Abstract
Enhancer of zeste homolog 2 (EZH2), a component of the polycomb repressive complex 2 that catalyzes trimethylation of H3K27 (H3K27me3), has been shown to promote tumor development and progression. Expression of EZH2 is associated with cell cycle regulation and cell proliferation in various neoplasms. Oral verrucous hyperplasia (OVH) and Oral verrucous carcinoma (OVC) are rare entities and share several clinical and histopathologic features. Problems distinguishing these lesions are added by a lack of adjacent normal tissue of the biopsy samples and poorly oriented tissue sections. The aim of this study was to investigate the expression of EZH2 and H3K27me3 in OVH and OVC and comparing the expression with normal oral mucosa and oral squamous cell carcinoma (OSCC). Seventy-eight samples, including 25 cases of OVC, 8 cases of OVH, 35 cases of OSCC and 10 cases of normal oral mucosa, were retrieved and submitted for immunohistochemical staining. The results demonstrated that the mean labeling indices (LIs) of EZH2 and H3K27me3 expression were highest in OSCC, followed by the OVC, OVH, and normal mucosa. Statistical differences in EZH2 LI were observed among these lesions whereas H3K27me3 LI was significantly different among OSCC, OVH and normal mucosa. EZH2 LI was found to have a sensitivity of 72.00% and specificity of 87.50% in distinguishing OVH from OVC, and a sensitivity of 57.14% and specificity of 84.00% in distinguishing OVC from OSCC. A positive correlation between EZH2 and H3K27me3 expression was significantly found in OVC but not in OVH and OSCC. These findings highlight the involvement of epigenetic regulation by EZH2-mediated H3K27me3 in the pathogenesis of OVH and OVC, and EZH2 expression indicates disease progression of these verrucous lesions. Diagnostic test analysis further suggests that EZH2 may be used as an additional test for differentiating OVH from OVC in questionable cases.
Electronic Supplementary Material
The online version of this article (10.1007/s12105-020-01209-0) contains supplementary material, which is available to authorized users.
Keywords: EZH2, H3K27me3, Oral verrucous carcinoma, Oral verrucous hyperplasia
Introduction
Epigenetic regulation through histone modification and DNA methylation involved in silencing of various tumor suppressor genes and facilitated tumorigenesis and tumor progression of human cancer [1]. EZH2 (enhancer of zeste homolog 2) is a histone methyl transferase and the core member of polycomb repressive complex 2 (PRC2) that catalyzes trimethylation of lysine 27 on histone 3 (H3K27me3). H3K27me3 functions to suppress the expression of specific target genes by altering the physical state of chromatin [2]. Overexpression of EZH2 has been reported in a variety of human malignancies including gastric, prostate, liver, breast and oral cancers [3, 4].
Oral verrucous carcinoma (OVC), a rare low-grade variant of oral squamous cell carcinoma (OSCC), accounted for 2–16% of oral squamous cell carcinoma [5]. This variant of OSCC is slow growing and exhibits rare regional and distant metastasis; however, it can grow large in size and destroy adjacent tissue [6]. Unlike OSCC, the prognosis of OVC is excellent with surgical treatment since the majority of OVC patients show five years disease-free survival [7]. Oral verrucous hyperplasia (OVH) is recognized as a premalignant lesion [8] and may transform into OVC or OSCC [9]. Both OVH and OVC share several clinicopathologic characteristics and often coexist with epithelial dysplasia [8]. Clinically, OVH and OVC may present as an extensive, thick, white plaque, or mass with exophytic cauliflower-like surface and they cannot thus be distinguished based on clinical appearances [10]. Histopathologically, these two lesions display parakeratotic and hyperplastic well-differentiated epithelium with bulbous rete ridges. No interruption of the basement membrane into the underlying connective tissue of the epithelial cells is recognized. The hyperplastic epithelium of OVC shows pushing margins at interface with underlying connective tissue whereas the epithelium of OVH is elevated in comparison to the adjacent normal epithelium [10, 11]. To establish an accurate diagnosis of these lesions, an adequate biopsy sample with lesional margin tissue, well oriented tissue sections and a communication between the clinician and the pathologist are therefore required [12].
