Abstract
Parathyroid carcinoma (PC) is a rare malignancy that poses a diagnostic challenge on histologic examination. We analyzed various clinicopathologic features of PC. Pathology reports and slides were reviewed to evaluate the diagnostic histopathologic features of archived cases of PC from the years of 2004–2018. The study cohort comprised twenty cases of PC. The median age was 49 years (range 21–73 years) with equal gender distribution (M:F = 1:1). Most patients presented with symptoms of hypercalcemia (n = 7, 54%). Serum calcium and serum parathyroid hormone were elevated in all but one patient. The right inferior parathyroid was commonly involved (n = 8/14, 57%). The mean tumor size was 2.4 cm (range 0.8–3.5 cm). On frozen section examination, PC was diagnosed in 8 out of 9 cases. Vascular (n = 19/20, 95%) and soft tissue invasion (n = 10/20, 50%) were the most common characteristic histologic findings. Capsular invasion was identified in all cases. Perineural invasion or metastasis at presentation was absent in all cases. Other histological features noted were intratumoral fibrous bands (70%), nodular growth pattern (70%), moderate nuclear atypia (30%), prominent nucleoli (20%), and necrosis (20%). Regional lymph nodes were negative for metastatic disease in all cases (n = 10). Eight out of 16 patients received adjuvant radiotherapy. Follow-up was available in 16 cases (median 21.5 months). Two patients died of disease. Vascular and soft tissue invasion are the most common diagnostic histologic features of PC. Capsular invasion is important to distinguish PC from its benign counterparts. Intraoperative frozen section examination can be used for accurate diagnosis and surgical management.
Keywords: Parathyroid carcinoma, Histopathology, Diagnostic criteria, Vascular invasion, Soft tissue invasion, Capsular invasion
Introduction
The spectrum of parathyroid lesions comprises parathyroid hyperplasia, parathyroid adenoma (PA), and the rare parathyroid carcinoma (PC). It is challenging to differentiate PC from PA based on clinicoradiologic findings and biochemical assay [1]. As a rare cause of primary hyperparathyroidism (PHPT), PC may be operated on under a presumptive diagnosis of PA [2, 3]. This is problematic as conservative surgery for PC is associated with a recurrence rate 13 times higher compared to en-bloc resection [4]. Often, the diagnosis of PC is made post-surgically on histopathologic examination [5]. A precise histopathologic diagnosis is pivotal in the management of PC; however, likely due to its rarity, no set of diagnostic criteria is universally accepted [5–7]. The data on PC is predominantly in the form of case reports and smaller case series. Moreover, even amongst larger series, only a few publications have evaluated the histopathologic characteristics [6–12]. The recently published World Health Organization (WHO) Classification of Tumors of Endocrine Organs has adapted diagnostic criteria proposed by Rosai and DeLellis. These criteria suggest the diagnosis of PC should be restricted to tumors that show evidence of invasive growth involving adjacent structures (thyroid or soft tissue), capsular and extracapsular blood vessels, perineural space, and/or to those with documented metastases [13, 14]. Being a tertiary cancer care hospital, we cater only to oncology patients and have a large database of various cancer types. We took this opportunity to review the histopathologic findings of PC cases diagnosed at our center and compare the results with the above proposed WHO recommendation.
Materials and Methods
A search query for the words ‘parathyroid and carcinoma’ was run on the Department of Pathology search engine. Cases of PC diagnosed between the years 2004–2018 were retrieved from the department’s archives. Clinical details obtained from the electronic medical records included age, sex, presenting symptoms, baseline serum calcium levels, baseline serum parathyroid hormone (PTH) levels, tumor (T)-size (radiographic T-size if pathologic T-size was unavailable), location of gland involved, date and type of surgery performed, details of adjuvant treatment received, details of recurrence and metastasis (upfront or subsequent), date of the last follow-up, outcomes, and any other concurrent pathology.
