Abstract
SMARCB1/INI1 deficient sinonasal carcinoma is a variant of sinonasal undifferentiated carcinoma (SNUC). There is a paucity of literature describing the histomorphological features of this relatively new entity. Herein we describe the histomorphological features of three such cases and review the literature. We retrospectively reviewed the cases of SNUC diagnosed in our institute in the last 6 years. Immunohistochemistry for INI1, NUT & p16 were performed on these cases. Three cases showed loss of INI1. The histomorphology and clinicopathological features of these cases were studied and compared with non INI1 deficient SNUC. A total of 9 cases of SNUC were identified. Three of these cases showed loss of INI1. These three cases had presented with large sinonasal mass and with intracranial extension. Histopathology of these cases showed a diffuse infiltrative pattern, nest, and islands of predominantly basaloid cells with focal rhabdoid morphology. Additional features like small cell carcinoma like pattern, pseudoalveolar and pseudoglandular patterns, clear vacuoles and pseudopapillary appearance were also noted. We conclude that in presence of a mixed pattern of cells with a predominance of basaloid morphology, the possibility of SMARCB1/INI1 deficient sinonasal carcinoma must be strongly suspected and immunohistochemistry for INI1 must be performed.
Electronic supplementary material
The online version of this article (10.1007/s12105-020-01246-9) contains supplementary material, which is available to authorized users.
Keywords: Sinonasal undifferentiated carcinoma, SMARCB1/INI1 deficient sinonasal carcinoma, Basaloid morphology, Rhabdoid morphology, p16
Introduction
Sinonasal undifferentiated carcinoma (SNUC) is defined as an undifferentiated carcinoma of the sinonasal tract without glandular and squamous features and not otherwise classifiable [1]. SNUC is rare, with about 0.02 cases per 100,000 people, accounting for only about 3–5% of all sinonasal carcinomas. There is no etiological role of EBV or HPV in SNUC.
Recent molecular studies have identified various variants of SNUC, which include NUT carcinoma, SMARCB1 (INI-1)–deficient sinonasal carcinoma (SDSC), and SMARCA4 (BRG1)–deficient sinonasal carcinoma [2–4]. These molecular alterations can be therapeutically targeted and hence the necessity of distinguishing them from SNUC.
SMARCB1/INI1 deficient sinonasal carcinoma is an uncommon and recently described entity. The first case was reported in 2014 by Agaimy et al. [5] as a SMARCB1 deficient basaloid carcinoma. Less than 100 cases have been reported till date, including a single study from India [3–13]. To the best of our knowledge, this is the second study highlighting the SMARCB1/INI1 deficient sinonasal carcinoma in India.
Materials and Methods
We retrospectively reviewed nine cases that were originally diagnosed as SNUC in our institute within a period of 6 years (January 2014 and December 2019). Clinical details were retrieved from the surgical case records. Hematoxylin and eosin stained slides along with their corresponding formalin fixed paraffin embedded tissue blocks were available in all cases. Immunohistochemistry (IHC) profile of these cases, which included Pan-CK, LCA, EMA, Synaptophysin, chromogranin, p40, p63, CK5/6, CD99, NSE, HMB45, CD56, Desmin and S100, was also available. Histomorphology of all these cases were reviewed. After a review of these cases, additional immunohistochemical stains were performed on all the cases. 2.5 μm thick sections were cut from representative formalin fixed paraffin embedded blocks. A further panel of IHC including INI1 (Ready-to-use, clone 25, Path-n-situ), NUT (1:100, clone C52B1, Cell Signalling Technology) and p16 INK4a (1:1500, clone S140, Sigma-Aldrich), were performed in the representative sections of index cases. The manufacturers instruction was followed for each of the above marker. Appropriate positive and negative controls were set up for each marker.
For the INI1 antibody, the presence of strong nuclear staining of stromal fibroblasts, inflammatory cells, vascular endothelial cells, or normal epithelial cells in the background was considered as a positive internal control. A complete loss of INI1 in the viable tumor cells with an appropriate positive internal control was interpreted as an INI1 deficient tumor. For the NUT antibody, strong nuclear expression in the tumor cells was considered as positive. Strong cytoplasmic and nuclear staining of p16 was considered positive.
Results
We reviewed nine cases of histologically and immunohistochemically proven SNUC, which were reported in the last 6 years. IHC for INI1, NUT and p16 were performed in all these cases and three of them were reclassified as SMARCB1/INI1 deficient sinonasal carcinoma. The clinicopathological features and immunohistochemistry findings of these cases are shown in Tables 1, 2, 3, 4.
Table 1.
