Abstract
Salivary gland tumors (SGT) represent an uncommon heterogeneous group of tumors with complex clinical and pathological characteristics. The prevalence of these lesions varies between studies but has been estimated between 3 and 6% of all tumors in the head and neck region. The present study aimed to evaluate the distribution and demographic findings of salivary gland tumors diagnosed in an oral pathology service in Mexico. A retrospective descriptive cross-sectional study was performed. A total of 164 cases of SGT from a private oral pathology service were diagnosed between 2000 and 2019 in Mexico City. All cases were reviewed histologically, and demographic data and histopathological diagnoses were collected. A total of 110 (67.1%) tumors were benign, and 54 (32.9%) were malignant. The majority of patients were female (n = 100, 61.0%) with an overall female:male ratio of 1.6:1. The minor salivary glands were affected more than the major salivary glands (68.9% vs. 25.6%). The palate (n = 67, 40.9%) was the most commonly affected site, followed by the parotid gland (n = 37, 22.6%), lips (n = 16, 9.8%), and buccal mucosa (n = 14, 8.5%). Pleomorphic adenoma (n = 88; 80.0%) and mucoepidermoid carcinoma (n = 16, 29.6%) were the most frequent benign and malignant tumors, respectively. The general features of SGT from the studied Mexican population shared some similarities and differences compared to previously reported series from various parts of the world.
Keywords: Salivary gland, Tumors, Epidemiology, Head and neck pathology
Introduction
The salivary glands are exocrine glands that produce secretions contributing to the lubrication, digestion, and protection of the upper aerodigestive tract [1]. They can be divided into major (parotid, submandibular, sublingual) and minor salivary glands [2]. Due to its complex histology, a variety of primary tumors can develop in these structures independently of the anatomical site [1, 2]. Also, the morphological diagnosis of these lesions is frequently challenging due to many histological subtypes, overlapping of morphological findings, and different classifications [2–4].
Although several epidemiological studies across the world have evaluated the frequency and incidence of these tumors [2, 3, 5–16], geographic variations have been observed in this group of lesions, particularly in relation to anatomical location and histological subtypes [2, 3, 8]. In addition, there are only a few studies about the incidence in the Mexican population, despite its large geographical size and population [14].
Thus, the objective of the present study was to describe the clinical and demographic aspects of salivary gland tumors (SGT) diagnosed in a private oral pathology service in Mexico City and to compare the findings with epidemiological data from different geographic locations obtained through the review of the literature.
Material and Methods
Study Design and Sample
In this study, the files of a private oral pathology service in Mexico were retrospectively reviewed during a 20-year period (between January 2000 and December 2019). All cases of SGT were retrieved, and data such as gender, age, anatomical location, and histopathological diagnosis were obtained from clinical records and analyzed. The lesions were reviewed histologically and were reclassified into benign and malignant tumors in accordance with the current WHO Classification of Head and Neck Tumours [17]. Microscopical slides of all cases were reexamined by two independent pathologists with more than 20 years of experience. Immunohistochemical, molecular, cytogenetic, and histochemical studies were performed during the reassessment of cases when routine staining (hematoxylin-eosin) was not sufficient to establish the final diagnosis.
Analysis
Descriptive and quantitative data analysis was performed using the Statistical Package for the Social Sciences for Windows 20.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean, median, and standard deviation values. Categorical variables were expressed as absolute number of cases and percentage values.
Results
The Private Oral Pathology Service received 11,017 surgical specimens between 2000–2019, of which 164 were diagnosed as SGT (1.48%). Of these, 110 (67.1%) were benign, and 54 (32.9%) malignant neoplasms with a benign:malignant ratio of 2.3:1, distributed among seven benign and ten malignant histologic subtypes (Table 1).
Table 1.
