Abstract
Myoepithelial neoplasms are rare tumors of the salivary glands with predominant myoepithelial differentiation and a broad histologic spectrum. Their histological features, immunohistochemical profile and biological behavior are not well characterized and pose a diagnostic challenge. A total of 15 myoepithelial tumors, diagnosed during 2012 and 2019 were subcategorized and correlated with MIB-1 labeling index (LI) and various histological parameters. Immunohistochemical stains for MIB-1 and other antibodies were performed. Statistical analysis was done by chi-square test, Fisher’s exact test and Kaplan Meier curve. Nine patients were male and six were female with the median age of 44 years (range 21–83 years). Of the 15 patients, 6 cases were classified as myoepithelioma (ME) and 9 cases as myoepithelial carcinoma (MECA). Parotid gland was the most common site (46.7%) followed by the palate. MEs showed well circumscribed tumor borders whereas MECAs exhibited focal capsular to extensive invasion into adjacent tissues. Epithelioid cell morphology was most common followed by mixed cell morphology. MIB-1 LI was significantly associated with invasive tumor borders, necrosis and high mitosis. Increased frequency of recurrence was noted with high MIB-1 LI, though it was not statistically significant. MIB-1 LI was high in nearly all MECAs with focal capsular to extensive invasion while low in MEs. Myoepithelial tumor with multinodular growth pattern and focal capsular invasion may have an indolent behavior if mitotic activity and MIB-1 LI is low. Early diagnosis and treatment of MECAs significantly improves the patient's survival and prognosis.
Keywords: Immunohistochemistry, Myoepithelioma, Myoepithelial carcinoma, MIB-1, Parotid, Salivary gland tumor
Introduction
Myoepithelial neoplasms are rare tumors of the salivary glands composed almost exclusively of cells with myoepithelial differentiation, which have contractile properties occurring in the middle to older age group. Myoepithelial tumors include benign myoepitheliomas (ME) and malignant myoepithelial carcinomas (MECA), which represent 1% and < 1% of all tumors in the major and minor salivary glands respectively. The most common sites include the parotid gland followed by the palate and the submandibular gland [1].
In 1943, Sheldon reviewed 57 mixed tumors of the salivary glands and was the first to categorize three salivary tumors as the MEs [2]. Later, Dardick et al. in 1989 best described the histological, immunohistochemical and ultrastructural features of myoepithelial tumors of salivary glands [3]. In 1991, myoepithelial tumors were included in the World Health Organization (WHO) classification of salivary gland tumors (second edition) as a separate entity [4]. In the recent WHO classification of Head and Neck Tumors (Fourth edition, 2017), nearly 31 distinct types of benign and malignant salivary gland neoplasms have been recognized [5].
ME is a benign salivary gland neoplasm composed almost exclusively of neoplastic myoepithelial cells with well-circumscribed tumor borders. The neoplastic myoepithelial cells can be epithelioid, plasmacytoid, clear cell, spindled or mixed morphology. MECA is a malignant neoplasm composed entirely of neoplastic myoepithelial cells with infiltrative growth. The infiltrative growth of MECA is often multinodular and can be seen in well-defined tumors within the capsule of the associated pleomorphic adenoma (PA) in cases of intracapsular MECA ex PA [5]. MECA can be distinguished from ME by demonstration of infiltrative growth, cytologic atypia, frequent mitoses and coagulative necrosis [6].
Nagao et al. [7] observed that myoepithelial tumors with high cell proliferative activity of > 7 mitoses per 10 high power fields (HPF) or MIB-1 LI > 10% suggests malignancy irrespective of histological appearance.
MECA is a histologically challenging entity that closely mimics PA or ME and seems to carry a significant risk of recurrence with aggressive behavior and unexpected metastasis [8].
We undertook this retrospective study to correlate the various histopathological parameters, recurrence and metastasis of myoepithelial tumors of salivary glands with MIB-1LI to objectively predict the biological behavior.
Materials and Methods
This was a retrospective study of all patients with myoepithelial tumors of the major and minor salivary glands. There were approximately 4000 salivary gland neoplasms diagnosed in the Department of General Pathology, Christian Medical College, Vellore between January 2012 and December 2019. Of these salivary gland neoplasms, only 22 cases of myoepithelial tumors were reported during this period, which accounts for 0.5% (22/4000) of all salivary gland tumors. Seven out of 22 patients were excluded from the study as their slides and blocks were not available. So a total of 15 patients with primary salivary gland tumors with exclusive myoepithelial differentiation on morphology and immunohistochemistry (IHC) were included in the study. The study was approved by the institutional review board (IRB No. 12663). The clinical details and follow-up information of each patient were obtained from the hospital medical records. Before analysis, all patients were contacted telephonically to enquire about the disease status on March 18th, 2020. Seven patients (Case Nos. 3, 4, 5, 7, 11, 12, 14) were not contactable, however, the last follow-up date available in the hospital medical records was noted.
Archived Hematoxylin and eosin (H&E) and immunohistochemically stained slides and blocks were retrieved from the department files. Wherever necessary, restaining of H&E slides or extra sections were cut from the blocks and fresh slides were prepared for staining and immunohistochemistry. All the archived slides were reviewed to assess various histopathological parameters including the tumor border, cell type, growth pattern/architecture, stroma (the type of matrix), cellular atypia, mitosis, necrosis, vascular invasion, perineural invasion and evidence of preexisting benign tumor. IHC was done on representative paraffin block(s) from each tumor.
Classification of Myoepithelial Tumors of Salivary Glands
All the 15 cases of myoepithelial tumors of salivary glands were classified as either ME or MECA based on the morphologic and IHC criteria [5, 6].