Clinicopathological features of the SNUC cases
| Case No. | Age (years) |
Sex | Signs and symptoms | Imaging findings | Histomorphological features | Treatment & follow up |
|---|---|---|---|---|---|---|
| 1 | 40 | F | Right sided nasal mass | Nasal mass with intracranial extension | Papillary pattern, perivascular arrangement, basaloid morphology, necrosis | NA |
| 2 | 38 | M | Swelling below left orbit | Mass in the maxillary sinus with extension into inferior orbital wall and infra-temporal fossa | Sheets of large cells with moderate cytoplasm, vesicular nucleus & conspicuous nucleoli | NA |
| 3 | 17 | M | Nasal mass with left cervical LN | CECT face and neck-large heterogeneously enhancing infiltrative soft tissue lesion centred in left nasopharynx, pterygo-maxillary space, left hemisphenoid, parasellar, paraclival region & left maxillary antrum | Diffuse infiltrative pattern, Trabecular and cords of tumor cells, small cell carcinoma like pattern |
No surgery, treated with chemo & radiotherapy DOD, 3 months after the biopsy diagnosis |
| 4 | 27 | M | Right sided nasal mass of 2-year duration | CECT–small polypoidal enhancing lesion extending from anterior aspect of inferior turbinate with contiguous extension to lateral nasal wall | Diffuse and nest like patterns, sarcomatoid areas, focal rhabdoid morphology | WLE of the mass. Alive without any recurrence (2 years duration) |
| 5 | 33 | M | Right sided polypoidal nasal mass | NA | Single cells, small nest, sheets of tumor cells variable amount of cytoplasm, round vesicular nucleus and prominent nucleoli, lymphoma like morphology | NA |
| 6 | 50 | M | Left nasal obstruction, bleeds on touch | NA | Nest of large tumor cells with moderate amount of cytoplasm, round nuclei & conspicuous nucleoli | Treatment details were not available. DOD, 1 year after the diagnosis |
SNUC sinonasal undifferentiated carcinoma, SDSC SMARCB1/INI1 deficient sinonasal carcinoma, NA not available, DOD died of diseases, WLE wide local excision
Table 2.
Immunohistochemical features of the SNUC cases
| Case No. | PanCK | EMA | CK5/6 | P40 | P63 | LCA | Synapto | Chromo | NSE | CD99 | CD56 | Desmin | HMB45 | S100 | INI1 | NUT | p16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | − | − | − | − | − | + | − | − | − | − | − | Retained | − | − |
| 2 | + | + | Focal weak + | − | − | − | + | − | + | − | − | − | − | − | Retained | − | − |
| 3 | + | + | − | − | − | − | − | − | + | − | − | − | − | − | Retained | − | − |
| 4 | + | + | − | − | − | − | − | − | + | − | − | − | − | − | Retained | − | − |
| 5 | + | + | − | − | − | − | − | − | + | Paranuclear dot like + | − | − | − | − | Retained | − | − |
| 6 | Focal + | + | − | − | − | − | − | + | + | Diffuse + | − | − | − | − | Retained | − | − |
+ positive, − negative, Synapto synaptophysin, Chromo chromogranin
Table 3.
Clinicopathological features of the SMARCB1/INI1 deficient sinonasal carcinoma cases
| Case No. | Age (years) |
Sex | Signs and symptoms | Imaging findings | Initial diagnosis | Histomorphological features | Treatment & follow up |
|---|---|---|---|---|---|---|---|
| 1 | 61 | M | Swelling in the fronto-ethmoid region | NA | SNUC | Nest, lobules, trabeculae pattern, small cell morphology, bone infiltration with a fibrous dysplasia like reaction |
Underwent surgery followed by chemo & radiotherapy DOD after 2 years of diagnosis |
| 2 | 35 | F | Left sinonasal mass associated with, bleeding, decreased vision | CT PNS-Mass occupying left sided nasal cavity with extension into sphenoid, posterior ethmoid sinuses | SNUC | Nest, lobules of tumor cells with basaloid morphology, vesicular nucleus, and prominent nucleoli, necrosis, perivascular arrangement | Underwent surgery followed by chemo& radiotherapy Alive without any recurrence (1 year & 8 months |
| 3 | 35 | F |
Right facial pain, epistaxis for 1 year, 6 × 7 cm swelling over right orbit with right eye pushed outward, right sided nasal mass |
CEMRI brain and PNS- A large heterogeneously enhancing mass centred over right ethmoid sinus, nasal cavity with intracranial extension |
SNUC | Sheets, lobules, and trabeculae, tumor cells with moderate amount of eosinophilic cytoplasm, round nucleus with prominent nucleoli. Focal pseudo-alveolar pattern, pseudoglandular pattern, empty vacuoles | Anterior cranio-facial resection of right sided nasal mass and right orbital exenteration followed by chemoradiotherapy. Alive with local recurrence (increase in swelling size) at 5 months |
SNUC sinonasal undifferentiated carcinoma, SDSC SMARCB1/INI1 deficient sinonasal carcinoma, NA not available, DOD died of diseases
Table 4.
Immunohistochemical features of the SMARCB1/INI1 deficient sinonasal carcinoma cases
| Case No. | PanCK | EMA | CK5/6 | P40 | P63 | LCA | Synapto | Chromo | NSE | CD99 | CD56 | Desmin | HMB45 | S100 | INI1 | NUT | p16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | Lost | − | − |
| 2 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | Lost | − | − |
| 3 | + | + | − | − | − | − | − | − | − | − | Focal + | − | − | − | Lost | − | − |
+ positive, − negative, Synapto synaptophysin, Chromo chromogranin
Patient Characteristics and Specimen Type
The median age of these patients was 35 years (ranged from 17 to 61 years). The male to female ratio was 2:1. All the cases presented with sinonasal mass and three of them showed intracranial extension. There was no significant cervical lymphadenopathy, except one case showed metastasis in the left cervical lymph node. In seven out the nine cases, a diagnostic biopsy sample was available. Wide local excision specimen was available in one case. A biopsy followed by anterior craniofacial resection of the right-sided nasal mass and right orbital exenteration specimen was available in one case. There was no distant metastasis in any of the cases.