Histologic and gender distribution of 164 salivary gland tumors
| n = 164 | %a | %b | Sex | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| n | % | n | % | ||||
| Benign tumors | |||||||
| Pleomorphic adenoma | 88 | 80.0 | 53.7 | 35 | 21.3 | 53 | 32.3 |
| Myoepithelioma | 13 | 11.8 | 7.9 | 7 | 4.3 | 6 | 3.7 |
| Warthin's Tumor | 3 | 2.7 | 1.8 | 2 | 1.2 | 1 | 0.6 |
| Canalicular adenoma | 2 | 1.8 | 1.2 | 1 | 0.6 | 1 | 0.6 |
| Cystadenoma | 2 | 1.8 | 1.2 | 2 | 1.2 | 0 | 0.0 |
| Basal cell adenoma | 1 | 0.9 | 0.6 | 0 | 0.0 | 1 | 0.6 |
| Sialadenoma papilliferum | 1 | 0.9 | 0.6 | 0 | 0.0 | 1 | 0.6 |
| Total | 110 | 100 | 67.1 | 47 | 28.7 | 63 | 38.4 |
| Malignant tumors | |||||||
| Mucoepidermoid carcinoma | 16 | 29.6 | 9.8 | 3 | 1.8 | 13 | 7.9 |
| Polymorphous adenocarcinoma | 10 | 18.5 | 6.1 | 0 | 0.0 | 10 | 6.1 |
| Adenoid cystic carcinoma | 8 | 14.8 | 4.9 | 2 | 1.2 | 6 | 3.7 |
| Acinic cell carcinoma | 7 | 13.0 | 4.3 | 6 | 3.7 | 1 | 0.6 |
| Carcinoma ex-pleomorphic adenoma | 5 | 9.3 | 3.0 | 2 | 1.2 | 3 | 1.8 |
| Clear cell hyalinizing carcinoma | 3 | 5.6 | 1.8 | 2 | 1.2 | 1 | 0.6 |
| Epithelial-myoepithelial carcinoma | 2 | 3.7 | 1.2 | 0 | 0.0 | 2 | 1.2 |
| Adenocarcinoma NOS | 1 | 1.9 | 0.6 | 0 | 0.0 | 1 | 0.6 |
| Salivary duct carcinoma | 1 | 1.9 | 0.6 | 1 | 0.6 | 0 | 0.0 |
| Myoepithelial carcinoma | 1 | 1.9 | 0.6 | 1 | 0.6 | 0 | 0.0 |
| Total | 54 | 100 | 32.9 | 17 | 10.3 | 37 | 22.6 |
aPercent in the group (benign or malignant); b Percent in relation to the total number of cases;
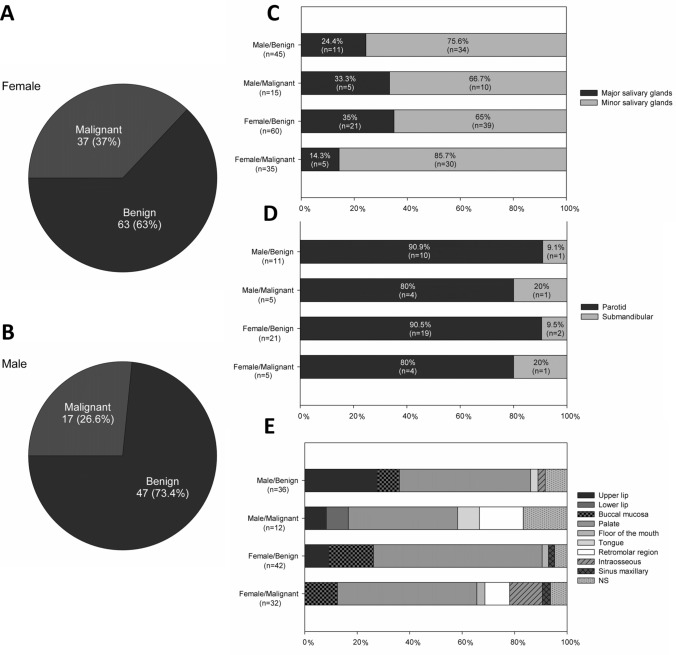
The majority of patients were female (n = 100, 61.0%) with an overall female:male ratio of 1.5:1 (Table 1). Most tumors occurred in patients between the fourth and sixth decades of life, with a mean age of 47.9 years (range 04–91 years). Table 2 shows the distribution of each salivary gland neoplasm, according to the age of patients.
Table 2.