Tumor subtypes were classified based on the predominant cell type (> 75% of cells) within the tumor and when 2 or more cell types predominated, the tumor was classified as mixed cell type. The mitotic rate was expressed as the number of mitoses seen per 10 HPFs (× 40 objective and × 10 ocular lens).
Immunohistochemistry (IHC)
The IHC was performed using the Ventana Benchmark XT (Ventana Medical Systems, Tucson, AZ, USA). The details of the primary antibodies used for IHC were. Pancytokeratin (Pan-CK) (AE1/AE3) (monoclonal clone AE1/AE3 [1:50]) (Dako), CK7 (monoclonalclone OV-TL/2/30 [1:50]) (Dako), epithelial membrane antigen (EMA) (monoclonal clone E29 [1:50]) (Dako), S-100 protein (polyclonal [1:200]) (Dako), smooth-muscle actin (SMA) (monoclonal clone 1A4 [1:100]) (Dako), p63 (monoclonal clone 4A4 [ready to use]) (Ventana), Glial fibrillary acid protein (GFAP) (polyclonal [1:200]) (Dako), SOX-10 (monoclonal clone EP268 [ready to use]) (Path in situ), Smooth muscle myosin heavy chain (SMMHC) (monoclonal clone Smms-1 [1:50]) (Dako) and MIB-1 antigen (monoclonal clone K-167 [1:100]) (Dako). Appropriate positive controls were included for each antibody throughout the study. The stain was considered to be positive if the tumor cells showed specific cytoplasmic, membrane and/or nuclear staining for the particular antibody. The MIB-1 LI was noted as the percentage of positively stained tumor nuclei per at least 1000 tumor cells in the regions of maximum immunoreactivity under a high-power objective [7].
Statistical Analysis
Descriptive statistics were used such as mean and standard deviation or median and range was used for age and follow up durations. Number and percentage was reported for categorical variables such as gender, metastasis, recurrence, tumor cell type etc. To find the association between MIB-1 LI (≥ 10% and < 10%) and gender, metastasis, recurrence, tumor cell type etc., chi-square test/Fisher’s exact test was used. Comparing the mean difference across the MIB-1 LI, Independent t-test was used and comparing 3 groups (ME, MECA) Kruskal–Wallis H test was used. Comparing two group and time to event outcome, Kaplan Meier curve was used. P value less than 0.05 was considered as statistically significant. All the analysis was carried out using SPSS 21.0 version.
Results
Clinical Findings
The clinical features of all the 15 patients with myoepithelial tumors of the major and minor salivary glands are summarized in Table 1. There were 9 males and 6 females with male to female ratio of 1.5:1. The age of the patients ranged from 21 to 83 years (median age 44 years). The myoepithelial tumors were subcategorized as ME (40%; 6/15) (case no. 1, 2, 3, 4, 5, 6) and MECA (60%; 9/15) (case no. 7, 8, 9, 10, 11, 12, 13, 14, 15).
Table 1.
Clinical characteristic features and outcome in the study group
| Pt. no. | Age (years)/gender | Primary site | Size (cm) | Prior treatment | Treatment | Recurrence | Metastases | Follow-Up (mo) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Cases of myoepithelioma | |||||||||
| 1 | 21/M | Parotid | 2.5 × 1.5 | Chemotherapy for T cell lymphoblatic leukemia | Superfical parotidectomy | No | No | 44 | NED |
| 2 | 44/M | Buccal cortex of mandible | 5 × 4 | Nil | Excision of lesion | No | No | 54 | NED |
| 3 | 60/M | Parotid | 2.5 × 1.5 | Nil | Superficial parotidectomy | No | No | 4 | NED |
| 4 | 21/F | Parotid | 1 × 1 | Nil | Superficial parotidectomy | NA | NA | NA | LOF |
| 5 | 56/F | Soft palate | 2 × 1 | Nil | Excision soft palate mass | NA | NA | NA | LOF |
| 6 | 30/M | Parotid | 6 × 4 | Nil | Superficial parotidectomy | No | No | 54 | NED |
| Cases of Myoepithelial carcinoma | |||||||||
| 7 | 74/M | Base of tongue | 2 × 2 | Nil | Wide local excision + Radiotherapy | No | No | 45 | NED |
| 8 | 56/M | Maxilla | 1 × 0.6 | Nil | Partial maxillectomy | No | No | 19 | NED |
| 9 | 43/F | Parotid | 6 × 5 | Parotid surgery- done elswhere | Total conservative parotidectomy + Endoscopic excision biopsy of nasal mass | Yes | Nasal cavity and brain metastastasis | 8 | DOD |
| 10 | 55/M | Parotid | 8 × 7 | Total conservative parotidectomy | Radical parotidectomy + Radiotherapy | Yes | No | 1 | Under follow-up |
| 11 | 83/M | Parotid | 10 × 8 | Parotid surgery- done elswhere | Radical parotidectomy | Yes | No | NA | LOF |
| 12 | 41/F | Nasopharynx | 3 × 2 | Nil | Endoscopic assisted biopsy + Concurrent Chemoradiation | No | No | 30 | NED |
| 13 | 33/F | Trachea | 3 × 2.5 | Tumor debulking done | Endobronchial debulking | Yes | No | 40 | DOD |
| 14 | 32/F | Hard palate | 6 × 4 | Excision of mass + Chemoradiotherapy done elswhere | Chemotherapy | NA | NA | NA | LOF |
| 15 | 51/M | Bronchus | 2 × 1 | Endobronchial debulking done elswhere | Underwent bronchoscopy | NA | NA | NA | LOF |
cm, centimeter; DOD, died of disease; F, female; LOF, lost to followup; M, male; mo, months; NA, not available; NED, no evidence of disease
Minor salivary gland involvement (53.3%; 8/15) was slightly more common than the major salivary gland involvement (46.7%; 7/15). However, the parotid gland was the most common site (46.7%; 7/15) followed by the palate (13.3%; 2/15), tracheobronchial region (13.3%; 2/15), sinonasal cavity (6.7%; 1/15), nasopharynx (6.7%; 1/15), tongue (6.7%; 1/15) and buccal cortex of mandible (6.7%; 1/15).