The histopathology slides [hematoxylin and eosin (HE)] were retrieved and reviewed by two pathologists (AP and AS), one of them a full-time dedicated head and neck pathologist (AP). The features noted from the histopathology reports were: T-size, frozen section diagnosis (if available), and the presence/absence of the histopathologic criteria proposed by Rosai et al.: (1) infiltration of adjacent structures (thyroid or soft tissue), (2) capsular/extracapsular vascular space invasion, and (3) perineural space invasion (PNI) [14]. Vascular invasion was identified when the capsular/extracapsular lymphovascular spaces had tumor covered with endothelium or when the tumor was adherent to the vessel wall with thrombus formation. Besides, the presence/absence of capsular invasion was also noted. Capsular invasion was defined by tumor invasion through the capsule and into adjacent soft tissue. Pseudoinvasion, invasion into the capsule or tumor cell entrapment within the capsule, was not classified as a capsular invasion. Slide review confirmed the presence/absence of these histopathologic criteria as well as the following features: tumor growth pattern (nodular, solid, trabecular), intratumoral fibrous bands, nuclear atypia (moderate or marked), prominent nucleoli, mitotic activity, necrosis, and lymph nodes assessment (when available). The consensus was reached by discussion and joint evaluation in discrepant cases.
Results
We reviewed a total of 20 cases with archived slides available. Of these, 16 cases had follow-up data: 10 were treated surgically at our institute and 6 were treated at other facilities with pathology material received for consultation/second opinion. The clinicopathologic details of individual patients are summarized in Table 1 and major clinicopathologic characteristics are summarized in Table 2. The median patient age was 49 years (range 21 to 73 years; interquartile range 41.75 to 56.5 years). There was no gender predominance with a male to female ratio of 1:1. Most of the patients (n = 7, 54%) presented with symptoms related to hypercalcemia such as anorexia, muscle, and joint pain, vomiting, and renal stones. Two additional patients (15%) presented with symptoms related to hyperparathyroidism in the form of jaw swelling and multiple lytic bony lesions. Two patients (15%) were diagnosed with parathyroid tumors after being evaluated for incidentally detected hypercalcemia while two others (15%) presented with a neck swelling.
Table 1.
Clinical, demographic and pathological data of the patients in this study
| Sr. no | Age (yr) | Sex | Ca level (mg/dL) | PTH (ng/L) | Symptoms | Max. size | FS | Pattern | Nuclear atypia and nucleoli | Fibrous band |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | 12.35 | 850 | Case of PA; muscle pain, anorexia | 1.8 | Favor PC | Nodular | Absent | + |
| 2 | 46 | F | 15.63 | 1098 | Right jaw swelling | 2.5 | Favor PC | Nodular | Moderate atypia, macronucleoli | + |
| 3 | 50 | M | 11.9 | 1389 | Backache, joint pain, left neck swelling | 3 | Favor PA | Nodular | Absent | + |
| 4 | 23 | F | 13.33 | 910 | Right shoulder pain | 3 | PC | Solid | Absent | − |
| 5 | 51 | F | NA | NA | NA | NA | ND | Nodular | Absent | + |
| 6 | 41 | F | 8.5 | 82 | Right neck swelling | 3.5 | Favor PC | Nodular | Nucleoli | + |
| 7 | 73 | F | 11.8 | 263 | Incidental d/t raise Ca | 2.5 | Favor PC | Nodular | Absent | + |
| 8 | 62 | M | 15.9 | 1150 | Right humerus fracture | 2 | ND | Nodular | Moderate atypia, macronucleoli | + |
| 9 | 32 | M | 15.8 | 1861 | Shoulder & back pain | 3.5 | ND | Solid | Moderate atypia | − |