Age group distribution (decade of life) of salivary gland tumors
| Age range | Mean agea | Age groups | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | > 70 | NS | n | % | |||
| Benign tumors | |||||||||||||
| Pleomorphic adenoma | 13–85 | 44.7 | 0 | 6 | 11 | 18 | 14 | 22 | 7 | 5 | 5 | 88 | 53.7 |
| Myoepithelioma | 04–77 | 45.3 | 1 | 1 | 1 | 2 | 1 | 0 | 4 | 1 | 2 | 13 | 7.9 |
| Warthin's Tumor | 54–82 | 65 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 3 | 1.8 |
| Canalicular adenoma | 69–78 | 73.5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 1.2 |
| Cystadenoma | 82–89 | 85.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 1.2 |
| Basal cell adenoma | – | 73 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.6 |
| Sialadenoma papilliferum | – | 55 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.6 |
| Total | 13–89 | 47.1 | 1 | 7 | 12 | 20 | 15 | 25 | 12 | 11 | 7 | 110 | 67.1 |
| Malignant tumors | |||||||||||||
| Mucoepidermoid carcinoma | 14–91 | 42.4 | 0 | 1 | 3 | 3 | 5 | 2 | 0 | 1 | 1 | 16 | 9.8 |
| Polymorphous adenocarcinoma | 26–76 | 57.2 | 0 | 0 | 1 | 0 | 2 | 3 | 2 | 2 | 0 | 10 | 6.1 |
| Adenoid cystic carcinoma | 29–63 | 49.2 | 0 | 0 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 8 | 4.9 |
| Acinic cell carcinoma | 29–68 | 49.7 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 7 | 4.3 |
| Carcinoma ex-pleomorphic adenoma | 25–78 | 50 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 5 | 3.0 |
| Clear cell hyalinizing carcinoma | 21–69 | 50 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 1.8 |
| Epithelial-myoepithelial carcinoma | 32–85 | 58.5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 1.2 |
| Adenocarcinoma NOS | - | 40 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Salivary duct carcinoma | - | 55 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.6 |
| Myoepithelial carcinoma | – | 37 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Total | 14–91 | 49.0 | 0 | 1 | 9 | 8 | 9 | 13 | 7 | 5 | 2 | 54 | 32.9 |
NS not specified
aYears
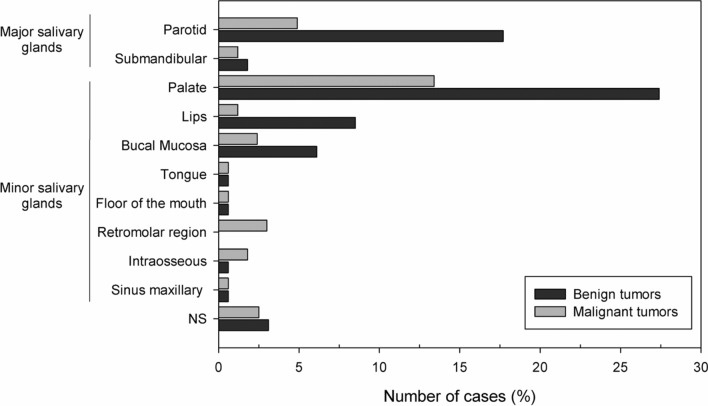
Regarding the anatomical site, 68.9% of the tumors occurred in the minor salivary glands (n = 113), while only 25.6% affected the major salivary glands (n = 42). The palate was the most commonly affected site, with a frequency of 40.9% (n = 67), followed by the parotid gland (n = 37, 22.6%), lips (n = 16, 9.8%), buccal mucosa (n = 14, 8.5%), and submandibular gland (n = 5, 3.0%). There were 9 cases with unspecified anatomic location (5.5%), and none affected the sublingual gland. Both benign and malignant neoplasms predominated in the soft and hard palate, followed by the parotid gland (Table 3).
Table 3.