The present study aims to examine the expression of EZH2 and H3K27me3 in OVH, OVC, and compare the expression with normal oral mucosa and OSCC. A correlation between the expression of EZH2 and H3K27me3 in these verrucous lesions was also evaluated.
Materials and Methods
Sample Selection
This study received ethical approval from the Institutional Review Board, Mahidol University (COE.No.MU-DT/PY-IRB 2018/060.1312). A total of 78 sample specimens, including 10 normal oral mucosae, 8 cases of OVH (2 cases showing no epithelial dysplasia; 6 cases showing mild epithelial dysplasia), 25 cases of OVC and 35 cases of OSCC were collected from the archive of the Department of Oral and Maxillofacial Pathology, Faculty of Dentistry, Mahidol University. The clinicopathologic information of all cases, including age, sex and intra-oral location, was retrieved from pathology requested forms. Histopathological slides of all specimens were reviewed and confirmed by a qualified oral pathologist (PL). Histopathological diagnosis of OVH and OVC was made based on criteria recommended by Lin et al. [11]. Specimens of normal oral mucosa were included as a control group. The normal oral mucosa samples were collected from the gingival flap raised during surgical removal of impacted mandibular third molars that were devoid of inflammation.
Immunohistochemistry
For immunohistochemical studies, the paraffin blocks were cut into approximately 4 µm thick sections on 3-aminopropyltriethoxysilane (Sigma-Aldrich, St. Louis, Missouri, USA) coated slides. The sections were deparaffinized with xylene and rehydrated. For antigen retrieval, the sections were microwaved in 10 mM Tris-EDTA buffer (pH 9.0) and cooled in room temperature. Endogenous peroxidase activity was blocked by incubating the sections with 3% H2O2. The slides were pre-incubated in blocking buffer (5% BSA in 0.1% Tween-20 in 1xPBS) and then incubated in primary antibody against EZH2 (EZH2 mouse monoclonal antibody, dilution 1:400, ab191080, Abcam, UK) and H3K27me3 (H3K27me3 rabbit monoclonal antibody, dilution 1:300, #9733, Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C in a humidified chamber. The sections were then washed and incubated in the labeled polymer (EnVision Detection Systems Peroxidase/DAB, Rabbit/Mouse, HRP Rabbit/Mouse (DAB+), Dako, Gilstrap, Denmark) for 30 min. Diaminobenzidine (Dako Liquid DAB + Substrate Chromogen System, Dako, Gilstrap, Denmark) was used as a chromogen and the sections were counterstained with hematoxylin. Negative control sections were done by omission of the relavant primary antibody. Positive controls for EZH2 (Tonsil tissue) and H3K27me3 (B-cell lymphoma) were also performed on each run.
Immunohistochemical Analysis
A brown nuclear staining was considered as positive for EZH2 and H3K27me3 immunoreactivity. At least 1000 epithelial cells or epithelial tumor cells were counted from five discrete representative areas at high magnification (×400). The percentage of positive-staining cells per 1000 counted cells was regarded as labeling index (LI) [11]. All the immunostained sections were independently evaluated by two oral pathologists (PS and PL). In the present study, the inter-observer reliability was 94% and 87% for EZH2 and H3K27me3, respectively. The slides with discrepant results were re-evaluated together, and a consensus was reached in all cases.
Statistical Analysis
The data was analyzed statistically using SPSS (version 18.0; SPSS Inc., Chicago, IL). The inter-observer reliability was assessed by intraclass correlation coefficient. Differences among groups were evaluated by ANOVA, followed by Tukey HSD pairwise comparisons. Receiver operating characteristic (ROC) curve analysis was performed to determine cutoff score between OVH and OVC as well as OVC and OSCC. The score was selected as the cutoff value which was closest to the point with both maximum sensitivity and specificity. The Pearson correlation analysis was used to evaluate a correlation between the expression of EZH2 and H3K27me3. The level of statistical significance is 0.05.