Histomorphological Features of SNUC: (Table 1)
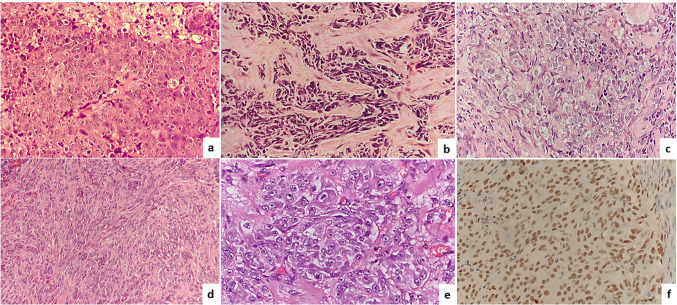
Most of these cases showed a diffusely infiltrative pattern. The cells were predominantly arranged in diffuse sheets, irregular nests, and small groups. The tumor cells in most cases showed a basaloid morphology with intermediate to large cells having scant to moderate amount of cytoplasm, round to oval nucleus with opened chromatin and conspicuous nucleoli (Fig. 1a). Apoptotic bodies and brisk mitotic activity were seen. Necrosis was seen in two cases. There was no evidence of glandular or squamous differentiation. Few cases showed unusual features. One case showed an exclusive small cell carcinoma like morphology (Fig. 1b). The tumor in this case showed tightly cohesive irregular clusters of cells with scanty cytoplasm, crushing artefact, nuclear moulding, and high rate of apoptosis. Another case showed mostly single cells or small discohesive clusters of round cells with pericellular halo akin to a high-grade Non-Hodgkin lymphoma (Fig. 1c). Another case, in addition to the usual morphology of SNUC, showed focal but significant areas of spindle cell morphology resembling a sarcoma (Fig. 1d). In the same case focal areas showed a rhabdoid morphology with large round cells having eccentric nucleus and prominent nucleoli (Fig. 1e). These rhabdoid appearing cells showed immunopositivity for INI1 (Fig. 1f). All these cases were evaluated with a large panel of immunohistochemistry marker and were confirmed to be SNUC (Table 2).
Fig. 1.
The histomorphology of SNUC cases showing a infiltrative nests and islands (Hematoxylin and Eosin, 200 ×), b small cell morphology with crushing artefact and moulding of tumor cells (Hematoxylin and Eosin, 100 ×), c single or small discohesive clusters of tumor cells with pericellular halo resembling a high grade Non Hodgkin Lymphoma, (Hematoxylin and Eosin, 200 ×), d sarcomatoid morphology (Hematoxylin and Eosin, 200 ×), and e Rhabdoid morphology in a case, (Hematoxylin and Eosin, 400 ×), f The rhabdoid cells were immunopositive for INI1 (Immunostain, 400x)
Immunohistochemistry Profile of SNUC (Table 2)
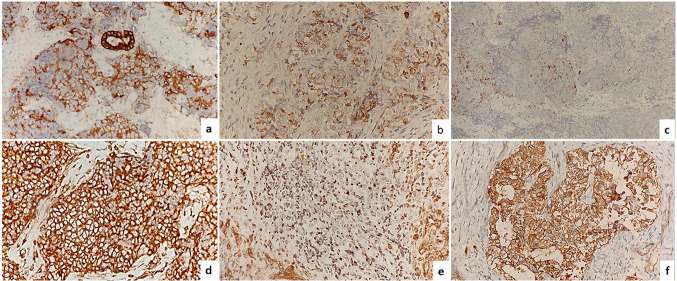
The tumor cells showed strong and diffuse cytoplasmic and membranous immunopositivity for Pan-CK and EMA in all cases. However, an occasional case showed a relative weak focal loss of expression of pan-CK (Fig. 2a) and EMA (Fig. 2b). All tumors were immunonegative for LCA, p40, p63, CK5/6, HMB45, Desmin, S-100, chromogranin and synaptophysin except for one case which showed focal synaptophysin positivity (Fig. 2c) and another showed focal chromogranin positivity. NSE was positive in all cases. Variable immunoexpression of CD99 was noted in the tumor cells. A strong diffuse membranous staining of CD99 was found in one case (Fig. 2d) and in one case a conspicuous paranuclear dot like positivity was noted (Fig. 2e). All these cases were immunonegative for NUT and p16 and showed retained INI1 immunoexpression. All tumors were found to show a membranous staining pattern for beta catenin (Fig. 2f).
Fig. 2.
