Abstract
Frank’s sign is a diagonal crease of the ear lobe, supposedly related to cardiac pathology, and has strongly been associated with coronary artery atherosclerosis. A total of 45 consecutive adult patients referred for autopsy in a one-and-a-half-year period were extensively studied. Samples from both the ear lobes were obtained for histopathology, as well as cardiac samples from all four cardiac compartments. When compared patients with Frank’s sign and those without it had no statistical difference in age (p = 0.0575). There was however a statistically significant increased cardiac weight (p = 0.0005), left ventricular wall thickness (p = 0.0002), and right ventricular wall thickness (p = 0.0043). Histopathology obtained from the ear lobes revealed myoelastofibrosis in an arterial vessel, located at the base of the crease, diffuse fibrosis, and Wallerian-like degeneration, with eosinophilic inclusions in the peripheral nerves. These changes suggest a time-related progression of the crease-associated changes. Our data suggest a significant correlation between the morphological changes of the myocardium and the presence of the ear lobe creases, with arterial myoelastofibrosis, Wallerian-like degeneration in peripheral nerves and deep tissue fibrosis found in the base of the crease.
Keywords: Frank’s sign, Pathology, Fibrosis, Heart failure, Hypoxia, Neural degeneration
Introduction
Frank’s sign, as described in 1973, is a diagonal crease of the ear lobe [1]. The characteristic is supposedly related to cardiac pathology and has strongly been associated with coronary artery atherosclerosis [2, 3]. Together with some more recently described auricular changes, developing in people with long-lasting Frank signs, such as paired ear creases of the helix (PECH), these changes are acquired and do not present a normal morphological variant of the human ear [4].
The etiology of Frank’s sign has not yet been established, with very few theories about its origin and how it correlates to cardiac pathology [5].
Well traced through history, Frank’s sign has seldom been investigated in depth clinically and to our knowledge has been morphologically evaluated only once in superficial biopsies [6].
We aimed to evaluate the gross morphology and histopathology of Frank’s sign and its relevance to cardiac changes in a cohort of autopsies.
Materials and Methods
A total of 45 consecutive adult patients referred for autopsy in a one-and-a-half-year period (February 2018–August 2019) were extensively studied. Samples from both the ear lobes of patients presenting Frank’s sign and those who did not were obtained for histopathology, as well as cardiac samples from all four cardiac compartments. The histopathological findings were later compared to one another and the clinical data.
Frank’s sign was defined as a bilateral, diagonal crease in the ear lobe extending from the tragus across the lobule to the rear edge of the auricle. Before the autopsy, all cases were carefully examined for the sign, with the goal of not misinterpreting scarring and lacerations from ear-ring holes. A total of ten patients had signs of ear-ring holes out of line with Frank’s sign. Patients with ear-ring holes were predominantly female, however, none of them showed a traumatic nature to the crease and it was accepted as Frank’s sign.
PECH were observed, though they were not studied in-depth, due to their development after Frank’s sign.
When obtaining specimens for histopathology, the ear lobule was severed horizontally from the ear, and the crease visibly diminished, although remaining somewhat visible. Hence, to mark the crease in further cases it was inked.
All specimens for histopathology were fixed in 10% neutral formaldehyde and later embedded in paraffin, stained with hematoxylin & eosin (H&E). All specimens were examined by a pathologist and were later independently blindly re-reviewed by two other pathologists.
Correlation with the clinical condition was carried out through the medical documentation of the patient and the overall autopsy findings.
The collected data was analyzed with MaxStat Pro, with a two-tailed t test p < 0.05 considered statistically significant.
Results
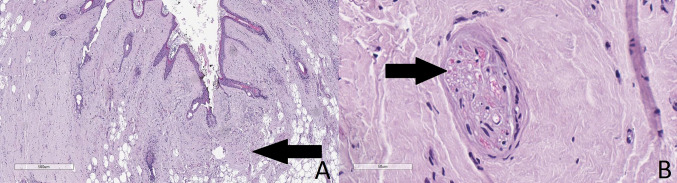
Of the 45 patients, 16 were female and 29 were male (Table 1). A total of 22 patients had a well-recorded clinical history of cardiac diseases and heart failure (HF), while the others did not. Upon general observation before the autopsy, 35 of the patients had well-formed Frank’s signs, some with PECH (Fig. 1; Table 1). The patients with ear-ring holes had no sign of ear-ring related injury to the lobule, so the creases observed were accepted as Frank’s sign.