Results
Demographic and Clinical Characteristics of OVH, OVC and OSCC
Seventy-eight samples, including 10 cases of control group (normal oral mucosa), 8 cases of OVH, 25 cases of OVC and 35 cases of OSCC, were included in our study. Table 1 presents demographic and clinical characteristics of patients with OVH, OVC and OSCC. Table S1 shows clinical presentations and types of specimens of OVH and OVC cases. Most patients with OVH were presented with an average age of 70.5 years (range 59–84) and a female predominance (M:F = 1:3). A half of OVH was presented at the buccal mucosa, followed by the gingiva/alveolar mucosa (37.5%). The majority of patients with OVC were in the eighth decade of life, with an average age of 76.1 years (range 54–103) and a female predilection (M:F = 1:4). OVC were mostly found in the buccal mucosa (32.0%), followed by the gingiva/alveolar mucosa (20.0%) and the tongue (16.0%). The average age of OSCC patients was 57.37 years (range 42–89) and a slightly female predilection was observed among OSCC patients. The most common site for OSCC was the tongue (37.1%), followed by the gingiva/alveolar mucosa (31.4%).
Table 1.
Demographic and clinical characteristics of patients with OVH, OVC and OSCC
| Characteristics | OVH (n = 08) |
OVC (n = 25) |
OSCC (n = 35) |
|---|---|---|---|
| Age | |||
| Mean | 70.5 | 76.1 | 57.37 |
| SD | 8.33 | 10.52 | 9.89 |
| Range | 59–84 | 54–103 | 42–89 |
| Sex | |||
| Male | 2 (25.0%) | 5 | 17 |
| Female | 6 (75.0%) | 20 | 18 |
| Site | |||
| Buccal mucosa | 4 (50.0%) | 8 (32.0%) | 5 (14.3%) |
| Gingiva/Alveolar mucosa | 3 (37.5%) | 5 (20.0%) | 11 (31.4%) |
| Tongue | 0 (0.0%) | 4 (16.0%) | 13 (37.1%) |
| Lip | 0 (0.0%) | 3 (12.0%) | 0 (0.0%) |
| Palate | 0 (0.0%) | 2 (8.0%) | 4 (11.4%) |
| Floor of mouth | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) |
| Retromolar area | 1 (12.5%) | 0 (0.0%) | 1 (2.9%) |
| Multiple sites | 0 (0.0%) | 3a (12.0%) | 0 (0.0%) |
aTwo cases were located at the lip and buccal mucosa whereas the other case was located at the ginigiva and buccal mucosa
EZH2 and H3K27me3 Expression in Normal Oral Mucosa, OVH, OVC and OSCC
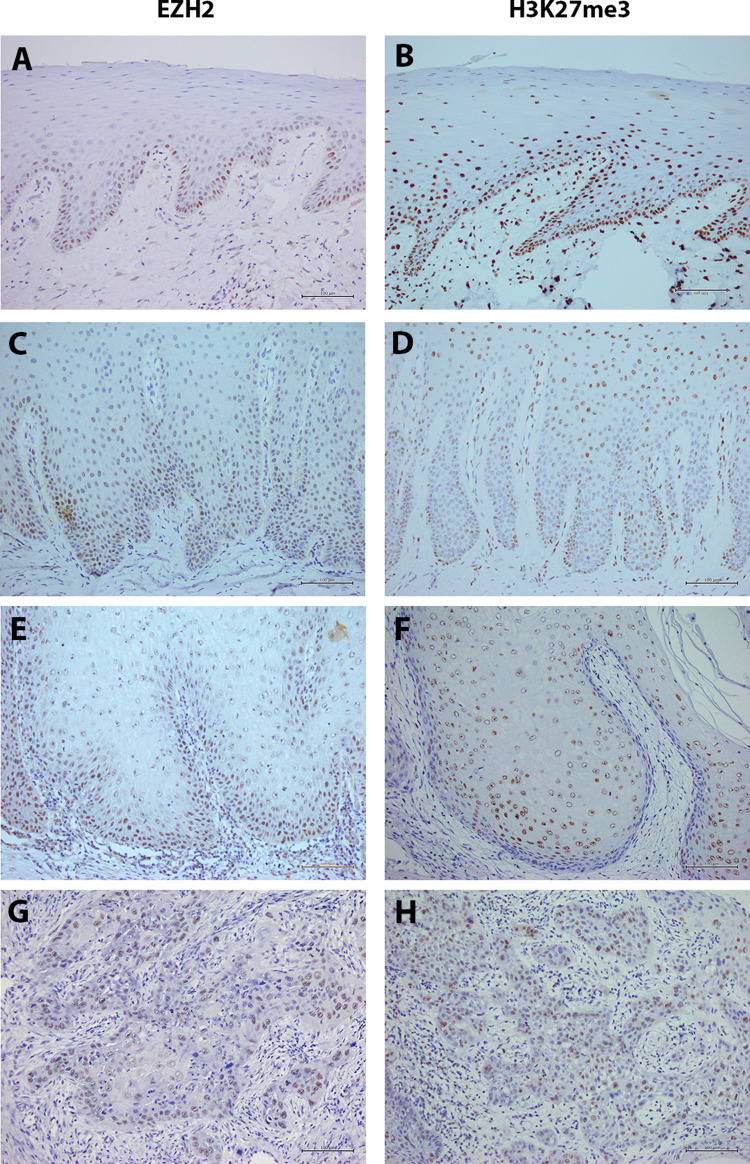
Immunoreactivity for EZH2 and H3K27me3 was detected in the nuclei of normal epithelial cells, epithelial cells of OVH, and tumor cells of OVC and OSCC (Fig. 1). In the normal oral epithelium, EZH2-positive cells were predominantly observed in the basal cell layer and a few cells in the parabasal layer (Fig. 1a). In OVH, EZH2-positive cells were observed in the basal and extend to the lower prickle cell layer (Fig. 1c), while in OVC EZH2 