Immunohistochemical profile of Sinonasal Undifferentiated carcinoma, a Pan-Ck, focal weak expression/loss was seen in one case, immunostain 200 ×. b Focal weak expression of EMA was noted in some cases, immunostain 200 ×. c Focal expression of synaptophysin is seen in some cases, immunostain 200 ×. d Diffuse membranous expression of CD99 was seen in one case, immunostain 200 ×. e Paranuclear dot like positivity of CD99 seen in one case, immunostain 200x, f Tumor cells showed membranous positivity for beta catenin. Immunostain 200 ×
Clinicopathological Features of SMARCB1/ INI1 Deficient Sinonasal Carcinoma (n = 3): (Tables 3 and 4)
Case 1
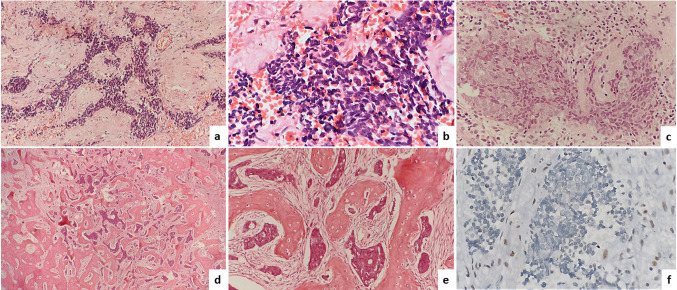
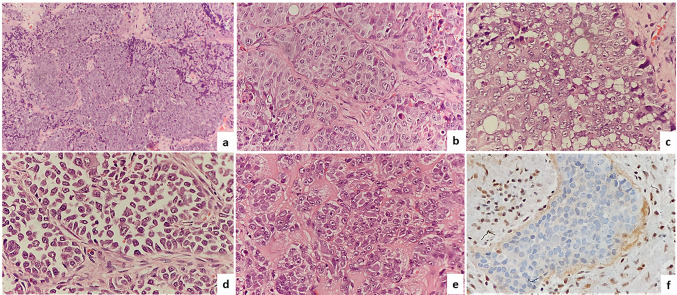
A 61-year-old male presented with swelling in the fronto-ethmoid region. Excision biopsy was done. After an initial panel of IHC (positivity for pan-CK, EMA and negativity for LCA, p40, Synaptophysin, chromogranin, CD99, S-100, HMB45), a diagnosis of SNUC was made. He underwent resection of the mass, followed by chemo and radiotherapy. The patient died due to the disease 2 years after the initial diagnosis. Review of the H & E stained slides of this case revealed a diffuse infiltrative pattern mostly comprising of small cells with scanty cytoplasm, (Fig. 3a, b) and focal areas with nests of larger cells intermixed with small cells (Fig. 3c). The small cells had high N/C ratio, hyperchromatic angulated nuclei, nuclear moulding, absence of nucleoli and crush artefacts resembling the morphology of a small cell carcinoma (Fig. 3a, b). Focal rhabdoid morphology, brisk mitosis, infiltration into skeletal muscles and bone were seen. Bone infiltration was seen as a fibrous dysplasia like reaction with small islands of tumor cells infiltrating within a fibroblastic stroma surrounded by irregular bone arranged in a Chinese letter like pattern (Fig. 3d, e). Additional IHC showed a loss of INI1 expression (Fig. 3f) along with immunonegativity for NUT protein and p16 and it was recategorized as SDSC.
Fig. 3.
The histomorphology of case 1-SMARCB1/INI1 deficient sinonasal carcinoma showing a diffuse infiltrative nests and cords (Hematoxylin and Eosin, 100 ×), b small cell carcinoma like pattern (Hematoxylin and Eosin, 200 ×), c intimate admixture of two population of cells; cells with moderate amount of cytoplasm, round vesicular nucleus and prominent nucleoli and basaloid cells (Hematoxylin and Eosin, 400 ×), d, e infiltration of islands of tumor cells in bone, there is marked fibroblastic proliferation and new bone formation giving rise to a fibrous dysplasia like reaction, (Hematoxylin & Eosin, 100x), f Complete loss of INI1 protein in the tumor cells with positive internal control (immunostain, 400 ×)
Case 2
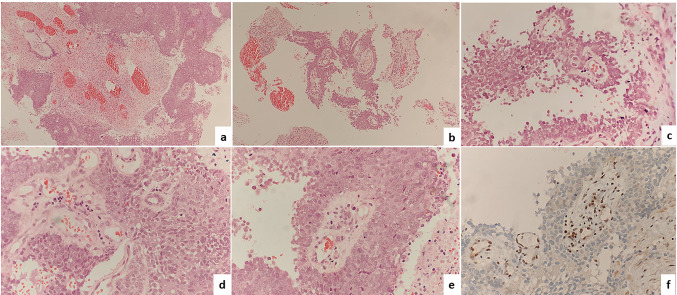
A 35-year-old female presented with left sinonasal mass associated with bleeding and decreased vision. CT PNS showed a mass occupying left-sided nasal cavity with extension into sphenoid and posterior ethmoid sinuses. Biopsy from the mass showed features of an undifferentiated carcinoma which was immunopositive for Pan-CK and negative for p40, LCA, Synaptophysin, NSE, HMB45, Desmin and S-100. A diagnosis of SNUC was made. She underwent resection of the mass, followed by chemo and radiotherapy. On follow up, the patient was alive without any recurrence at 1 year and 8 months after the initial diagnosis. Review of the H & E stained slides of this case showed island and nest like pattern of basaloid cells with pushing as well as infiltrative margins (Fig. 4a). Focal perivascular arrangement of tumor cells resembling pseudo-rosettes, were noted (Fig. 4b, c). Brisk mitosis and extensive areas of necrosis were seen. The tumor cells were small to intermediate sized with high N/C ratio, vesicular chromatin, and prominent nucleoli (Fig. 4d, e). The tumor cells showed a complete loss of INI1 expression (Fig. 4f) and immunonegativity for NUT protein, and p16. Based on these features, a revised diagnosis of SMARCB1/ INI1 deficient sinonasal carcinoma was made.