Pathologic Features
Gross Findings
The tumor size ranged from 1 cm to 10 cm with the maximum number of tumors in the range of 1 cm to 6 cm (86.7%; 13/15). The mean tumor size was 4.1 cm. The tumors were completely or partially encapsulated with single or multinodular appearance. The cut-section of the tumors were soft to firm with grey-white to tan appearance (Fig. 1). Two tumors showed grossly cystic change (case no. 1, 3).
Fig. 1.
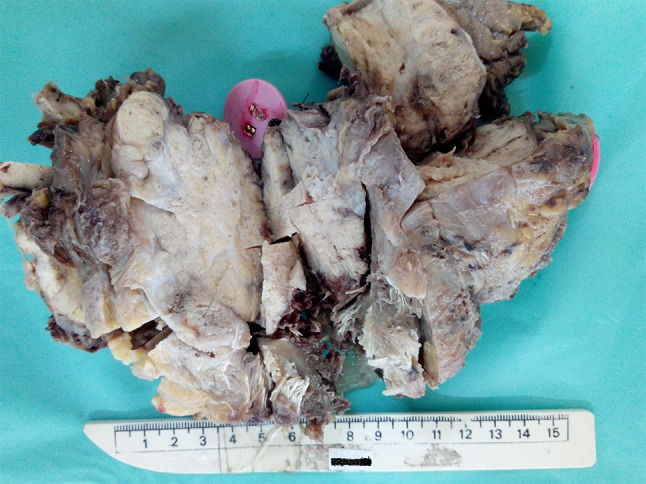
Gross specimen of myoepithelial carcinoma of salivary gland with multinodular unencapsulated tumor and firm grey white cut surface
Histopathological Features
The light microscopic histological findings of the 15 myoepithelial tumors of major and minor salivary glands are summarized in Table 2 and various parameters among myoepithelial tumors were compared in Table 3.
Table 2.
Summary of histologic features and MIB-1 LI of myoepithelial tumors
| Pt. no. | Cell type | Architecture | Matrix | Tumor borders | Cytologic atypia | Necrosis | Mitosis/10 HPF | LVI | PNI | Assoc. BT | MIB-1 LI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases of myoepithelioma | |||||||||||
| 1 | Mix | Sheet like | Hyalinized | Circumscribed | Mild | No | 2 | No | No | No | 2 |
| 2 | Mix | Sheet like | Myxoid | Circumscribed | Mild | No | 1 | No | No | No | 3 |
| 3 | Ep | Sheet like | Hyalinized | Circumscribed | Mild | No | 1 | No | No | No | 1 |
| 4 | Ep | Trabecular | Hyalinized | Circumscribed | Mild | No | 1 | No | No | No | 2 |
| 5 | Ep | Sheet like | Hyalinized | Circumscribed | Mild | No | 3 | No | No | No | 3 |
| 6 | Ep | Sheet like | Myxoid | Circumscribed | Mild | No | 3 | No | No | No | 1 |
| Cases of myoepithelial carcinoma | |||||||||||
| 7 | Mix | Multinodular | Hyalinized | Focal invasive | Moderate | No | 3 | No | No | No | 3 |
| 8 | Ep | Multinodular | Hyalinized | Focal invasive | Moderate | No | 7 | No | No | No | 10 |
| 9 | Mix | Sheet like | Myxoid | Invasive pattern | Marked | Yes | 8 | No | No | PA | 15 |
| 10 | C | Multinodular | Myxoid | Invasive pattern | Moderate to marked | Yes | 10 | No | No | PA | 30 |
| 11 | C | Multinodular | Hyalinized | Invasive pattern | Moderate | Yes | 9 | Yes | Yes | PA | 30 |
| 12 | Ep | Sheet like | Hyalinized | Invasive pattern | Moderate | Yes | 8 | No | No | No | 30 |
| 13 | Mix | Sheet like | Myxoid | Invasive pattern | Marked | Yes | 10 | Yes | Yes | ME | 60 |
| 14 | Ep | Sheet like | Hyalinized | Invasive pattern | Marked | Yes | 3 | No | No | No | 15 |
| 15 | Ep | Multinodular | Hyalinized | Invasive pattern | Marked | Yes | 20 | No | No | No | 20 |
Assoc. BT associated benign tumor, C clear cell, Ep epithelioid, HPF high power field, LVI lymphovascular invasion, LI labelling index, Me myoepithelioma, Mix mixed, PA pleomorphic adenoma, PNI perineural invasion
Table 3.
Comparison of various parameters among myoepithelial tumors
| Variables | ME | MECA | P value |
|---|---|---|---|
| Total cases | 6 | 9 | |
| Age range (Median) | 37(21, 60) | 51(32, 83) | 0.272 |
| Gender | 2:1 | 1:1.2 | |
| Male | 4(66.7) | 5(44.4) | 0.608 |
| Female | 2(33.3) | 4(55.6) | |
| Tumor cell type | 0.776 | ||
| Epithelioid | 4(66.67) | 4(44.44) | |
| Clear cell | 0(0) | 2(22.22) | |
| Mixed | 2(33.3) | 3(33.33) | |
| Architecture | 0.053 | ||
| Multinodular | 0(0) | 5(55.56) | |
| Sheet like | 5(83.33) | 4(44.44) | |
| Trabecular | 1(16.67) | 0(0) | |
| Tumor matrix | 1.000 | ||
| Hyalinized | 4(66.67) | 6(66.67) | |
| Myxoid | 2(33.33) | 3(33.33) | |
| Cytologic atypia | Mild | Moderate to marked | |
| Tumor borders | |||
| Invasive pattern (focal to extensive invasion) | 0 | 9 | |
| Circumscribed | 6 | 0 | |
| Necrosis | 0 | 7 | |
| Lymphovascular invasion | 0 | 2 | |
| Perineural invasion | 0 | 2 | |
| Mitosis/10 HPF (range) | 1–3 | 3–20 | |
| MIB-1 LI range (%) | 1–3 | 3–60 | |
| Prognosis |
4 NED and followed for 4, 44, 54 and 54 mo 0 DOD 0 REC 0 METS 2 LOF |
3 NED and followed for 19, 30 and 45 mo 2 DOD at 8 & 40 mo 4 REC 1 METS 3 LOF |
DOD died of disease, HPF high power field, LOF lost to followup, LI labelling index, ME myoepithelioma, MECA myoepithelial carcinoma, METS metastasis, mo months, NED no evidence of disease, REC recurrence
Tumor Border
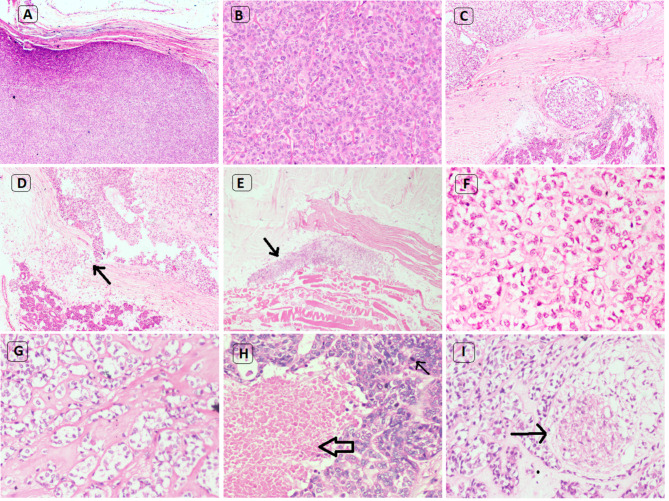
40% of myoepithelial tumors (6/15) showed circumscribed tumor borders (Fig. 2a). The remaining 60% tumors (9/15) exhibited varying degrees of invasive pattern ranging from focal capsular to extensive invasion into the surrounding salivary gland or other tissues (Fig. 2d, e).
Fig. 2.
Histologic findings of myoepithelial tumors of salivary gland. a Myoepithelioma with well circumscribed tumor border (H&E* × 40). b Myoepithelioma with plasmacytoid morphology, insignificant mitosis and no necrosis (H&E* × 100). c Myoepithelial carcinoma with multinodular growth pattern (H&E* × 100). d Myoepithelial carcinoma with focal capsular invasion (narrow arrow) (H&E* × 100). e Myoepithelial carcinoma showing extensive invasion into adjacent skeletal muscle (narrow arrow) (H&E* × 100). f Myoepithelial carcinoma with epithelioid morphology (H&E* × 100). g Myoepithelial carcinoma with clear cell morphology (H&E* × 100). h Myoepithelial carcinoma with necrosis (broad arrow) and mitotic activity (narrow arrow) (H&E* × 100). i Myoepithelial carcinoma with perineural invasion (narrow arrow) (H&E* × 100).*Hematoxylin and eosin
Growth Pattern/Architecture
A variety of growth patterns were identified in these tumors; predominantly sheet-like (60%; 9/15) (Fig. 2b) followed by multinodular (33.3%; 5/15) (Fig. 2c) and trabecular (6.7%; 1/15) pattern. The nodules characteristically showed peripheral hypercellular and central hypocellular regions.
Cell Types
The tumor cells showed a varied morphology with predominantly epithelioid appearance (53.3%; 8/15). The morphology of the various tumor cells were as follows:
Epithelioid Cells 53.3% of tumors (8/15) showed predominantly epithelioid cell morphology (Fig. 2f) characterized by the cells with abundant eosinophilic cytoplasm and centrally placed round to ovoid nuclei. Almost all the tumors with epithelioid cells showed hyalinized stroma in varying amounts.
Clear Cells 13.3% of tumors (2/15) showed only clear cells with centrally placed round nuclei and abundant clear cytoplasm (Fig. 2g). These tumors showed predominantly solid sheets with a multinodular appearance surrounded by hyalinized and myxoid matrix.
Plasmacytoid 20% of tumors (3/15) showed a few cells displaying plasmacytoid morphology with eccentrically placed round to ovoid nuclei and abundant eosinophilic cytoplasm (Fig. 2b).
Mixed Cells Some tumors showed a mixture of cell types, i.e. more than one cell type which combined imperceptibly with each other. In 33.3% of tumors (5/15), there was significant number of epithelioid, clear cell types (each making up more than 25% of the total number of cells) and a few cells with plasmacytoid morphology and therefore these tumors were classified as mixed-cell type.
Stroma The intervening stromal matrix between tumor nests or sheets showed a hyalinized and myxoid matrix. 66.7% of tumors (10/15) showed hyalinized stromal matrix and 33.3% of tumors (5/15) showed myxoid stromal matrix. The myxoid stromal matrix appeared bluish-gray whereas the hyalinized component was eosinophilic and fibrillar.