Distribution of the 164 salivary gland tumors according to the location (major and minor salivary glands)
| Major salivary glands | Minor salivary glands | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parotid | Submandibular | Palate | Lips | Buccal mucosa | Tongue | Floor of the mouth | Retromolar | IO | Maxillary sinus | NS | n | % | |
| Benign tumors | |||||||||||||
| Pleomorphic adenoma | 27 | 3 | 35 | 9 | 8 | 1 | 0 | 0 | 0 | 0 | 5 | 88 | 53.7 |
| Myoepithelioma | 0 | 0 | 9 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 13 | 7.9 |
| Warthin's Tumor | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.8 |
| Canalicular adenoma | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.2 |
| Cystadenoma | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1.2 |
| Basal cell adenoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.6 |
| Sialadenoma papilliferum | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Total | 29 | 3 | 45 | 14 | 10 | 1 | 1 | 0 | 1 | 1 | 5 | 110 | 67.1 |
| Malignant tumors | |||||||||||||
| Mucoepidermoid carcinoma | 2 | 0 | 9 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 16 | 9.8 |
| Polymorphous adenocarcinoma | 0 | 0 | 6 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 10 | 6.1 |
| Adenoid cystic carcinoma | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 8 | 4.9 |
| Acinic cell carcinoma | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 7 | 4.3 |
| Carcinoma ex-pleomorphic adenoma | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3.0 |
| Clear cell hyalinizing carcinoma | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1.8 |
| Epithelial-myoepithelial carcinoma | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1.2 |
| Adenocarcinoma NOS | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Salivary duct carcinoma | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Myoepithelial carcinoma | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| Total | 8 | 2 | 22 | 2 | 4 | 1 | 1 | 5 | 4 | 1 | 4 | 54 | 32.9 |
NS not specified, IO intraosseous
Among the benign salivary gland tumors, pleomorphic adenoma (PA) was the most frequent (n = 88; 80.0%), followed by myoepithelioma (n = 13, 11.8%), and Warthin's Tumor (n = 3, 2.7%) (Table 1). Most tumors were diagnosed in the fourth and fifth decades of life (Fig. 1); however, the age ranged from 4 to 89 years, with an average of 47.1 years (SD ± 18.9). Most cases occurred in the palate (n = 45, 40.9%) and female patients (n = 63; 57.3%), with a female:male ratio of 1.3:1 (63 female and 47 male).
Fig. 1.
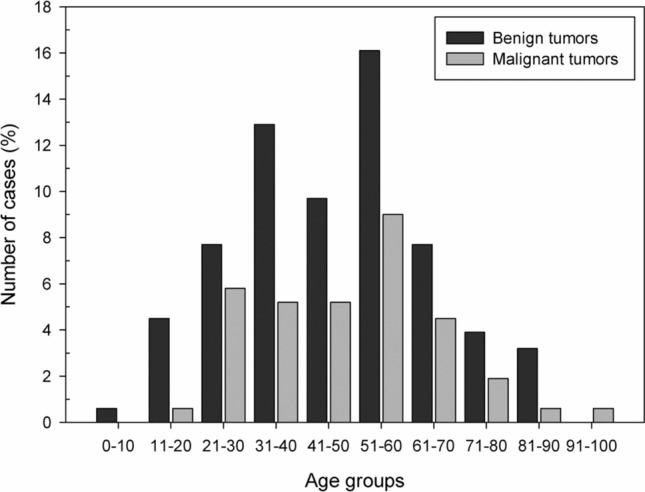
Distribution of 164 salivary gland tumors according to the age group (decade of life)
Regarding the malignancies (n = 54), mucoepidermoid carcinoma was the most frequent malignant tumor (n = 16, 29.6%), followed by polymorphous adenocarcinoma (n = 10, 18.5%), and adenoid cystic carcinoma (n = 8, 14.8%) (Table 1). The age ranged from 14 to 91 years, with a mean of 49.0 years (SD ± 17.3) (Table 2). Most cases also occurred in the minor salivary glands of the palate (n = 22, 40.7%) and female patients (n = 37; 68.5%), with a female:male ratio of 2.2:1 (37 female and 17 male) (Tables 1 and 3).
Immunohistochemical reactions (IHC) were used in 6 cases (3.7%). In 4 of the cases, IHC was used to determine the proliferative index; in only 2 of the cases, it aimed to identify cells and structures in order to facilitate the diagnosis. After reevaluation of morphology, immunohistochemical, molecular, and cytogenetic studies, six cases (3.7%) were reclassified. Of these, four benign tumors (2.4%) were reclassified as malignant neoplasms, and two malignant tumors (1.2%) reclassified according to the morphological subtype. Four cases of PA were reclassified as carcinoma ex-pleomorphic adenomas. One case of adenocarcinoma NOS as clear cell hyalinizing carcinoma. Also, one case of ACC and two of polymorphous adenocarcinomas were compatible as cribriform adenocarcinoma of minor salivary glands (CAMSG), a variant of polymorphous adenocarcinoma. However, cases compatible with CAMSG were maintained as polymorphous adenocarcinomas based on the current WHO Classification of Head and Neck Tumors [17].