Fig. 4.
The histomorphology of case 2-SMARCB1/INI1 deficient sinonasal carcinoma showing a diffuse sheets of tumor cells with geographic areas on necrosis. (Hematoxylin and Eosin, 40 ×), b, c perivascular arrangement of tumor cells (Hematoxylin and Eosin, 200 ×), d, e The tumor cells were small to intermediate sized with high N/C ratio, vesicular chromatin, and prominent nucleoli (Hematoxylin and Eosin, 400 ×), f Complete loss of INI1 protein in tumor cells with positive internal control (immunostain, 400 ×)
Case 3
A 35-year-old female presented with right-sided facial pain and epistaxis for 1 year. Local examination showed a 6 × 7 cm swelling over right orbit with right eye pushed outward and right-sided nasal mass. CE MRI brain and PNS revealed a large heterogeneously enhancing mass cantered over the right ethmoid sinus and nasal cavity with intracranial extension. Incisional biopsy showed an undifferentiated malignant tumor with brisk mitosis, numerous apoptotic bodies, and necrosis. These cells were positive for Pan-CK and focally positive for CD56 and negative for LCA, p40, HMB45, Desmin, NSE, CD 99, S-100, Synaptophysin, chromogranin, p40 and CK5/6. The initial diagnosis was given as SNUC, following which the patient underwent anterior craniofacial resection of the right-sided nasal mass, right orbital exenteration, and chemoradiotherapy. On follow up, she was alive 5 months after the initial diagnosis. Review of its H & E stained slides showed a mixture of patterns including predominantly of nests and lobules (Fig. 5a, b). Focal pseudo glandular pattern (Fig. 5c) and pseudo-alveolar pattern (Fig. 5d) were noted. The predominant population were cells with a moderate amount of eosinophilic cytoplasm, round to oval nucleus with vesicular chromatin and prominent nucleoli (Fig. 5b). Some of them showed a rhabdoid appearance with eccentrically placed nuclei and prominent nucleoli (Fig. 5e). Focal basaloid morphology was also noted. Comedo type of central necrosis and brisk mitosis were also noted. Clear cell changes and empty vacuoles were also seen focally (Fig. 5c). IHC showed complete loss of INI1 expression (Fig. 5f), and immunonegativity for NUT protein, and p16. A revised diagnosis of SMARCB1/ INI1 deficient sinonasal carcinoma was made.
Fig. 5.
The histomorphology of case 3-SMARCB1/INI1 deficient sinonasal carcinoma showing a, b nests and lobules of tumor cells (Hematoxylin and Eosin, 400 ×, c pseudoglandular pattern containing empty vacuoles, (Hematoxylin and Eosin, 400 ×) d pseudoalveolar pattern (Hematoxylin and Eosin, 400 ×), e lobules of tumor cells with rhabdoid morphology of cells (Hematoxylin and Eosin, 200 ×), f Complete loss of INI1 protein with positive internal control (immunostain, 200 ×)
Clinical Follow Up
Follow up was available in six cases. Out of three cases of SMARCB1/INI1 deficient sinonasal carcinoma, one patient died after 2 years of initial diagnosis, the second patient was alive without recurrence after 1 year and 8 month of the diagnosis, and the third patient was alive with local recurrence at 5 months after diagnosis. Among the six cases of SNUC, one patient died after 1 year of the diagnosis. Another patient was alive without recurrence after 2 years of the diagnosis. One patient died after 3 months of diagnosis and the remaining three cases were lost follow up after initial diagnosis.
Discussion
SMARCB1-deficient sinonasal carcinoma was initially described as a tumor predominated by basaloid cells with focal rhabdoid/plasmacytoid morphology. As a greater number of cases are being reported (Table 5), the morphologic spectrum of this tumor has gradually expanded. Many different morphological features have been described in the recent literature. Many of these features are seen focally in addition to the basaloid and/or rhabdoid morphology. Agaimy et al. [3] described squamoid, spindle cell, sarcomatoid, adenoid, and focal clear cell features and presence of non-specific empty vacuoles [10]. Kakkar et al. [11] have mentioned oncocytoid adenocarcinoma like morphology in two of their cases.
Table 5.