Atypical Histological Features 53.3% of myoepithelial tumors (8/15), categorized as MECAs, showed features of multinodular/sheet like growth pattern, focal capsular to extensive invasion, moderate to marked nuclear pleomorphism, increased mitosis, necrosis (Fig. 2h) and high MIB-1 LI. However, in 6.7% of tumors (1/15) (case no. 7), although the mitosis (3/10 HPF) and MIB-1 LI (3%) were low, in view of multinodular growth pattern and focal capsular invasion, it was considered as MECA.
Associated/Pre-existing Benign Tumor 73.3% of myoepithelial tumors (11/15) developed de novo while the remaining 26.7% (4/15) of patients had a history of previous benign salivary gland tumor. 20% of tumors (3/15) (case no. 9, 10, 11) had recurrent pleomorphic adenoma of the parotid and later developed MECA (Carcinoma ex pleomorphic adenoma) and 6.7% (1/15) of patients (case no. 13) had myoepithelioma of the tracheal region which later developed into MECA.
Other Histologic Features 13.3% of myoepithelial tumors (2/15) (case no. 9, 13) showed squamous metaplasia. The mitotic activity of all the myoepithelial tumors ranged from 1 to 20 (average value 5.9) per 10 HPF. The mitotic activity ranged from 1 to 3 (average value 1.8) and 3–20 (average value 8.7) per 10 HPF among ME and MECAs respectively. 22.2% MECAs (2/9) showed both vascular invasion and perineural invasion (Fig. 2i) (case no. 11, 13).
Immunohistochemical Analysis
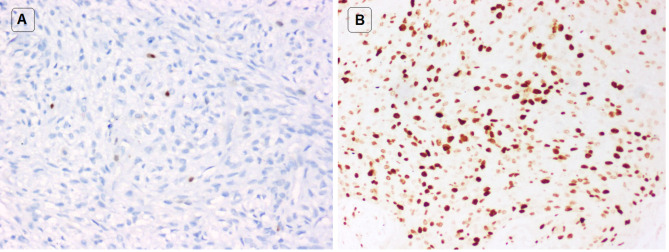
The immunohistochemical profiles of all the 15 myoepithelial tumors showed no major difference with respect to the different cell types, except for MIB-1 labeling index. The tumor cells showed positive immunoreaction for S100 (100%; 6/6 cases), SOX10 (100%; 3/3 cases), p63 (91.7%; 11/12 cases), CK7 (80%; 4/5 cases), smooth muscle actin (42.8%; 3/7 cases), pancytokeratin (75%; 3/4 cases), EMA (66.7%; 2/3 cases), GFAP (50%; 2/4 cases), SMMHC (57.1%; 4/7 cases) and MIB-1 (100%; 15/15 cases) (Fig. 3). The MIB-1 LI of all the myoepithelial tumors ranged from 1 to 60% (average value 15%). The MIB-1 LI of MEs and MECAs ranged from 1 to 3% (average value 2%) and 3–60% (average value 23.7%) respectively.
Fig. 3.
MIB-1 immunohistochemical stain of myoepithelial tumors. a Myoepithelioma with very low MIB-1 labelling index (IHC* × 100). b Myoepithelial carcinoma with very high MIB-1 labelling index (IHC* × 100). *Immunohistochemistry
Statistical Analysis
Table 4 shows the correlation of MIB-1LI with tumor cell type, architecture, tumor matrix, tumor border, necrosis, mitosis, vascular invasion, perineural invasion, recurrence and metastasis.
Table 4.
Myoepithelial tumors of salivary glands with MIB-1 association [7]
| MIB-1 LI ≥ 10% (n = 8) | MIB-1 LI < 10% (n = 7) | p value | |
|---|---|---|---|
| Gender | |||
| Male | 3(37.5) | 5(71.4) | 0.315 |
| Female | 5(62.5) | 2(28.6) | |
| Age | 43.71 ± 20.63 | 49.25 ± 16.43 | 0.573 |
| Range(years) | 32–83 | 21–74 | |
| Median (years) | 47 | 44 | |
| Tumor cell type | |||
| Epithelioid | 4(50.0) | 4(57.14) | 0.608 |
| Mixed | 2(25.0) | 3(42.86) | |
| Clear cell | 2(25.0) | 0(0) | |
| Architecture | |||
| Multinodular | 4(50.0) | 1(14.29) | 0.282 |
| Sheet like | 4(50.0) | 5(71.43) | |
| Trabecular | 0(0) | 1(14.29) | |
| Matrix | 1.000 | ||
| Hyalinized | 5(62.50) | 5(71.43) | |
| Myxoid | 3(37.50) | 2(28.57) | |
| Tumor borders | 0.001 | ||
| Invasive pattern | 8(100) | 1(14.29) | |
| Circumscribed | 0(0) | 6(85.71) | |
| Necrosis | 0.001 | ||
| Present | 7(87.5) | 0(0) | |
| Absent | 1(12.5) | 7(100) | |
| Mitosis/10 HPF | |||
| ≥ 7/10 HPF | 8(100) | 0(0) | < 0.001 |
| < 7/10 HPF | 0(0) | 7(100) | |
| Vascular invasion | |||
| Present | 2(25.0) | 0(0) | 0.155 |
| Absent | 6(75.0) | 7(100) | |
| Perineural invasion | |||
| Present | 2(25.0) | 0(0) | 0.155 |
| Absent | 6(75.0) | 7(100) | |
| Recurrence | |||
| Present | 4(50.0) | 0(0) | 0.077 |
| Absent | 4(50.0) | 7(100) | |
| Metastasis | |||
| Present | 1(12.5) | 0(0) | 1.000 |
| Absent | 7(87.50) | 7(100) |
HPF high power field, LI labelling index
Cell proliferative activity as assessed by MIB-1 LI was significantly associated with invasive tumor borders, necrosis and higher mitosis per 10 HPFs (p ≤ 0.001). Patients with high MIB-1 LI had an increased frequency of tumor recurrence, though it did not attain statistical significance (p 0.077). There was no statistically significant difference of MIB-1 LI between the gender, age, tumor cell type, architecture, tumor matrix, vascular invasion, perineural invasion and metastasis.