is diffusely expressed from the basal cell layer to the prickle cell layer (Fig. 1e). In contrast, in OSCC samples EZH2-positive cells were found frequently in the peripheral cells of tumor nests, but no positive cell was found in keratin pearls (Fig. 1g). Similar to EZH2, immunoreactivity for H3K27me3 was observed in the nuclei of normal epithelial cells, epithelial cells of OVH, and tumor cells of OVC and OSCC. In the control group of normal oral epithelium, H3K27me3 were more dispersed compared to EZH2 expression (Fig. 1b). It was found in the basal and the lower prickle cells. In OVH, OVC and OSCC, H3K27me3 expression pattern was relatively similar to EZH2 expression (Fig. 1d, f, h).
Fig. 1.
Representative immunohistochemical staining for EZH2 in a Normal oral epithelium, c OVH, e OVC, g OSCC; for H3K27me3 in b Normal oral epithelium, d OVH, f OVC, h OSCC. (Original magnification, ×20)
Table 2 presents the mean LIs of EZH2 and H3K27me3 in normal oral epithelium, OVH, OVC and OSCC. We found that the mean LI of EZH2–positive cells in normal oral mucosa, OVH, OVC and OSCC was 31.36 ± 3.0, 47.53 ± 4.1, 65.62 ± 2.2 and 75.05 ± 1.3, respectively. Statistically significant differences were observed among these lesions. The EZH2 LI in OSCC was significantly higher than that in OVC (p = 0.002), OVH (p < 0.001), and normal oral mucosa (p < 0.001), respectively. The LI of EZH2 expression was significantly higher in OVC than in OVH (p < 0.001) and normal oral mucosa (p < 0.001), respectively. Statistical difference was also observed between EZH2 LI in OVH and in normal oral mucosa (p = 0.003).
Table 2.
The mean labeling indices of EZH2 and H3K27me3 in normal oral mucosa, OVHs, OVCs and OSCCs
| Diagnosis | Number of case | EZH2 LI (mean ± SE) |
H3K27me3 LI (mean ± SE) |
|---|---|---|---|
| Normal oral mucosa | 10 | 31.36 ± 3.0a | 42.85 ± 4.0a |
| OVH | 8 | 47.53 ± 4.1b | 44.89 ± 4.7a,b |
| OVC | 25 | 65.62 ± 2.2c | 57.55 ± 2.5b,c |
| OSCC | 35 | 75.05 ± 1.3d | 65.55 ± 2.6c |
a−d Different letters indicate significant difference between the diagnosis groups
For H3K27me3, the mean LIs of H3K27me3 was highest in OSCC (65.55 ± 2.6), followed by OVC (57.55 ± 2.5), OVH (44.89 ± 4.7) and normal oral mucosa (42.85 ± 4.0), respectively (Table 2). The H3K27me3 LI in OSCC was significantly higher than that in OVH (p = 0.002) and normal oral mucosa (p < 0.001), respectively. Additionally, the H3K27me3 LI in OVC was also significantly higher than that in normal oral mucosa (p = 0.031). No significant difference of H3K27me3 LI was present in the remaining compared groups (OSCC vs. OVC and OVC vs. OVH).