Clinicopathological features of SMARCB1/INI1-deficient sinonasal carcinoma (SDSC) with original diagnosis of SNUC
| S No. |
Author, year ref | Age (years) |
Sex | Clinical presentation | Initial diagnosis | P16 IHC |
Revised diagnosis | Treatment & follow up |
|---|---|---|---|---|---|---|---|---|
| 1 | Bishop JA et.al., 2014 [6] | 33 | M | Proptosis, vision defect, mass in the nasal, ethmoid and frontal sinus | SNUC | ± | SDSC |
Local recurrence and distant metastasis DOD (30 months) |
| 2 | Bishop JA et.al., 2014 [6] | 54 | F | Headache, nasal discharge, mass in the nasal, ethmoid and maxillary sinus | SNUC | − | SDSC |
Local recurrence DOD (15 months) |
| 3 | Bell D et.al., 2015 [7] | 64 | F | Diplopia, mass in the frontal sinus | SNUC | Focal + | SDSC | NED (3 months) |
| 4 | Bell D et.al., 2015 [7] | 51 | F | Sinusitis, mass in the left temporal fossa and sphenoid | SNUC | Focal + | SDSC |
DOD (24 months) Local recurrence and distant metastasis |
| 5 | Zeng M et.al., 2016 [8] | 86 | M | Left maxillary sinus and nasal mass | SNUC | − | SDSC | Died of colorectal cancer (21 months) |
| 6 | Shatzkes DR et.al. 2016 [9] | 62 | M | NA | SNUC | ND | SDSC | NED (3 months) |
| 7 | Wasserman JK et.al.,2017 [10] | 34 | M | Facial swelling, loose dentition, right maxillary mass extending into orbital floor | SNUC | ND | SDSC | Local recurrence and distant metastasis to lung liver and bone. DOD (26 months) |
| 8 | Kakkar A et.al., 2018 [11] | 23 | M | Epistaxis, upper jaw pain, loosening of teeth | SNUC | ND | SDSC | Biopsy followed by 2 NACT (cisplatin + 5FU) followed by radical maxillectomy and PORT 60gy in 30 fractions; achieved CR; NED at 17 months |
| 9 | Kakkar A et.al 2018 [11] | 60 | M | Mass in the left nasal cavity | SNUC | ND | SDSC | Biopsy only; no follow-up information available |
| 10 | Kakkar A et.al., 2018 [11] | 40 | M | Vascular mass in the right nasal cavity | SNUC | ND | SDSC | Biopsy only; no follow-up information available |
| 11 | Fard EV et.al.,2019 [13] | 61 | M | Large sinonasal mass with orbital and brain extension, possible lung metastasis | SNUC | + | SDSC | AWD 17 months, with multiple bone metastasis |
| 12 | Fard EV et.al.,2019 [13] | 61 | M | Large sinonasal mass with brain, liver and lung metastasis | SNUC | − | SDSC | AWD (11 months) |
| 13 | Fard EV et.al.,2019 [13] | 75 | M | Large sinonasal mass with bilateral orbital and skull base involvement | SNUC | + | SDSC | AWD (7 months) |
| 14 | Present study | 61 | M | Swelling in the fronto-ethmoid region | SNUC | − | SDSC |
Underwent surgery followed by chemo & radiotherapy DOD after 2 years of diagnosis |
| 15 | Present study | 35 | F | Left sinonasal mass associated with, bleeding, decreased vision, CT PNS-Mass occupying left sided nasal cavity with extension into sphenoid, posterior ethmoid sinuses | SNUC | − | SDSC | Underwent surgery followed by chemo& radiotherapy. Alive without any recurrence (1 year 8 months duration) |
| 16 | Present study | 35 | F | Right facial pain, epistaxis for 1 year, 6 × 7 cm swelling over right orbit with right eye pushed outward, right sided nasal mass. CEMRI brain and PNS—A large heterogeneously enhancing mass cantered over right ethmoid sinus, nasal cavity with intracranial extension | SNUC | − | SDSC |
Anterior cranio-facial resection of right sided nasal mass and right orbital exenteration followed by chemoradiotherapy. Alive with local recurrence (c/o increase in swelling size) 5 months |
SNUC sinonasal undifferentiated carcinoma, SDSC SMARCB1/INI1 deficient sinonasal carcinoma, + positive, − negative, NA not available, ND not done, DOD died of diseases, NED no evidence of disease, AWD alive with disease
We have observed, in our SMARCB1-deficient sinonasal carcinoma cases, varying proportion of basaloid cells admixed with larger tumor cells having a moderate amount of eosinophilic cytoplasm. In addition, we found a small cell carcinoma like pattern and focal pseudo-alveolar patterns in two of the cases. These patterns were not described previously.