| 10 | 25 | M | 17.8 | 1072 | Abdominal pain & vomiting | 3.5 | Favor PC | Nodular | Absent | + |
| 11 | 61 | F | 12.2 | 365 | Joint pain, lethargy, renal stones | 0.8 | Favor PC | Nodular | Moderate atypia | + |
| 12 | 53 | M | 13.9 | 363 | Incidental d/t raise Ca | 1.9 | ND | Nodular | Absent | + |
| 13 | 49 | M | 12.1 | 971 | Multiple lytic lesion | 1.4 | Favor PC | Solid | Moderate atypia | − |
| 14 | 48 | F | NA | NA | NA | NA | ND | Nodular | Absent | + |
| 15 | 53 | F | 14.4 | NA | Right flank pain, renal stone | NA | NA | Solid | Marked atypia | − |
| 16 | 42 | F | NA | NA | NA | NA | NA | Nodular | Absent | + |
| 17 | 55 | F | NA | NA | NA | NA | NA | Nodular | Absent | + |
| 18 | 21 | M | NA | NA | NA | NA | NA | Solid | Moderate atypia, nucleoli | − |
| 19 | 42 | M | NA | NA | NA | 1.5 | ND | Solid | Absent | − |
| 20 | 63 | M | NA | NA | NA | 2.4 | NA | Nodular | Moderate atypia | + |
| Sr. no | Mitosis (/10HPF) | Necrosis | Cap. inv | Vas. Inv | ST invasion | Gland involved | Adj treatment | Follow-up (months) | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Absent | − | + | + | − | Right inf | Adj RT | 09 | Alive | |
| 2 | < 1 | − | + | + | + | Left Ectopic (upper med) | No RT | 41 | Alive | |
| 3 | < 1 | − | + | + | − | Left inf PC | No RT | 58 | Alive | |
| 4 | < 1 | − | + | + | − | Left inf | No RT | 36 | Alive | |
| 5 | < 1 | − | + | + | + | Left | No RT | 25 | Alive | |
| 6 | 1–2 | + large | + | + | − | Right inf | Adj RT, later Pall RT | 06 | DOD | |
| 7 | 3–4 | − | + | + | + | Right inf | Adj RT | 19 | Alive | |
| 8 | < 1 | − | + | + | + | Right inf | Adj RT | 44 | DOD | |
| 9 | 1–2 | − | + | + | − | Right inf | No RT | 12 | Alive | |
| 10 | < 1 | + focal | + | + | + | Right inf | Adj RT | 24 | Alive | |
| 11 | Absent | − | + | + | − | Right sup | Adj RT | 12 | Alive | |
| 12 | Absent | − | + | + | + | Left inf | Adj RT | 10 | Alive | |
| 13 | < 1 | − | + | + | + | Right inf | No RT | 09 | Alive | |
| 14 | < 1 | − | + | − | − | Right | No RT | 40 | Alive | |
| 15 | < 1 | − | + | + | + | Right inf | No RT | 10 | Alive | |
| 16 | < 1 | − | + | + | + | NA | NA | NA | NA | |
| 17 | < 1 | − | + | + | + | Left inf | Pall RT | 16 | Alive | |
| 18 | < 1 | + focal | + | + | − | NA | NA | NA | NA | |
| 19 | < 1 | − | + | + | − | NA | NA | NA | NA | |
| 20 | 1–2 | + focal | + | + | − | NA | NA | NA | NA | |
Adj, adjuvant; Ca, calcium; Cap, capsular; DOD, dead of disease; d/t, due to; FS, frozen section; Inf, inferior; Max, maximum; Med, mediastinal; NA, not available; ND, not done; PA, parathyroid adenoma; Pall, palliative; PC, parathyroid carcinoma; PTH, paratharmone; RT, radiotherapy; ST, soft tissue; sup, superior; Vas, vascular; Yr, years; +, present; −, absent; /10HPF, per 10 high power fields
Table 2.
Major clinicopathological characteristics and their frequencies in this study
| Parameter | Data |
|---|---|
| Age | |
| Range | 21 to 73 years |
| Median | 49 years |
| Sex | |
| Male | 10/20 (50%) |
| Female | 10/20 (50%) |
| Tumor location | |
| Right inferior parathyroid | 08/14 (57%) |
| Left inferior parathyroid | 04/14 (29%) |
| Right superior parathyroid | 01/14 (07%) |
| Left ectopic parathyroid (upper mediastinum) | 01/14 (07%) |
| Tumor laterality | |
| Right | 10/15 (67%) |
| Left | 05/15 (33%) |
| Frozen section diagnosis | |
| Parathyroid carcinoma | 08/09 (89%) |
| Parathyroid adenoma | 01/09 (11%) |
| Histopathological features | |
| Growth pattern | |
| Nodular | 14/20 (70%) |
| Solid | 06/20 (30%) |
| Capsular invasion | 20/20 (100%) |
| Vascular invasion | 19/20 (95%) |
| