate of 60 to 70%, while the 5-year survival rate ranged from 30 to 40% [5, 8, 17, 18]. Our systematic review corroborates the findings of other studies by demonstrating a 5-year survival rate of only 44.6%.
The lack of specific clinical and radiographic features of PIOSCC hinder early diagnosis, a factor that may be directly associated with the poor prognosis of the tumor. Cortical bone expansion, tooth mobility, pain, and paresthesia were relatively common findings in the selected articles. However, early-stage tumors are commonly included in the differential diagnosis with benign odontogenic lesions, delaying the diagnosis and, consequently, appropriate treatment [5, 19, 20]. Lukandu et al. [19] also pointed out that the late diagnosis of PIOSCC is frequently associated with the absence of imaging tests before exodontia procedures.
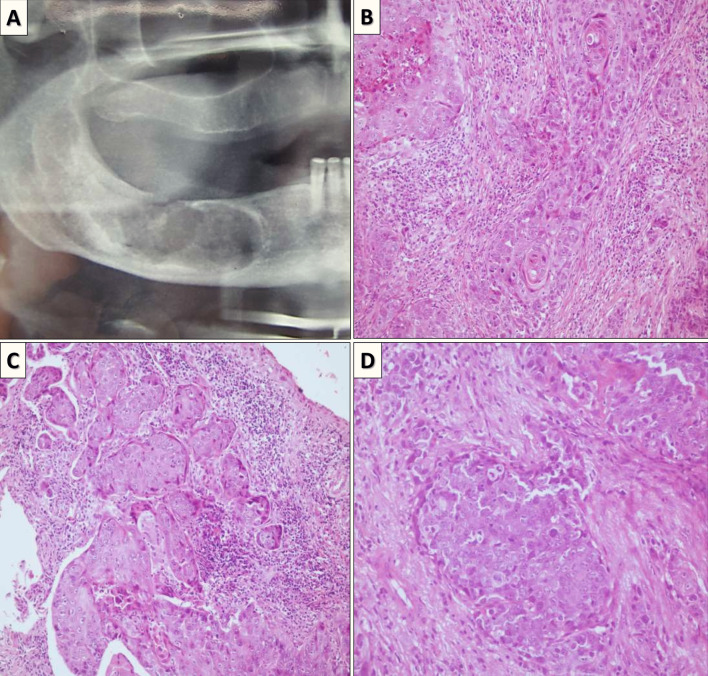
The histopathological pattern or subtype of PIOSCC was not associated with the survival of the patients analyzed in our systematic review (Fig. 4). However, unlike observed in the present systematic review, the histological classification of PIOSCC had a strong survival impact in other studies [5, 8, 21]. The lack of histopathological grading data in a significant number of cases included in our review (42.8%) may explain the absence of a significant association. However, histopathological parameters, e.g., tumor budding, lymphovascular invasion, perineural invasion and extraosseous extension into surrounding structures can be promising markers of prognosis.
Fig. 4.
a Sectional orthopantomogram showing irregular radiolucent areas in the right mandible. b–d Microscopic features. Proliferation of islands, cords, and solid masses of malignant epithelial cells. Cellular and nuclear pleomorphism, scarce intracellular, extracellular keratinization, and increased mitosis are observed in the same specimen
In our study, most cases were tumors arising de novo (subtype I) or from an odontogenic cyst. Careful analysis of the surgical specimen by an experienced pathologist is important to identify the true origin of the tumor [3].
There is still no specific staging system for PIOSCC and the staging system proposed by the American Joint Committee on Cancer (AJCC) for oral cancer is used for this purpose [22]. Specific treatment guidelines for PIOSCC are also not yet available. However, Naruse et al. [18] believe that, since the overall survival in PIOSCC is similar to that of stage-IV oral squamous cell carcinoma, the treatment of PIOSCC should be compatible with that of T3N0 oral cancer. Future studies evaluating therapeutic protocols with the greatest impact on the survival of patients with PIOSCC will be a decisive factor to prolong patient survival.
The present study is the first systematic review aimed at evaluating the clinicopathological features of PIOSCC and possible etiological factors associated with its prognosis. We observed that, although less frequent, cases of PIOSCC in the maxilla are associated with a better prognosis than those involving the mandible. In addition, recurrence was strongly associated with poor survival. Future multicenter studies for the long-term follow-up of patients with PIOSCC are necessary to expand our knowledge about this neoplasm.
The limitations of our systematic review include the lack of clinicopathological data for a large number of cases diagnosed as PIOSCC since such data are useful to better understand the characteristics of this tumor and possible factors associated with its prognosis. In addition, 5-year follow-up data, which are important for survival analysis, were only available for a small number of cases.
In conclusion, the prognosis of patients with PIOSCC is poor, with high rates of regional lymph node metastases, recurrence and mortality. In view of the lack of specific clinical and radiographic features, PIOSCC should be included in the differential diagnosis of intraosseous lesions of the jaws. Specific staging and therapeutic protocols should be elaborated for PIOSCC in order to improve the prognosis of the tumor. Furthermore, future studies with sample sizes adequate are necessary to investigate possible correlations between clinical, radiographic aspects and morphological parameters and tumor behavior.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 25 kb)
Electronic supplementary material 2 (DOCX 21 kb)
Acknowledgements
This study was supported by Postgraduate Program in Oral Pathology of the UFRN and Coordination for the Improvement of Higher Education Personnel—CAPES. RAF is Research Productivity Fellows at National Council for Scientific and Technological Development—CNPq.
Funding
This study was supported by Postgraduate Program in Oral Pathology of the UFRN and Coordination for the Improvement of Higher Education Personnel—CAPES. RAF is Research Productivity Fellows at National Council for Scientific and Technological Development—CNPq.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geetha P, Avinash Tejasvi ML, Babu BB, Bhayya H, Pavani D. Primary intraosseous carcinoma of the mandible: a clinicoradiographic view. J Cancer Res Ther. 2015;11(3):651. doi: 10.4103/0973-1482.140814. [DOI] [PubMed] [Google Scholar]
- 2.Woolgar JA, Triantafyllou A, Ferlito A, Devaney KO, Lewis JS, Rinaldo A, et al. Intraosseous carcinoma of the jaws: a clinicopathologic review. Part III: primary intraosseous squamous cell carcinoma. Head Neck. 2013;35(6):906–9. doi: 10.1002/hed.22922. [DOI] [PubMed] [Google Scholar]
- 3.Bodner L, Manor E, Shear M, van der Waal I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst: a clinicopathologic analysis of 116 reported cases. J Oral Pathol Med. 2011;40(10):733–8. doi: 10.1111/j.1600-0714.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. World Health Organization classification of head and neck tumours. 4. Lyon: IARC Press; 2017. [Google Scholar]
- 5.Wenguang X, Hao S, Xiaofeng Q, Zhiyong W, Yufeng W, Qingang H, et al. Prognostic factors of primary intraosseous squamous cell carcinoma (PIOSCC): a retrospective review. PLoS ONE. 2016;11(4):e0153646. doi: 10.1371/journal.pone.0153646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur A, Tupkari JV, Joy T, Gogri AA. Primary intraosseous squamous cell carcinoma—a rare odontogenic malignancy. J Oral Maxillofac Pathol. 2017;21(1):320. doi: 10.4103/jomfp.JOMFP_25_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Ide F, Takizawa S, Suzuki S, Sakashita H, Li TJ, et al. Initial-stage primary intraosseous squamous cell carcinoma derived from odontogenic keratocyst with unusual keratoameloblastomatous change of the maxilla: a case report and literature discussion. Case Rep Otolaryngol. 2018;2018:7959230. doi: 10.1155/2018/7959230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li K, Yang L, Qiao YJ, Liang YJ, Wang X, Liao GQ. Risk factors and prognosis for the primary intraosseous carcinoma of the jaw. Int J Oral Maxillofac Surg. 2019;48(2):157–62. doi: 10.1016/j.ijom.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2(5):38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles M, Barr T, Leong I, Ngan BY, Forte V, Sándor GK. Primary intraosseous malignancy originating in an odontogenic cyst in a young child. J Oral Maxillofac Surg. 2008;66(4):813–9. doi: 10.1016/j.joms.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Jain M, Mittal S, Gupta DK. Primary intraosseous squamous cell carcinoma arising in odontogenic cysts: an insight in pathogenesis. J Oral Maxillofac Surg. 2013;71(1):e7–14. doi: 10.1016/j.joms.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Boni P, Sozzi D, Novelli G, Pagni F, Valente G, Bozzetti A. Primary intraosseous squamous cell carcinoma of the jaws: 6 new cases, experience, and literature comparison. J Oral Maxillofac Surg. 2016;74(3):541–6. doi: 10.1016/j.joms.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Hamakawa H, Kayahara H, Sumida T, Tanioka H. Mandibular intraosseous carcinoma coexisting with ameloblastoma. J Oral Maxillofac Surg. 2000;58(4):430–3. doi: 10.1016/S0278-2391(00)90929-7. [DOI] [PubMed] [Google Scholar]
- 15.van der Waal I, Rauhamaa R, van der Kwast WA, Snow GB. Squamous cell carcinoma arising in the lining of odontogenic cysts. Report of 5 cases. Int J Oral Surg. 1985;14(2):146–52. doi: 10.1016/S0300-9785(85)80086-7. [DOI] [PubMed] [Google Scholar]
- 16.Alevizos I, Blaeser B, Gallagher G, Ohyama H, Wong D, Todd R. Odontogenic carcinoma: a functional genomic comparison with oral mucosal squamous cell carcinoma. Oral Oncol. 2002;38(5):504–7. doi: 10.1016/S1368-8375(01)00093-8. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Luo H, Li Q, Li T. Primary intraosseous squamous cell carcinoma of the jaws: clinicopathologic presentation and prognostic factors. Arch Pathol Lab Med. 2009;133(11):1834–40. doi: 10.5858/133.11.1834. [DOI] [PubMed] [Google Scholar]
- 18.Naruse T, Yanamoto S, Sakamoto Y, Ikeda T, Yamada S, Umeda M. Clinicopathological study of primary intraosseous squamous cell carcinoma of the jaw and a review of the literature. J Oral Maxillofac Surg. 2016;74(12):2420–7. doi: 10.1016/j.joms.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Lukandu OM, Micha CS. Primary intraosseous squamous cell carcinoma arising from keratocystic odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(5):e204-9. doi: 10.1016/j.oooo.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Bajpai M, Chandolia B, Pardhe N, Arora M. Primary intra-osseous basaloid squamous cell carcinoma of mandible: report of rare case with proposed diagnostic criteria. J Coll Phys Surg Pak. 2019;29(12):1215–7. doi: 10.29271/jcpsp.2019.12.1215. [DOI] [PubMed] [Google Scholar]
- 21.Nomura T, Monobe H, Tamaruya N, Kishishita S, Saito K, Miyamoto R, et al. Primary intraosseous squamous cell carcinoma of the jaw: two new cases and review of the literature. Eur Arch Otorhinolaryngol. 2013;270(1):375–9. doi: 10.1007/s00405-012-2235-9. [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Edge SB. American Joint Committee on Cancer. AJCC cancer staging manual. 8. Switzerland: Springer; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 25 kb)
Electronic supplementary material 2 (DOCX 21 kb)