Treatment
Surgical excision of the tumor was done in 73.3% of patients (11/15). Cervical lymph node dissection was performed in 13.3% patients (2/15) (case no. 7, 10). 6.7% of patients (1/15) received postoperative concurrent chemoradiation (case no. 12). 13.3% patients (2/15) received postoperative radiation therapy (case no. 7, 10) and 6.7% of patients (1/15) received chemotherapy (case no. 14).
20% patients (3/15) had a history of prior parotid surgery (case no. 9, 10, 11) and 6.7% of patients (1/15) completed chemotherapy for T cell acute lymphoblastic leukemia (case no. 1). 6.7% of patients (1/15) came for a second opinion (confirmation of disease), underwent only a bronchoscopic biopsy but refused to take any treatment (case no. 15).
Follow-up
The follow-up period of myoepithelial tumors ranged from 4 to 54 months with the median follow up of 45 months. 66.7% of ME patients (4/6) were followed up for 4, 44, 54 and 54 months respectively with no evidence of disease at last follow up (case no. 3, 1, 2, 6) and the remaining 33.3% of patients (2/6) were lost to follow up (case no. 4, 5).
33.3% of MECA patients (3/9) followed up for 19, 30 and 45 months showed no evidence of disease at last follow up (case no. 8, 12, 7), 44.4% of patients (4/9) had recurrence of disease (case no. 9,10,11,13), 11.1% of patients (1/9) had tumor metastasis to nasal cavity and brain (case no. 9), 22.2% of patients (2/9) succumbed to disease at 8 months and 40 months respectively (case no. 9, 13) and 33.3% of patients (3/9) were lost to follow up (case no. 11, 14, 15). 11.1% of patients (1/9) were still being followed-up (case no.10) at 1 month.
On an average median survival probability of all myoepithelial tumors by Kaplan–Meier curve was 44 months (95% CI 26.67–61.33). Mean survival probability in the MECA group was 40% (Fig. 4). However, since the sample size is small, there is no significant difference across the groups (p = 0.157).
Fig. 4.
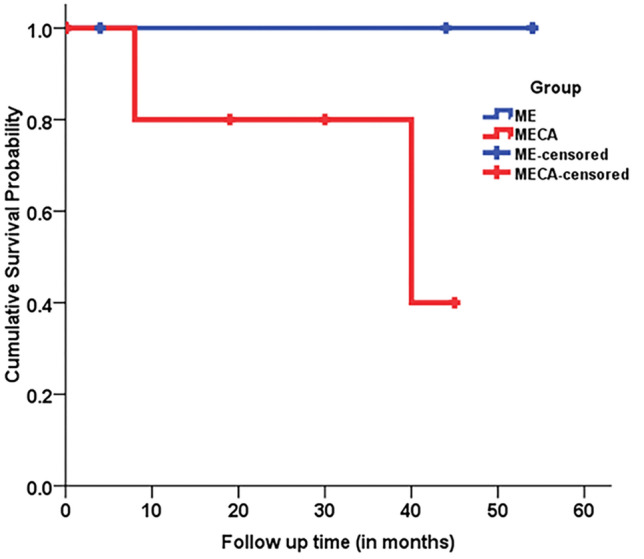
Kaplan Meier survival curve of patients with myoepithelioma (ME) and myoepithelial carcinoma (MECA) of salivary glands
Discussion
Salivary glands normally contain myoepithelial cells in the acini and intercalated ducts. Myoepithelial tumors are rare tumors that originate from the myoepithelial cells which were first described in 1898 by Zimmerman [9]. The MEs represent an extreme form in the continuum of the spectrum of pleomorphic adenoma–basal cell adenoma–myoepithelioma family without ductal component [6].
The majority of salivary gland myoepithelial tumors are benign MEs but the transformation to atypical features and malignancy is rare, however, it can take place in recurrent cases and cases left untreated. The MEs are seen more commonly in the parotid gland than in the minor salivary glands [6, 10]. In the present series also benign MEs most commonly occurred in the parotid gland.
Benign or malignant myoepithelial tumors are mostly seen in the major salivary glands which include parotid gland (50%), sublingual gland (33%) and submandibular gland (13%). The other common sites include breast, nasopharynx, larynx, lung, retroperitoneum, skin and soft tissue [11]. In the present study of 15 myoepithelial tumors, 53.3% tumors developed in the minor salivary glands rather than the major salivary glands (46.7%). However, the parotid gland was still the most common site (46.7%) followed by the palate (13%), tracheobronchial region (13%), nasopharynx (6.7%), tongue (6.7%), sinonasal cavity (6.7%) and buccal cortex of mandible (6.7%).
Benign MEs are mostly well-circumscribed and encapsulated. Benign satellite nodules or protrusions are seen outside the capsule which is described in hypocellular myxoid pleomorphic adenoma and should not be confused with the malignant nodular pattern of MECA. Other features that favor benign salivary gland tumor include the lack of the expansile myoepithelial cellular growth and the presence of heterogeneous arrangement of ducts, myoepithelial cells and stroma within the tumor. Recurrent PA can present with multiple tumor nodules, most often as a miliary pattern of hypocellular nodules which is typical of PA morphology and should not be confused with the malignant nodular pattern of MECA [8].
Vilar et al. [11] noted that, MECAs display varied cellular morphology accompanied by myxoid and/or hyalinised extracellular matrix with infiltrative growth and characteristic multinodular architecture with cellular periphery and central myxoid zones. The mitotic rate ranged from 3 to 51 mitoses per 10 HPF. IHC or ultra-structural studies are often required, since recognition of myoepithelial cell differentiation is not easy on routine H&E stained sections.
Malignant transformation in a pre-existing PA, known as carcinoma ex pleomorphic adenoma (2–4% of all salivary gland tumors), may follow a stepwise sequence classified as in situ or intracapsular, minimal/focal invasion (≤ 1.5 mm) and frank invasion (> 1.5 mm invasion beyond capsule). Malignant change is usually seen in the setting of long-standing pleomorphic adenoma or a tumor with multiple recurrences. Common sites of carcinoma ex pleomorphic adenoma are the parotid glands followed by the submandibular glands, minor salivary glands and rarely, the sublingual salivary gland. The most common malignancies seen in carcinoma ex pleomorphic adenoma include adenocarcinoma not otherwise specified, ductal adenocarcinoma, adenosquamous carcinoma, epithelial-myoepithelial carcinoma, myoepithelial carcinomas and adenoid cystic carcinoma [6, 12].
In our study, 20% of patients (3/15) (case no. 9, 10, 11) presented with recurrent parotid gland tumors operated multiple times and diagnosed as PA. Later, these tumors developed a malignant transformation termed as carcinoma ex pleomorphic adenoma and the malignant component was diagnosed as MECA. Another 6.7% of patients (1/15) (case no. 13) developed MECA from a pre-existing benign ME.
In a review of MECAs of the major and minor salivary glands in 2015, Wang et al. observed that tumor cells exhibited sheet-like (50%) and multinodular growth pattern (50%) with epithelioid (50%), clear cell (18%), plasmacytoid (18%), mixed (11%) and spindle cell (3%) morphology. Tumor related stroma was predominantly hyalinized (68%) than myxoid (32%). Mitotic activity ranged from 3 to 9 per 10 HPF in low-grade tumors and 5 to 26 per 10 HPF in high-grade tumors [13].
The present series reveals that multinodular and sheet-like architecture were most common in MECAs and sheet-like architecture in MEs. Epithelioid cell morphology was the most common subtype followed by mixed cell type. The hyalinized matrix occurred more frequently than the myxoid matrix in both MEs and MECAs. Overall, there was no significant difference between the tumor architecture, cell type and tumor matrix among ME and MECAs. The mitotic activity ranged from 1 to 3 per 10 HPFs in the MEs and 3–20 per 10 HPFs in the MECAs. Almost all the MECAs exhibited mitotic activity > 7 per 10HPF except one case.
Savera et al. reviewed 25 MECAs of salivary glands and observed that the mitotic activity ranged from 3 to 51 mitoses per 10 HPFs, necrosis (60%), perineural invasion (44%) and vascular invasion (16%). The tumor cells were immunopositive for vimentin (100%), S-100 protein (100%), AE1:AE3 (100%), 34βE12 (92%), CAM5.2 (89%), cytokeratin 7 (21%), cytokeratin 14 (53%), smooth muscle actin (50%), calponin (75%), muscle-specific actin (31%), glial fibrillary acidic protein (31%), and epithelial membrane antigen (21%) [14].
In our study, tumor necrosis, vascular invasion and perineural invasion were seen in the MECAs but not in the MEs. The tumor cells were immunopositive for S100 (100%), SOX10 (100%), p63 (91.7%), CK7 (80%), smooth muscle actin (42.8%), pancytokeratin (75%), EMA (66.7%), GFAP (50%), and SMMHC (57.1%). There was no significant difference in the pattern of immunostaining among ME and MECAs.
According to Nagao et al. [7], myoepithelial tumors with MIB-1 LI > 10% can be categorized as malignant. Kane et al. [15] observed MIB-1 LI ranging from 4 to 10% among MECAs; Xu et al. [8], also noted MIB-1 LI ranging from < 5 to 10% in MECAs; and Wang et al. [13] also noted MIB-1 LI ranging from < 5 to 20%. MECAs with MIB-1 LI < 5% can be misleading with bland cytological features. The mitotic activity among the MECAs showed a significant correlation with the prognosis (p = 0.042) while tumor size, tumor site, cell type, grade, pre-existing benign tumor and necrosis did not achieve any statistical significance [13].
In the present series, the MIB-1 LI ranged from 1 to 3% in the MEs and 3 to 60% in the MECAs. In our study, a statistically significant correlation was noted between high MIB-1 LI and invasive tumor borders (p < 0.001), necrosis (p < 0.001) and high mitotic activity (p < 0.001). Though statistical significance was not achieved, MECAs with high MIB-1 LI have an increased frequency of tumor recurrence (p 0.077).
None of the other parameters such as the age, gender, cell type, architecture, tumor matrix, vascular invasion, perineural invasion and the presence of metastases showed statistically significant correlation at 5% level of significance with MIB-1 LI using the Fisher exact and independent t-tests.