EZH2 Expression for Differentiating Between OVH Versus OVC and OVC Versus OSCC
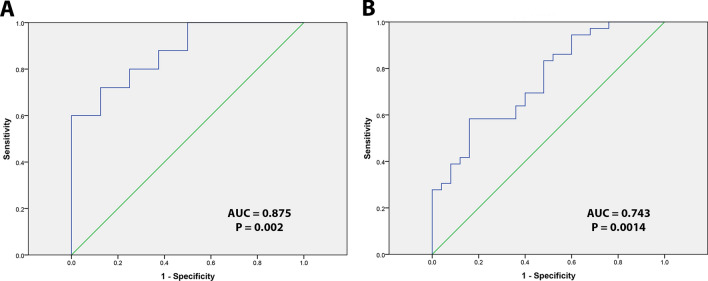
Figure 2 presents receiver operating characteristic (ROC) curve analysis for evaluating diagnostic test between OVH versus OVC and OVC versus OSCC. The cut-off value for EZH2 expression between OVC and OVH was 59.30%. Diagnostic test analysis showed that the sensitivity was 72.00% and specificity was 87.50% with a positive predictive value of 94.73% and negative predictive value of 50.00%.
Fig. 2.
Receiver operating characteristic curve analysis was employed to determine the cutoff score for the high EZH2 LI. The sensitivity and specificity for differentiating OVH versus OVC (a) and for differentiating OVC versus OSCC (b) were plotted
Between OVC and OSCC, the cut-off level of EZH2 LI was 74.84%. Diagnostic test analysis showed that the sensitivity was 57.14% and specificity was 84.00% with a positive predictive value of 83.33% and negative predictive value of 58.34%.
Correlation Between the Expression of EZH2 and H3K27me3 in OVH, OVC and OSCC
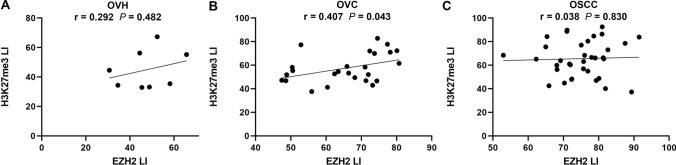
We further evaluate the potential correlation between expression of EZH2 and H3K27me3 in OVH, OVC and OSCC. We found that EZH2 expression showed a positive correlation with H3K27me3 expression in OVC (r = 0.407, p = 0.043) (Fig. 3) whereas no correlation between EZH2 and H3K27me3 expression was significantly observed in OVH (r = 0.29, p = 0.48) and OSCC (r = 0.038, p = 0.83).
Fig. 3.
Scatterplot showing the correlation between EZH2 LI and H3K27me3 LI in OVH (a), OVC (b) and OSCC (c) samples
Discussion
EZH2 has been known to epigenetically silence tumor-suppressor genes by catalyzing tri-methylation of histone H3 at Lys 27 (H3K27me3). It is involved in the development and progression of several human malignancies such as melanoma, endometrial, lung, and prostate cancer [2]. EZH2 was strongly associated with advanced stage and poor prognosis in squamous cell malignancies including cervical cancer [13], esophageal squamous cell carcinoma [14], and head and neck cancer [15]. The expression of EZH2 has never been studied in oral verrucous carcinoma and oral verrucous hyperplasia samples. In the present study, we found that EZH2 staining was present differently in the nuclei of the epithelial cells of these lesions. In normal mucosa, EZH2-positive cells were primarily located in the basal cell layer. Similar result was also reported by Kidani et al. [4]. The basal cells are known to possess the ability to divide. After dividing, the divided cells undergo differentiation process, move superficially and are ultimately sloughed off the surface [16]. This result suggests that EZH2 may play a role in cell proliferation or differentiation of the oral epithelium. To support this notion, EZH2-positive cells in OSCC were observed frequently in the peripheral cells of tumor nests. This EZH2 expression corresponds to the expression pattern of Ki-67. Two previous studies showed that the peripheral cells of tumor nests expressed Ki-67, whereas the central cells with ability of keratinization showed no Ki-67 expression [4, 17]. In OVH, EZH2-positive cells were observed in the basal and the parabasal cell layer, while in OVC EZH2 positive cells were found extending from the basal cells to the upper part of prickle cell layer. This staining pattern of EZH2 also resembles to that of Ki-67 expression in OVC and OVH [18]. The higher expression of EZH2 in OVC compared to OVH, suggests that EZH2 may play a role in the pathogenesis of OVH and OVC and reflect the progression from benign to malignant epithelial tumor.
A previous study found that the LI of EZH2 was highest in OSCC, followed by epithelial dysplasia and normal mucosa with significant differences [4]. In agreement with previous reports, we found that the mean LI of EZH2–positive cells was highest in OSCC, followed by OVC, OVH and normal epithelium, respectively. These results indicate that EZH2 is possibly related to the proliferative activity of oral epithelial tumor cells and further support that EZH2 functions as an oncogene in oral epithelial malignancies. Additionally, increased expression of EZH2 in these verrucous lesions may indicate disease progression and EZH2 LI of OVC being between OVH and OSCC may contribute to its low aggressiveness of this rare cancer. However, the mean LI of EZH2 in OSCC (75.05 ± 1.3) and normal oral mucosa (31.36 ± 3.0) in our study is higher than that in Kidani’s study (50.7 ± 2.1 and 19.4 ± 1.4, respectively) [4]. This difference could be attributed to different primary antibody used in immunohistochemical staining as well as different specimen cohort.
As with EZH2, H3K27me3 were also overexpressed in OVH, OVC and OSCC. The expression of EZH2 in our specimens is relatively similar to that of H3K27me3 as the mean H3K27me3 LIs was highest in the OSCC, followed by OVC, OVH and the normal mucosa. To our knowledge, there has been no known report studying any histone modifications in OVH and OVC. For OSCC, the previous report has shown that the high level of H3K27me3 was positively correlated with OSCC stage [19]. Although high expression of H3K27me3 was observed in these lesions, a positive correlation between EZH2 and H3K27me3 labeling indices was found in only OVC specimens whereas the correlation between EZH2 and H3K27me3 was not significantly present in normal mucosa, OVH and OSCC. These results point out that the molecular mechanism involved in the EZH2 regulation are different but may be partly overlapped among these lesions. EZH2-mediated H3K27me3 may contribute to the pathogenesis of OVC while EZH2 regulation in OSCC may be more complicated and not solely involved its methyltransferase activity. Our results are also consistent with a previous study conducting protein levels of EZH2 and H3K27me3 in a tissue microarray of 59 patients with Head and Neck SCC and 12 normal oral epithelial tissue sections. No association between the high levels of EZH2 and H3K27me3 was found in their SCC cohort [20]. In the literature, the correlations of EZH2 and H3K27me3 in human cancers were different depending on cancer type. For example, a positive correlation between EZH2 and H3K27me3 was found in esophageal carcinoma [14] whereas an inverse correlation was observed in extranodal NK/T-cell lymphoma [21]. Nevertheless, some limitations still exist in the present study including the small number of OVH and OVC samples, Further study with a larger sample cohort is warranted.
Difficulty can be incurred for pathologists to distinguish either OVC from OVH or OVC from OSCC. Frequently, these entities are clinically similar and establishing the histopathological diagnosis may be troublesome due to poorly oriented tissue sections, small size of tissue specimens and devoid of the adjacent normal tissue [10, 11, 22]. However, it is necessary to distinguish these lesions since these lesions ranged from potentially malignant lesions to oral malignancies and possess different biological behaviors and prognosis [22]. A systematic review revealed several diagnostic biomarkers that have been published and could be applied for differentiating these oral verrucous lesions. These molecular biomarkers mostly studied are proliferative and apoptotic biomarkers such as p53, Ki67, PCNA and Cyclin D1 [22]. Our result showing statistically significant differences of EZH2 LI among these oral lesions raises a possibility of using EZH2 to help distinguishing OVH from OVC in difficult cases. From our diagnostic test analysis, EZH2 LI showed high specificity (87.5%) and acceptable sensitivity (72%) in distinguishing OVH from OVC. Using EZH2 to differentiate OVC from OSCC may not be applicable due to its low sensitivity (57.14%). However, an adequate surgical specimen with adequate depth and adjacent normal epithelium is still the gold standard for establishing the definite diagnosis of these lesions. EZH2 has previously been proposed for differential diagnosis in several diseases, particularly differential diagnosis between malignant and benign tumors [23, 24]. For example, in malignant and benign myogenic tumors, EZH2 protein was suggested to use for differentiating well-differentiated leiomyosarcoma from cellular leiomyoma with a sensitivity of 91.30% and specificity of 100% [23].
In summary, our findings demonstrate that EZH2 and H3K27me3 are overexpressed in OVH and OVC, suggesting the involvement of epigenetic regulation by EZH2 and H3K27me3 in the pathogenesis of these oral verrucous lesions. Expression of EZH2 and H3K27me3 was highest in OSCC, followed by OVC, OVH and normal mucosa, respectively. A positive correlation of EZH2 and H3K27me3 was observed in OVC, indicating the canonical EZH2 regulation in OVC. In addition, EZH2 LI may be helpful to facilitate the differential diagnosis between OVH and OVC.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 15 kb)
Acknowledgements
This work was supported by International Dental Collaboration of The Mekong River Region (IDCMR) scholarship, Faculty of Dentistry, Mahidol University.
Abbreviations
- EZH2
Enhancer of zeste homolog 2
- H3K27me3
Histone 3 lysine 27 trimethylation
- OVH
Oral verrucous hyperplasia
- OVC
Oral verrucous carcinoma
- OSCC
Oral squamous cell carcinoma
- LI
Labeling index
Compliance with Ethical Standards
Conflict of interest
All authors have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 2.Christofides A, Karantanos T, Bardhan K, Boussiotis VA. Epigenetic regulation of cancer biology and anti-tumor immunity by EZH2. Oncotarget. 2016;7(51):85624–40. doi: 10.18632/oncotarget.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647(1–2):21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Kidani K, Osaki M, Tamura T, Yamaga K, Shomori K, Ryoke K, et al. High expression of EZH2 is associated with tumor proliferation and prognosis in human oral squamous cell carcinomas. Oral Oncol. 2009;45(1):39–46. doi: 10.1016/j.oraloncology.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Rekha KP, Angadi PV. Verrucous carcinoma of the oral cavity: a clinico-pathologic appraisal of 133 cases in Indians. Oral Maxillofac Surg. 2010;14(4):211–8. doi: 10.1007/s10006-010-0222-0. [DOI] [PubMed] [Google Scholar]