Table 1.
Patient data and gross cardiac parameters
CHF chronic heart failure, AMI acute myocardial infarction
*Patient excluded from statistical analysis due to cardiac changes from leukemia infiltrate
Fig. 1.
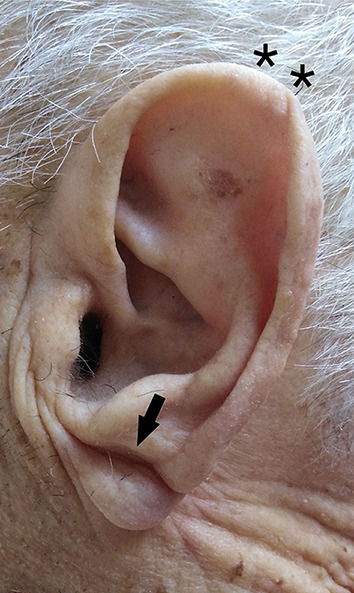
Frank’s sign (arrow) and PECH (asterisk) as seen on observation before the autopsy
One patient not expressing Frank’s sign had cardiac histopathology changes – infiltration from acute leukemia, whilst the others had no histopathological changes in the myocardium. Due to the short clinical course and cardiac pathology due to infiltration from tumor cells, the case was excluded from the statistical analysis.
Gross evaluation of the heart between the two groups, Frank’s sign (+), n = 35, mean age 64.29 ± 13.33, mean cardiac weight 527.69 ± 155.08; mean left ventricular wall thickness 19 ± 4.27, mean right ventricular wall thickness 7.49 ± 3.74 and Frank’s sign (−), n = 09, mean age 54.33 ± 14.86, mean cardiac weight 303.23 ± 32.41; mean left ventricular wall thickness 12.33 ± 1.32, mean right ventricular wall thickness 3.67 ± 0.71, revealed no statistical difference in age (p = 0.0575), statistically significant increased cardiac weight (p = 0.0005), left ventricular wall thickness (p = 0.0002) and right ventricular wall thickness (p = 0.0043) in the group with evident Frank’s sign.
There were no gender-specific parameters that could be attributed to the presence of Frank’s sign.
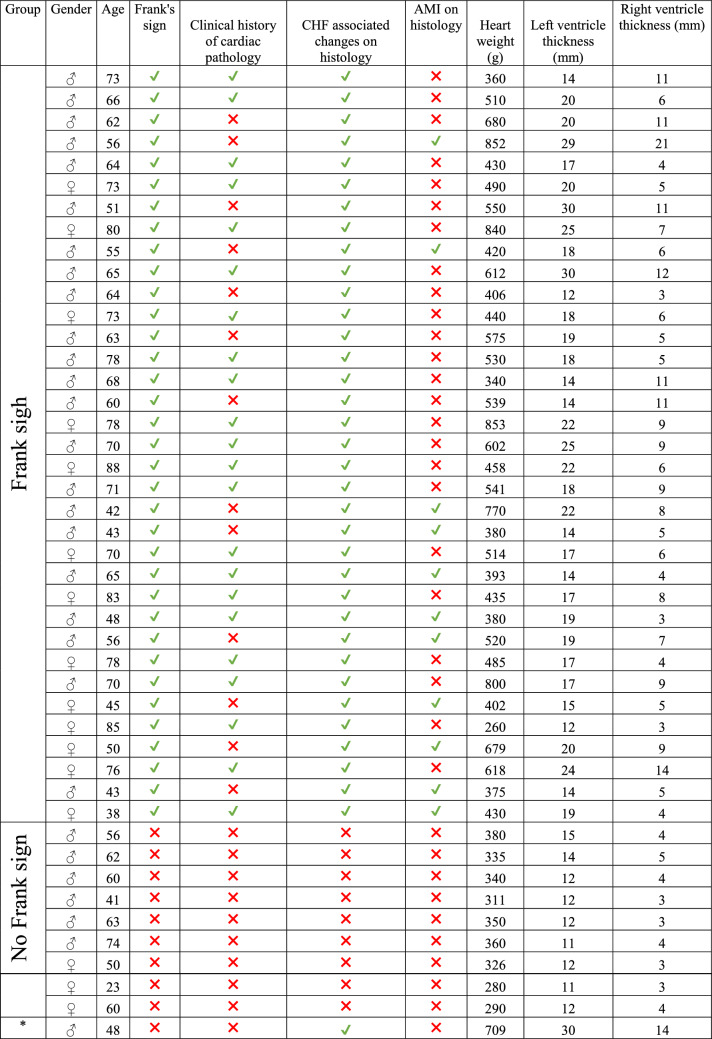
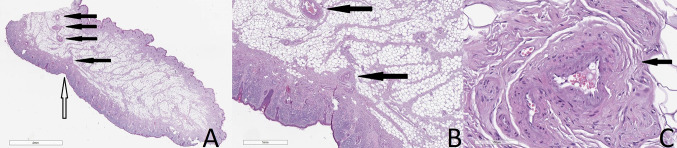
Histopathology obtained from the ear lobes of individuals aged 65 or less revealed myoelastofibrosis in an arterial vessel, located at the base of the crease, while at the base of the crease of older individuals there was diffuse fibrosis (Figs. 2 and 3). At higher magnification in both cohorts, the peripheral nerves revealed Wallerian-like degeneration, with eosinophilic inclusions (Fig. 3). These changes suggest a time-related progression of the crease-associated changes.
Fig. 2.
Histopathology obtained from the same patient from Fig. 1. Frank’s sight (hollow arrow), showing no fibrosis in the interstitium and a corkscrew-like twisted arterial vessel with myoelastofibrosis (black arrows) located at the base of the crease (a), macro view of the histology slide; the blood vessel (black arrows), crease and surrounding tissues (b), original magnification 100x; the blood vessels showing myoelastofibrotic changes (c), original magnification ×200. b and c are magnified sections of a, hematoxylin and eosin stain
Fig. 3.