Perivascular pseudo-rosettes pattern was noted in one of our cases, similar to the observation of Agaimy et al. [5], which they found in all their three cases. Comedo-pattern of necrosis was seen in one of our cases which is similar to the cases reported by Wasserman et al. [10]
A comparison of the morphology of SMARCB1-deficient sinonasal carcinoma with SNUC showed that the distinction between the two may be difficult on morphology. Both these tumors share many morphological features including an infiltrative pattern of growth, tumor cells arrangement in varying sized nests, islands or diffuse pattern, and a high-grade morphology with areas of necrosis, apoptosis, and brisk mitosis. Both tumors show a predominance of cells with basaloid morphology. Among our SNUC cases, in additional to the above general features, we have noted focal sarcomatoid and rhabdoid features in few cases. The rhabdoid appearing cells were immunopositive for INI1, indicating that the rhabdoid morphology is not unique to SMARCB1-deficient sinonasal carcinoma. Nasopharyngeal undifferentiated carcinoma like morphology and small cell carcinoma like pattern were found in both SMARCB1-deficient sinonasal carcinoma and SNUC cases. However, the pseudo-alveolar pattern and pseudopapillary patterns, that were seen in the two cases of SMARCB1-deficient sinonasal carcinoma, were not found in any the SNUC cases. Focal pseudo glandular pattern, clear cell change, and empty cytoplasmic vacuoles were the additional features seen in SMARCB1/INI1 deficient sinonasal carcinoma.
Immunohistochemical profile of SNUC and SMARCB1-deficient sinonasal carcinoma were remarkably similar. Both showed diffuse Pan-CK and EMA positivity. Focal loss or weak expression of pan-CK and EMA was observed in two cases of SNUC. Focal expression of synaptophysin, chromogranin, NSE, and CD99 was seen in two cases of SNUC. Expression of CD99 showed a peculiar paranuclear dot like pattern in one case, the significance of which is not clear. Both the tumors were uniformly immunonegative for LCA, S100, HMB45, p40, CD56, NUT and p16. Immunophenotypic distinction between the two tumors required the demonstration of complete loss of INI1 in the latter. The results of p16 expression by IHC in SNUC and SMARCB1/INI1 deficient sinonasal carcinoma are variable. The reported frequency of p16 expression in SNUC ranges from 20 to 100% [14–16]. In a recent study by Fard et al. [13], p16 positivity was found in five out of six SNUC cases, while all cases of SNUC mimickers were immunonegative. This contrasts with our findings, where all the SNUC cases showed immunonegativity for p16. The reported expression of p16 in SMARCB1/ INI1 deficient sinonasal carcinoma cases, ranges from 13.8 to 66.7% [3, 5, 13]. Kakkar et al. [11] and Truei et al. [12] have noted p16 negativity in all their cases. We also noted the absence of p16 expression in all our three cases. These contrasting findings might be due to the different clones of p16 antibodies which were used in these studies (clone E6H4 in study by Fard et al. [13], clone JC8 in study by Agaimy et al. [4], INK4a in study by Bishop et al. [6]). Nevertheless, the role of p16 expression in SMARCB1/INI1 deficient sinonasal carcinoma has been shown to be related to cell cycle mechanisms rather than the HPV infection status.
SMARCB1/INI1 deficient sinonasal carcinoma shares many of its morphological features with the newly described SMARCA4 deficient sinonasal carcinoma. Agaimy et al. [17] observed a similar histology in these two tumors with an undifferentiated high grade cytological features and basaloid pattern. However, the latter tumor showed predominance of large cells with frequent immunoexpression of neuroendocrine markers, much like neuroendocrine carcinomas. These tumors are characterized by a loss of immunoexpression of SMARCA4 whereas SMARCB1/INI1 immunoexpression is retained.
Loss of INI1 is the defining feature of SMARCB1/INI1 deficient sinonasal carcinoma. Loss of INI1 plays an important role in the pathogenesis of SMARCB1/INI1 deficient sinonasal carcinoma, where in INI1 deficiency causes G0/G1 cell cycle arrest by transcriptional repression of Cyclin D1, induction of p16, and hypo-phosphorylation of RB [18–21]. Clinical presentation of SNUC and SMARCB1-deficient sinonasal carcinoma were found to be very similar in our cases and is in concordance with the previously reported series [5, 13]. Both these tumors present as progressively enlarging, locally infiltrative and destructive sinonasal masses. The age of patients ranges from 17 to 86 years with a mean age at presentation of 35 years. There is no gender bias. Prognosis of these cases is generally poor. The largest series of SMARCB1/INI1 deficient sinonasal carcinoma by Agaimy et al. [3] have found that 56% of patients died of their disease within a few days to 102 months after diagnosis (median, 15 months). We observed two of our cases were alive with a follow-up duration of 20 months and 5 months, respectively. Another patient died 2 years after the diagnosis. Details of the cause of death, recurrence, and metastasis for this case are not available. We noted no significant differences in the follow up of SNUC cases and the SMARCB1/INI1 deficient sinonasal carcinoma. This might be because all these cases were initially diagnosed as SNUC and received similar multimodal therapy. A larger study with a greater number of cases and follow up data is required to delineate the prognosis of these patients. Though SMARCB1/INI1 deficient sinonasal carcinoma certainly overlaps with SNUC histomorphologically, it also does with other high grade sinonasal neoplasms. Screening SNUC to identify SMARCB(INI1)-deficient sinonasal carcinoma cases may not yield maximum result as well as finding the extended histomorphological features. Hence, INI1 staining must be employed routinely in all cases of sinonasal neoplasms with a high grade poorly differentiated/ undifferentiated morphology.