- 6.Koch BB, Trask DK, Hoffman HT, Karnell LH, Robinson RA, Zhen W, et al. National survey of head and neck verrucous carcinoma: patterns of presentation, care, and outcome. Cancer. 2001;92(1):110–20. doi: 10.1002/1097-0142(20010701)92:1<110::aid-cncr1298>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Walvekar RR, Chaukar DA, Deshpande MS, Pai PS, Chaturvedi P, Kakade A, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases. Oral Oncol. 2009;45(1):47–51. doi: 10.1016/j.oraloncology.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Shear M, Pindborg JJ. Verrucous hyperplasia of the oral mucosa. Cancer. 1980;46(8):1855–62. doi: 10.1002/1097-0142(19801015)46:8<1855::aid-cncr2820460825>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Wang YP, Chen HM, Kuo RC, Yu CH, Sun A, Liu BY, et al. Oral verrucous hyperplasia: histologic classification, prognosis, and clinical implications. J Oral Pathol Med. 2009;38(8):651–6. doi: 10.1111/j.1600-0714.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu LK, Ding YW, Liu W, Zhou YM, Shi LJ, Zhou ZT. A clinicopathological study on verrucous hyperplasia and verrucous carcinoma of the oral mucosa. J Oral Pathol Med. 2012;41(2):131–5. doi: 10.1111/j.1600-0714.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin HP, Wang YP, Chiang CP. Expression of p53, MDM2, p21, heat shock protein 70, and HPV 16/18 E6 proteins in oral verrucous carcinoma and oral verrucous hyperplasia. Head Neck. 2011;33(3):334–40. doi: 10.1002/hed.21452. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira DT, de Moraes RV, Fiamengui Filho JF, Fanton Neto J, Landman G, Kowalski LP. Oral verrucous carcinoma: a retrospective study in Sao Paulo Region, Brazil. Clin Oral Investig. 2006;10(3):205–9. doi: 10.1007/s00784-006-0050-7. [DOI] [PubMed] [Google Scholar]
- 13.Jin M, Yang Z, Ye W, Yu X, Hua X. Prognostic significance of histone methyltransferase enhancer of zeste homolog 2 in patients with cervical squamous cell carcinoma. Oncol Lett. 2015;10(2):857–62. doi: 10.3892/ol.2015.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Gu L, Cao Y, Fan X, Zhang F, Sang M. Aberrant overexpression of EZH2 and H3K27me3 serves as poor prognostic biomarker for esophageal squamous cell carcinoma patients. Biomarkers. 2016;21(1):80–90. doi: 10.3109/1354750x.2015.1118537. [DOI] [PubMed] [Google Scholar]
- 15.Chang JW, Gwak SY, Shim GA, Liu L, Lim YC, Kim JM, et al. EZH2 is associated with poor prognosis in head-and-neck squamous cell carcinoma via regulating the epithelial-to-mesenchymal transition and chemosensitivity. Oral Oncol. 2016;52:66–74. doi: 10.1016/j.oraloncology.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Jones KB, Klein OD. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int J Oral Sci. 2013;5(3):121–9. doi: 10.1038/ijos.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takkem A, Barakat C, Zakaraia S, Zaid K, Najmeh J, Ayoub M, et al. Ki-67 prognostic value in different histological grades of oral epithelial dysplasia and oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2018;19(11):3279–86. doi: 10.31557/apjcp.2018.19.11.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klieb HB, Raphael SJ. Comparative study of the expression of p53, Ki67, E-cadherin and MMP-1 in verrucous hyperplasia and verrucous carcinoma of the oral cavity. Head Neck Pathol. 2007;1(2):118–22. doi: 10.1007/s12105-007-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YW, Kao SY, Wang HJ, Yang MH. Histone modification patterns correlate with patient outcome in oral squamous cell carcinoma. Cancer. 2013;119(24):4259–67. doi: 10.1002/cncr.28356. [DOI] [PubMed] [Google Scholar]
- 20.Gannon OM, Merida de Long L, Endo-Munoz L, Hazar-Rethinam M, Saunders NA. Dysregulation of the repressive H3K27 trimethylation mark in head and neck squamous cell carcinoma contributes to dysregulated squamous differentiation. Clin Cancer Res. 2013;19(2):428–41. doi: 10.1158/1078-0432.ccr-12-2505. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Liang L, Huang S, Nong L, Li D, Zhang B, et al. Aberrant differential expression of EZH2 and H3K27me3 in extranodal NK/T-cell lymphoma, nasal type, is associated with disease progression and prognosis. Hum Pathol. 2019;83:166–76. doi: 10.1016/j.humpath.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinpour S, Mashhadiabbas F, Ahsaie MG. Diagnostic biomarkers in oral verrucous carcinoma: a systematic review. Pathol Oncol Res. 2017;23(1):19–32. doi: 10.1007/s12253-016-0150-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, Zeng Z, Li S, Wang F, Huang P. High expression of EZH2 as a marker for the differential diagnosis of malignant and benign myogenic tumors. Sci Rep. 2018;8(1):12331. doi: 10.1038/s41598-018-30648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinozaki-Ushiku A, Ushiku T, Morita S, Anraku M, Nakajima J, Fukayama M. Diagnostic utility of BAP1 and EZH2 expression in malignant mesothelioma. Histopathology. 2017;70(5):722–33. doi: 10.1111/his.13123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 15 kb)