The variable morphological appearances of benign and malignant myoepithelial tumors lead to a wide variety of differential diagnoses. The ME with plasmacytoid morphology mimics plasmacytoma, rhabdomyoma and oncocytoma. The ME with predominantly clear cells must be differentiated from the deposits of the renal cell carcinoma. The spindle cell ME should be differentiated from the nerve sheath tumors, fibrohistiocytic tumors and nodular fasciitis. The MECAs must be differentiated from the myoepithelioma, epithelial myoepithelial carcinoma, clear-cell adenocarcinoma, synovial sarcoma, malignant peripheral nerve sheath tumor and malignant melanoma. The ancillary studies such as special stains and IHC help differentiate these tumors [14, 16].
The treatment of myoepithelial tumors is complete surgical excision with free margins with or without nodal dissection [15, 17]. In the patients with the MECAs with extensive dissemination or after palliative surgery, adjuvant radiotherapy or chemotherapy might be beneficial [18].
In conclusion, myoepithelial tumors of the salivary gland are rare tumors with a male predominance in or around middle age. These tumors are seen most often in the parotid gland. These tumors showed a broad spectrum of histological features. However, there is no significant difference between the tumor architecture, cell type and tumor matrix among ME and MECAs. All MEs expressed low cell proliferative activity compared to MECAs. MECAs were significantly associated with invasive borders, necrosis and high mitosis. Tumors with focal capsular invasion, multinodular growth pattern with low mitosis and MIB-1 LI may show an indolent behavior. Identification of such tumors is important, since MECAs usually behave aggressively with an increased risk of recurrence and metastases. Early diagnosis and treatment of MECAs can significantly reduce the rate of recurrence and metastasis thus improving the survival and prognosis of patients.
However, this study is limited by the small number of cases, short follow up and/ or lack of follow up data for several cases.
Acknowledgements
The authors thank the staff Mrs. Annie Rebecca and Mrs. Priya in the Immunohistochemical laboratory, Department of General Pathology at Christian Medical College, Vellore for their technical help.
Funding
No funding obtained.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflicts of interest of any type.
Ethical Approval
This study was approved by the Christian Medical College, Vellore Institutional review board (IRB No. 12663).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vilar-González S, Bradley K, Rico-Pérez J, Vogiatzis P, Golka D, Nigam A, Sivaramalingam M, Kazmi S. Salivary gland myoepithelial carcinoma. Clin Transl Oncol. 2015;17(11):847–855. doi: 10.1007/s12094-015-1329-4. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon WH. So-called mixed tumors of the salivary glands. Arch Pathol. 1943;35:1–20. [Google Scholar]
- 3.Dardick I, Thomas MJ, VanNostrand P. Myoepithelioma—new concepts of histology and classification: a light and electron microscopic study. Ultrastruct Pathol. 1989;13(2–3):187–224. doi: 10.3109/01913128909057442. [DOI] [PubMed] [Google Scholar]
- 4.Seifert G, Sobin LH, editors. World Health Organization International Histological Classification of Tumours. 2. Berlin: Springer; 1991. [Google Scholar]
- 5.El-Naggar AK, Chan JKC, Grandis JR, et al., editors. World Health Organization Classification of Head and Neck Tumours. 4. Lyon: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 6.Fletcher CD, Bridge JA, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone. 4. Lyon: IARC Press; 2013. pp. 277–298. [Google Scholar]
- 7.Nagao T, Sugano I, Ishida Y, et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83(7):1292–1299. doi: 10.1002/(SICI)1097-0142(19981001)83:7<1292::AID-CNCR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Xu B, Mneimneh W, Torrence DE, Higgins K, Klimstra D, Ghossein R, Katabi N. Misinterpreted myoepithelial carcinoma of salivary gland: a challenging and potentially significant pitfall. Am J SurgPathol. 2019;43(5):601–609. doi: 10.1097/PAS.0000000000001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savera AT, Zarbo RJ. Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol. 2004;11(2):69–85. doi: 10.1097/00125480-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Weitzel M, Cohn JE, Spector H. Myoepithelioma of the parotid gland: a case report with review of the literature and classic histopathology. Case Rep Otolaryngol. 2017;2017:6036179. doi: 10.1155/2017/6036179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilar S, et al. Salivary gland myoepithelial carcinoma. Clin Transl Oncol. 2015;17:847–855. doi: 10.1007/s12094-015-1329-4. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Manipadam MT, Michael R. Myoepithelial carcinoma arising in recurrent pleomorphic adenoma in maxillary sinus. J Oral Maxillofac Pathol. 2013;17(3):427–430. doi: 10.4103/0973-029X.125213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Zhang Z, Ge Y, Liu Z, Sun J, Gao Z, Li L. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 29 patients. J Oral Maxillofac Surg. 2015;73(10):1938–1945. doi: 10.1016/j.joms.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24(6):761–774. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Kane SV, Bagwan IN. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 51 cases in a tertiary cancer center. Arch Otolaryngol Head Neck Surg. 2010;136(7):702–712. doi: 10.1001/archoto.2010.104. [DOI] [PubMed] [Google Scholar]
- 16.Gore CR, Panicker N, Chandanwale S, Singh BK. Myoepithelioma of minor salivary glands - A diagnostic challenge: Report of three cases with varied histomorphology. J Oral Maxillofac Pathol. 2013;17(2):257–260. doi: 10.4103/0973-029X.119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Li L, Zeng M, Zhu X, Zhang J, Chen X. Myoepithelial carcinoma of intraoral minor salivary glands: a clinicopathological study of 7 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(1):85–93. doi: 10.1016/j.tripleo.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Ni S, Zhu Y, Wang J. Analysis of the diagnosis and treatment of myoepithelial carcinoma of the parotid gland: report of 17 cases. Zhonghua Zhong Liu Za Zhi. 2015;37(5):392–394. [PubMed] [Google Scholar]