Conclusion
We conclude that many cases diagnosed previously as SNUC represent SMARCB1/INI1 deficient sinonasal carcinomas. Pathologist must be aware of the varied morphological appearances of this tumor to avoid its underdiagnosis. Apart from the common basaloid morphologic features and rhabdoid morphology, presence of mixed morphologic features like small cell-like pattern, pseudoalveolar and pseudoglandular pattern, and perivascular pseudo rosette like morphology within a given tumor must raise a suspicion of a SMARCB1/INI1 deficient sinonasal carcinoma. Immunohistochemistry with INI1 antibody must be employed in all such cases to arrive at the proper diagnosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (TIF 1288 kb)
Author Contributions
PA: Data curation, investigation, visualization, project administration, writing—review and editing. PM: Conceptualization, Data curation, methodology, formal analysis, and writing—original draft, review and editing. CP: Conceptualization, Data curation, investigation, visualization, project administration, writing—review and editing. AKA: Conceptualization, Data curation, methodology, formal analysis, and writing—original draft, review and editing.
Funding
This study did not receive any funding.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical approval was waived by the local Ethics Committee of All India Institute of Medical sciences Bhubaneswar, in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. [Google Scholar]
- 2.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 3.Agaimy A, Hartmann A, Antonescu CR, et al. SMARCB1 (INI-1)–deficient sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol. 2017;41:458–471. doi: 10.1097/PAS.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agaimy A, Weichert W. SMARCA4-deficient sinonasal carcinoma. Head Neck Pathol. 2017;11:541–545. doi: 10.1007/s12105-017-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agaimy A, Koch M, Lell M, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–1281. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell D, Hanna EY, Agaimy A, Weissferdt A. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Arch. 2015;467:649–656. doi: 10.1007/s00428-015-1853-1. [DOI] [PubMed] [Google Scholar]
- 8.Zeng M, Chen C, Yang S, Chen X. SMARCB1 (INI1)-deficient sinonasal carcinoma: a newly described entity. Int J Clin Exp Pathol. 2016;9:3454–3458. [Google Scholar]
- 9.Shatzkes DR, Ginsberg LE, Wong M, et al. Imaging appearance of SMARCB1 (INI1)-deficient sinonasal carcinoma: a newly described sinonasal malignancy. AJNR Am J Neuroradiol. 2016;37:1925–1929. doi: 10.3174/ajnr.A4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasserman JK, Dickson BC, Perez-Ordonez B, et al. INI1 (SMARCB1)-deficient sinonasal carcinoma: a clinicopathologic report of 2 cases. Head Neck Pathol. 2017;11:256–261. doi: 10.1007/s12105-016-0752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakkar A, Antony VM, Pramanik R, Sakthivel P, Singh CA, Jain D. SMARCB1 (INI1)-deficient sinonasal carcinoma: a series of thirteen cases with assessment of histological patterns. Hum Pathol. 2019;83:59–67. doi: 10.1016/j.humpath.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Trieu V, Aulet RM, Ciolino A, Rimash T. SMARCB1-deficient sinonasal carcinoma: a case report and discussion of the clinical implications. Ann Otol Rhinol Laryngol. 2019;128:676–680. doi: 10.1177/0003489419836668. [DOI] [PubMed] [Google Scholar]
- 13.Fard EV, Zhang S, Cai Z, et al. Sinonasal undifferentiated carcinoma: clinicopathological spectrums and diagnosis reappraisal. Hum Pathol. 2019;89:62–70. doi: 10.1016/j.humpath.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 14.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth B, Bumpous JM, Martin AW, Nowacki MR, Jenson AB, Farghaly H. Expression of p16 in sinonasal undifferentiated carcinoma (SNUC) without associated human papillomavirus (HPV) Head Neck Pathol. 2011;5:349–354. doi: 10.1007/s12105-011-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray ST, Herr MW, Sethi RK, et al. Treatment outcomes and prognostic factors, including human papillomavirus, for sinonasal undifferentiated carcinoma: a retrospective review. Head Neck. 2015;37:366–374. doi: 10.1002/hed.23606. [DOI] [PubMed] [Google Scholar]
- 17.Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma: A series of 10 cases expanding the genetic spectrum of SW1/SNF-driven sinonasal malignancies. Am J Surg Pathol. 2020;44:703–710. doi: 10.1097/PAS.0000000000001428. [DOI] [PubMed] [Google Scholar]
- 18.Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108:547–552. doi: 10.1111/cas.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohashi K, Oda Y, Yamamoto H, et al. Highly aggressive behaviour of malignant rhabdoid tumor: a special reference to SMARCB1/INI1 gene alterations using molecular genetic analysis including quantitative real-time PCR. J Cancer Res Clin Oncol. 2007;133:817–824. doi: 10.1007/s00432-007-0223-z. [DOI] [PubMed] [Google Scholar]
- 20.Versteege I, Medjkane S, Rouillard D, Delattre O. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene. 2002;21:6403–6412. doi: 10.1038/sj.onc.1205841. [DOI] [PubMed] [Google Scholar]
- 21.Betz BL, Strobeck MW, Reisman DN, Knudsen ES, Weissman BE. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21:5193–5203. doi: 10.1038/sj.onc.1205706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (TIF 1288 kb)