Histology of the crease revealing deep tissue fibrosis in a patient aged more than 65 (a), original magnification ×40, Wallerian degeneration in peripheral nerves (b), original magnification ×400. Hematoxylin and eosin stain
Cardiac histology of the 22 cases with a clinical history of cardiac disorders, revealed a variety of pathological changes—old myocardial infarctions, diffuse myocardial micro-scarring (myocardial fibrosis), lipomatosis and mild to moderate coronary atherosclerosis. All these changes are the morphological substrate for chronic HF (Table 1).
Of the other 23 cases without a clinical history of HF, nine did not have Frank’s sign and their histology revealed no myocardial changes, except the additional excluded case with leukemic infiltration in the myocardium, whilst the remaining 14 who had Frank’s sign also had significant myocardial histopathological changes (Table 1).
Cause of death in patients without Frank’s signed varied from malignancy, to severe inflammatory processes and peptic ulcer disease, with none dying as a result of cardiovascular disease. Cause of death in patients with Frank’s sign, although predominantly cardiovascular, also included malignancy, pulmonary diseases, respiratory infections, and peptic ulcer disease.
Discussion
Although only recently described, the sign has been traced back through history back to the second century A.D. to the Roman Emperor Hadrian, who was presented in his later life on statues having the typical mark on the ear lobe [7]. Hadrian’s well-chronicled life also showed a steadily deteriorating condition and eventual death, which can be interpreted as exacerbated HF [7].
Several clinical studies have shown a correlation between coronary atherosclerosis and Frank’s sign, whilst a few have reputed the finding, especially those focusing on conditions exacerbating atherosclerosis, such as type two diabetes mellitus [2, 3, 8–10]. Several studies have also investigated the effect of age, gender and non-cardiovascular conditions, such as diabetes and chronic kidney failure [11–13].
A drawback of the current study is the lack of a classification of Frank’s sign based on those of several previous studies. The combination of crease morphology, as well as evaluation of unilateral Frank creases, together with the detailed autopsy report could have revealed further correlations of the condition [11, 12].
However, there is only one histological study, on biopsy material, showing myoelastofibrosis and elastic fiber disposition, which can be attributed to solar elastosis [6]. Most studies, however, suggest a mechanical formation of the crease due to sleeping position in patients with HF [14].
From an embryological standpoint, the diagonal line connecting the tragus to the tip of the ear lobe is a tissue weak point, as that is a point of merger of preformed structures [15, 16]. As such several congenital anomalies have been identified in this topographical area, such as auricular clefts and sinuses [17, 18].
The nature of the changes seems to be based on the physiologically lower oxygen saturation of the ear lobule, due to its embryological development. The ear lobe is formed by the fusion of the first and sixth hillocks of His on the first and second pharyngeal arches. This represents a relatively late fetal fusion site, with decreased vasculature [15, 16]. These changes make it more susceptible to chronic hypoxia and reoxygenation injury before any observable changes can be seen in other surface structures, other than the ear which also show early and late hypoxia changes such as cyanosis, in acute events, and epidermal-dermal degeneration, in chronic events, observed on the lips, nose and fingertips and their chronic hypoxia changes.
The presented results show a significant association between Frank’s sign and histopathological changes in the myocardium. Despite this and several other studies supporting the correlation between HF and Frank’s sign, there is disputable information on its relevance, with some authors stating that it has significance in patients age less than 50, whilst others believe it’s relevant after the age of 50, especially in males [2, 4, 6, 8–11]. Age acquired changes of the ear lobe also need to be assessed in elderly patients as age-related changes have been well established and possibly mimic the crease, without the presence of cardiovascular disease [19].
The chronic hypoxia and reoxygenation changes in patients with HF, such as during and after physical stress, even without an angina attack can affect the auricular blood vessels with segmental myoelastofibrosis and lead to nerve damage with Wallerian-like degeneration, leading to the formation of the crease on top of the changed tissues. This can also explain why when the lobule is dissected, although still visible, the crease flattens out.
Wallerian degeneration has classically been associated with nerve damage, predominantly due to injury. Such changes, however, have been described in the cardiac muscle both after myocardial infarction and in the context of HF [20].
Similar changes can explain the later development of PECH, as the same changes can be observed on the tip of the helix, however, due to its rigid cartilaginous support, a longer period is needed for the changes to become grossly visible [4].
The progression of Frank’s sign from myoelastofibrosis and Wallerian-like degeneration to tissue fibrosis further proves the chronic course of the condition [21].
Genetic and environmental conditions, such as smoking and medications, cannot be ruled out as causative factors in the development of the crease. However, based on our limited clinical data a correlation with them could not be established.
Histological evaluation of Frank’s sing is a novel approach and further research in this area is needed to better understand the mechanisms of its development, with the possible implementation of histological classification, akin to the clinical one [11, 13]. Comparison between these changes and evaluation of patient conditions and their severity can hence lead to the establishment of Frank’s sign, not as a clinical myth and area for dispute, but as a diagnostic and prognostic factor with possible prophylactic implications [5].
Conclusions
Our data suggest a significant correlation between the morphological changes of the myocardium and the presence of the ear lobe creases, with arterial myoelastofibrosis, Wallerian-like degeneration in peripheral nerves and deep tissue fibrosis found in the base of the crease. The proposed model of hypoxia/reoxygenation in HF and the ear lobule’s physiologically lower oxygen saturation and embryological development, being responsible for the observed changes is the first model explaining the development of Frank’s sign and its correlation to clinically observable changes.
Acknowledgements
The authors would like to thank the reviewers for their comment and suggestion, which represent an enormous contribution to the manuscript.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by GS, LP, and DD. The first draft of the manuscript was written by GS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interest.
Ethical Approval
All procedures contributing to this work complied with the ethical standards of the Helsinki declaration of 1964 and its seventh revision from 2013, the ethical standards of the Bulgarian Ministry of Healthcare, as well as the ethical standards and protocols of the St. Marina University Hospital, Varna, Bulgaria.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frank ST. Aural sign of coronary-artery disease. N Engl J Med. 1973;289:327–8. doi: 10.1056/nejm197308092890622. [DOI] [PubMed] [Google Scholar]
- 2.Baboujian A, Bezwada P, Ayala-Rodriguez C. Diagonal earlobe crease, a marker of coronary artery disease: a case report on Frank’s sign. Cureus. 2019;11:e4219. doi: 10.7759/cureus.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffing G. Frank’s sign. N Engl J Med. 2014;370:e15. doi: 10.1056/NEJMicm1213868. [DOI] [PubMed] [Google Scholar]
- 4.Pathmarajah P, Rowland Payne C. Paired Ear Creases of the Helix (PECH): a possible physical sign. Cureus. 2017;9:e1884. doi: 10.7759/cureus.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin AN, Lin K, Kyaw H, Abboud J. A myth still needs to be clarified: a case report of the Frank’s sign. Cureus. 2018;10:e2080. doi: 10.7759/cureus.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoenfeld Y, Mor R, Weinberger A, Avidor I, Pinkhas J. Diagonal ear lobe crease and coronary risk factors. J Am Geriatr Soc. 1980;28:184–7. doi: 10.1111/j.1532-5415.1980.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 7.Petrakis NL. Diagonal earlobe creases, type A behavior and the death of Emperor Hadrian. West J Med. 1980;132:87–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Davis TM, Balme M, Jackson D, Stuccio G, Bruce DG. The diagonal ear lobe crease (Frank’s sign) is not associated with coronary artery disease or retinopathy in type 2 diabetes: the Fremantle Diabetes Study. Aust N Z J Med. 2000;30:573–7. doi: 10.1111/j.1445-5994.2000.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 9.Edston E. The earlobe crease, coronary artery disease, and sudden cardiac death: an autopsy study of 520 individuals. Am J Forensic Med Pathol. 2006;27:129–33. doi: 10.1097/01.paf.0000221067.73173.d7. [DOI] [PubMed] [Google Scholar]
- 10.Nazzal S, Hijazi B, Khalila L, Blum A. Diagonal earlobe crease (Frank’s sign): a predictor of cerebral vascular events. Am J Med. 2017;130:1324. doi: 10.1016/j.amjmed.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 11.Wakasugi M, et al. Prevalence of earlobe creases and their association with history of cardiovascular disease in patients undergoing hemodialysis: a cross-sectional study. Ther Aphere Dial. 2017;21:478–84. doi: 10.1111/1744-9987.12567. [DOI] [PubMed] [Google Scholar]
- 12.Patel V, Champ C, Andrews PS, Gostelow BE, Gunasekara NP, Davidson AR. Diagonal earlobe creases and atheromatous disease: a postmortem study. J R Coll Physcians Lond. 1992;26:274–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-López C, Garlito-Díaz H, Madroñero-Mariscal R, Sánchez-Cervilla PJ, Graciani A, López-Sendón JL, López-de-Sá E. Earlobe crease shapes and cardiovascular events. Am J Cardiol. 2015;116:286–93. doi: 10.1016/j.amjcard.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Agouridis AP, Elisaf MS, Nair DR, Mikhailidis DP. Ear lobe crease: a marker of coronary artery disease? Arch Med Sci. 2015;11:1145–55. doi: 10.5114/aoms.2015.56340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood-Jones F, I-Chuan W. The development of the external ear. J Anat. 1934;68:525–353. [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CG. Development of the human external ear. J Am Acad Audiol. 1997;8:379–82. [PubMed] [Google Scholar]
- 17.Fraser FC, Sproule JR, Halal F. Frequency of the branchio-oto-renal (BOR) syndrome in children with profound hearing loss. Am J Med Genet. 1980;7:341–9. doi: 10.1002/ajmg.1320070316. [DOI] [PubMed] [Google Scholar]
- 18.Fumiiri M, Hyakusoku H. Congenital auricular cleft. Plast Reconstr Surg. 1983;71:249–50. doi: 10.1097/00006534-198302000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Heathcote JA. Why do old men have big ears? BMJ. 1995;311:1668. doi: 10.1136/bmj.311.7021.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garikipati VNS, Verma SK, Kishore R. The nervous heart: role of sympathetic reinnervation in cardiac regeneration. Circul Res. 2015;117:980–1. doi: 10.1161/CIRCRESAHA.115.307637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overfield T, Call EB. Earlobe type, race, and age: effects on earlobe creasing. J Am Geriatr Soc. 1983;31:479–81. doi: 10.1111/j.1532-5415.1983.tb05121.x. [DOI] [PubMed] [Google Scholar]