Discussion
In the last 20 years, the histological classification of salivary gland tumors has been modified twice, in 2005 and 2017, and several studies have been published on the incidence and characteristics of SGT worldwide [2–5, 18–23, 30]. However, the relative frequency and distribution of salivary gland neoplasms remain topics of discussion in the scientific literature. In the present study, the sample represented about 1.48% of all lesions diagnosed in the referred oral pathology service. Studies conducted in other oral pathology services reveal that SGT accounts for about 0.08% to 2.6% of all diagnosed lesions [2, 16], which are data similar to our results, and variations in frequency depend on the referral sources and type of diagnostic services (private, public, hospital-based, etc).
Overall, according to the WHO Histological Classification of Head and Neck Tumors (2017), female patients are slightly more affected by SGT than male patients [17]. However, some variations can be found when analyzing specific tumor subtypes [2, 3, 8, 15]. In the present study, the female-to-male ratio was 2.3:1, which is in agreement with most studies [13, 19], including Mexican reports [14].
In this study, most tumors were benign (67.1%), data similar to other studies where these tumors correspond to about 64.7 to 80.0% of all salivary gland neoplasms [11, 15]. However, some studies have shown a higher incidence of malignancies [20–23] and suggest geographic variation in the frequency of these tumors. In addition, benign neoplasms presented a female-to-male ratio of 1.3:1, while malignant neoplasms demonstrated a female-to-female ratio of 2.2:1, indicating that both benign and malignant tumors were more common in female patients. These data are in accordance with a previous study performed in Mexico that also showed that female patients were more affected by malignant neoplasms than male patients [14]. However, unlike our results, other studies show that men are mainly affected by malignant salivary gland tumors, but no explanation has been offered for this data [3, 8].
Regarding the benign neoplasms, PA was the most common tumor in this study, accounting for 80.0% of all benign neoplasms, followed by myoepithelioma (11.8%). In fact, PA is the most common benign neoplasm [2, 3, 5–16, 18–20]. However, in contrast to our results, most studies have shown Warthin's Tumor [3, 5–13, 18] and basal cell adenoma [2, 10–13, 15, 19, 22] as the second or third most common benign tumor, respectively. The fact that the present study was carried out in an oral pathology service may explain this apparent difference. Warthin's tumor is a neoplasm that affects almost exclusively the parotid gland, and some studies show that most cases of surgical pathology centers affect the major salivary glands, especially the parotid gland. In contrast, tumors of the minor salivary glands represent the majority of the cases diagnosed in oral pathology services [3, 8]. These results suggest that multicenter studies can better characterize the heterogeneity of tumors and contribute to the comprehension of epidemiological differences in the population [3]. In addition, some other benign tumors were very rare, such cystadenoma (n = 2, 1.8%) and sialadenoma papilliferum (n = 1, 0.9%), in accordance with previous studies [12, 13].
Regarding malignancies, the most common Tumor was MEC, accounting for 29.6% of the cases, similar to previous studies [2, 3, 6–13, 16, 19]. However, other series found ACC as the most frequent malignant tumor [14, 18, 20, 21], including Brazilian reports, where ACC corresponded to approximately 58.3% of all malignant neoplasms of the salivary glands [5]. In our study, the second most common malignant tumor was polymorphous adenocarcinoma, accounting for 18.5% of all malignant neoplasms. Interestingly, only one of the previously published studies shows polymorphous adenocarcinoma among the three most common malignancies [2]. In general, the most frequent malignant tumors are mucoepidermoid carcinoma, adenoid cystic carcinoma, acinic cell carcinoma, and adenocarcinoma not otherwise specified [2, 3]. In our study, some other malignant tumors were rare, such as salivary duct carcinomas (n = 1, 1.9%) and myoepithelial carcinoma (n = 1, 1.9%). Although these entities are well recognized, they are also rarely reported in studies with small samples [2, 3]. This difference may be explained perhaps due to lack of uniform histomorphological criteria for diagnosis, different classifications, and time of experience and familiarity of pathologists with these lesions.
In addition, it is essential to be aware of the recent changes in terminology of SGTs, as some primary malignant epithelial SGTs were included in the current WHO classification (2017), such as secretory carcinoma, intraductal carcinoma, and poorly differentiated carcinoma [17]. Also, polymorphous low-grade adenocarcinoma (PLGA) is now termed polymorphous adenocarcinoma [3, 17] because of the variable biological behavior of this neoplasm. The purpose of this modification, in particular, is to facilitate the choice of treatment and to avoid possible terminological confusion, especially in the most unusual cases [2, 3, 17]. In the present study, we reevaluated the morphologic diagnosis of all tumors according to the latest WHO classification (2017) [17], and six cases (3.7%) were reclassified based on morphological characteristics and immunohistochemical and molecular studies.
Four cases of PA were reclassified as carcinoma ex-pleomorphic adenomas and one case of adenocarcinoma NOS as clear cell hyalinizing carcinoma after reevaluation of morphology and immunohistochemistry analysis. Also, one case of ACC and two of polymorphous adenocarcinomas were compatible as cribriform adenocarcinoma of minor salivary glands (CAMSG). The first description of this tumor was done by Michal et al. (1999) under the term cribriform adenocarcinoma of the tongue (CAT) [24]. Later, it was renamed by Skalova et al. as "cribriform adenocarcinoma of minor salivary gland origin" in a series of 23 new cases because they affected other minor salivary glands, including those of the palate, retromolar region, tonsils, and upper lip [25].
Currently, CAMSG is a provisional entity without a clear statement as to whether it represents a genuine entity or is merely a possible variant of polymorphous adenocarcinoma [17, 26]. However, it is essential to emphasize that although some cases of CAMSG have an indolent clinical course such as polymorphous adenocarcinoma, it presents a higher probability to metastasize to cervical lymph nodes [17]. Although polymorphous adenocarcinoma shares some histologic similarities with CAMSG, polymorphous adenocarcinoma has more diverse histology and the characteristic ground glass nuclei [17, 25]. Moreover, recent molecular studies indicate that rearrangements of PRKD1-3, including ARID1A-PRKD1 and DDX3X-PRKD1 gene fusions, are seen in about 80% of cases of CAMSG, as also observed in our cases, and in less than 10% of cases with classic morphology of polymorphous adenocarcinoma [27]. In contrast, PRKD1 E710D mutations are largely restricted to classic polymorphous adenocarcinoma, with only about 10% of CAMSG showing a mutation [28, 29].
Like previous authors [24–26], we agree that CAMSG is a distinct tumor entity that differs from polymorphous adenocarcinoma by location (most often arising on the posterior region of the tongue), histological architecture, and biological behavior, with frequent metastases at the time of presentation of the primary tumor [25, 26]; however, the counterpoints to these arguments are that the findings in the literature are still scarce, there is some morphological and genotypic overlap between these lesions, and despite the regional aggressiveness, it has not been established differences in survival rates [17]. For these reasons, despite the extensive debate, the decision of the WHO was to maintain a more conservative and unifying approach and leave the CAMSG as a variant of polymorphous adenocarcinoma for this edition. This is also consistent with the fact that both polymorphous adenocarcinoma and CAMSG are driven by genes of the same family, which possibly indicates that are variants of the same spectrum [17].
The classification of SGT is dynamic, and with the recent advances in immunohistochemistry and application of in situ fluorescence hybridization for molecular cytogenetic analysis, specific and refined changes continue to occur [30]. Thus, continuous epidemiological studies of SGT are essential because they help to improve understanding of their clinical and pathological characteristics and are essential to keep physicians and surgeons up to date, especially when the classification of these tumors undergoes some change [2, 3].
Regarding the anatomical location, most of the SGT of this study were diagnosed in the minor salivary glands of the palate (Figs. 2, 3). In general, this result was also reported by other studies [22, 23]. Nonetheless, some studies have shown that SGT preferentially affects the parotid gland [2, 3, 5–16, 18–21]. This difference maybe can be explained by the fact that most surgical specimens sent for an oral pathology service are incisional biopsies or relatively small surgical specimens managed by oral and maxillofacial surgeons, while larger lesions from major salivary glands tend to be treated in hospitals that also perform the histopathological diagnosis [8]. In the present study, benign tumors were greater in number than malignant tumors in all decades of life in both major and minor salivary glands. However, no benign or malignant tumor occurred in the sublingual gland, perhaps because these tumors have a low prevalence in the sublingual glands, as shown by some studies [12, 16]. On the other hand, when these lesions occur in this region, 70–90% of the tumors are malignant [16].
Fig. 2.
Distribution of 164 salivary gland tumors according to the primary site of involvement. NS not specified
Fig. 3.
a and b Distribution of benign and malignant salivary gland tumors by sex; c, d, and e. Distribution of benign and malignant salivary gland tumors by location (major vs. minor salivary glands). NS not specified
In summary, the data from the present study suggest slight variations in the relative frequency and distribution of SGT among populations in Mexico and other regions of the world. Further research is needed to clarify whether such differences derive from the peculiar characteristics of the populations analyzed or the particularities of the service in which the study was conducted. Also, despite the rarity in which SGTs are encountered in the practice of medicine and dentistry, it is essential that physicians and dentists be informed about salivary gland function, abnormalities, and the diseases that can affect these glands, contributing to early diagnosis and effective treatment of these lesions and cancer prevention.
Acknowledgments
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES).
Funding
No funding sources to disclose.
Compliance with Ethical Standards
Conflict of interest
No conflicts of interest to disclose.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of the School of Dentistry of Piracicaba (Protocol nº 20726819.6.0000.5418).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ogle OE. Salivary gland diseases. Dent Clin N Am. 2020;64(1):87–104. doi: 10.1016/j.cden.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 2.da Silva LP, Serpa MS, Viveiros SK, Sena DAC, de Carvalho Pinho RF, de Abreu Guimarães LD, et al. Salivary gland tumors in a Brazilian population: a 20-year retrospective and multicentric study of 2292 cases. J Craniomaxillofac Surg. 2018;46(12):2227–2233. doi: 10.1016/j.jcms.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Cunha JLS, Coimbra AC, Silva JV, Nascimento IS, Oliveira CR, Andrade ME, et al. Epidemiologic analysis of salivary gland tumors over a 10-years period diagnosed in a northeast Brazilian population. Med Oral Patol Oral Cir Bucal. 2020;25(4):e516–e522. doi: 10.4317/medoral.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooper LM. Challenges in minor salivary gland biopsies: a practical approach to problematic histologic patterns. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasconcelos AC, Nör F, Meurer L, Salvadori G, Souza LB, Vargas PA, et al. Clinicopathological analysis of salivary gland tumors over a 15-year period. Braz Oral Res 30:pii:S1806-83242016000100208. 2016. [DOI] [PubMed]
- 6.Vargas PA, Gerhard R, AraújoFilho VJ, de Castro IV. Salivary gland tumors in a Brazilian population: a retrospective study of 124 cases. Rev Hosp Clin Fac Med Sao Paulo. 2002;57(6):271–6. doi: 10.1590/S0041-87812002000600005. [DOI] [PubMed] [Google Scholar]
- 7.Ito FA, Ito K, Vargas PA, de Almeida OP, Lopes MA. Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;34(5):533–536. doi: 10.1016/j.ijom.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca FP, de Carvalho MV, de Almeida OP, Rangel AL, Takizawa MC, Bueno AG, et al. Clinicopathologic analysis of 493 cases of salivary gland tumors in a Southern Brazilian population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):230–9. doi: 10.1016/j.oooo.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Bittar RF, Ferraro HP, Moraes Gonçalves FT, Couto da Cunha MG, Biamino ER. Neoplasms of the salivary glands: analysis of 727 histopathological reports in a single institution. Otolaryngol Pol. 2015;69(4):28–33. doi: 10.5604/00306657.1163578. [DOI] [PubMed] [Google Scholar]
- 10.Wang YL, Zhu YX, Chen TZ, Wang Y, Sun GH, Zhang L, et al. Clinicopathologic study of 1176 salivary gland tumors in a Chinese population: experience of one cancer center 1997–2007. Acta Otolaryngol. 2012;132(8):879–886. doi: 10.3109/00016489.2012.662715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao M, Hao Y, Huang MX, Ma DQ, Chen Y, Luo HY, et al. Salivary gland tumours in a northern Chinese population: a 50-year retrospective study of 7190 cases. Int J Oral Maxillofac Surg. 2017;46(3):343–349. doi: 10.1016/j.ijom.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang XD, Meng LJ, Hou TT, Huang SH. Tumours of the salivary glands in northeastern China: a retrospective study of 2508 patients. Br J Oral Maxillofac Surg. 2015;53(2):132–137. doi: 10.1016/j.bjoms.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol. 2008;44(2):187–192. doi: 10.1016/j.oraloncology.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Mejía-Velázquez CP, Durán-Padilla MA, Gómez-Apo E, Quezada-Rivera D, Gaitán-Cepeda LA. Tumors of the salivary gland in Mexicans. A retrospective study of 360 cases. Med Oral Patol Oral Cir Bucal. 2012;17(2):e183–e189. doi: 10.4317/medoral.17434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sentani K, Ogawa I, Ozasa K, Sadakane A, Utada M, Tsuya T, Kajihara H, Yonehara S, Takeshima Y, Yasui W. Characteristics of 5015 salivary gland neoplasms registered in the Hiroshima Tumor Tissue Registry over a period of 39 years. J Clin Med. 2019;8(5):566. doi: 10.3390/jcm8050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinheimer A, Vieira DS, Cordeiro MM, Rivero ER. Retrospective study of 124 cases of salivary gland tumors and literature review. J Clin Exp Dent. 2019;11(11):e1025–e1032. doi: 10.4317/jced.55685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017;11(1):55–67. 10.1007/s12105-017-0795-0. [DOI] [PMC free article] [PubMed]
- 18.de Oliveira FA, Duarte EC, Taveira CT, Máximo AA, de Aquino EC, Alencar Rde C, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3(4):271–275. doi: 10.1007/s12105-009-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araya J, Martinez R, Niklander S, Marshall M, Esguep A. Incidence and prevalence of salivary gland tumours in Valparaiso. Chile. Med Oral Patol Oral Cir Bucal. 2015;20(5):e532–e539. doi: 10.4317/medoral.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fomete B, Adebayo ET, Ononiwu CN. Management of salivary gland tumors in a Nigerian tertiary institution. Ann Afr Med. 2015;14(3):148–54. doi: 10.4103/1596-3519.152071. [DOI] [PubMed] [Google Scholar]
- 21.Lawal AO, Adisa AO, Kolude B, Adeyemi BF, Olajide MA. A review of 413 salivary gland tumours in the head and neck region. J Clin Exp Dent. 2013;5(5):e218–e222. doi: 10.4317/jced.51143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussari S, Shedam TM, Debta P, Shaikh NA, Yeli NR, Jeergal PA, et al. A retrospective clinicopathological study of salivary gland tumors with particular reference to histological types. Site. Age. and Sex Distribution. J Int Oral Health. 2016;8(1082):1088. [Google Scholar]
- 23.Tilakaratne WM, Jayasooriya PR, Tennakoon TM, Saku T. Epithelial salivary tumors in Sri Lanka: a retrospective study of 713 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:90–98. doi: 10.1016/j.tripleo.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Michal M, Skálová A, Simpson RHW, Raslan WF, Curik R, Leivo I, et al. Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology. 1999;35:495–501. doi: 10.1046/j.1365-2559.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 25.Skalova A, Sima R, Kaspirkova-Nemcova J, Simpson RH, Elmberger G, Leivo I, et al. Cribriform adenocarcinoma of minor salivary gland origin principally affecting the tongue: characterization of new entity. Am J Surg Pathol. 2011;35(8):1168–1176. doi: 10.1097/PAS.0b013e31821e1f54. [DOI] [PubMed] [Google Scholar]
- 26.Michal M, Kacerovska D, Kazakov DV. Cribriform adenocarcinoma of the tongue and minor salivary glands: a review. Head Neck Pathol. 2013;7 Suppl 1(Suppl 1):S3–S11 [DOI] [PMC free article] [PubMed]
- 27.Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–856. doi: 10.1002/gcc.22195.(A). [DOI] [PubMed] [Google Scholar]
- 28.Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. doi: 10.1038/ng.3096.(B). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinreb I, Chiosea SI, Seethala RR, Reis-Filho JS, Weigelt B, Piscuoglio S, et al. Genotypic and phenotypic comparison of polymorphous and cribriform adenocarcinomas of salivary gland. Mod Pathol. 2015;28(S2):333. [Google Scholar]
- 30.Mahomed Y, Meer S. Primary epithelial minor salivary gland tumors in South Africa: a 20-year review. Head Neck Pathol. 2020;14(3):715–723. doi: 10.1007/s12105-019-01111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]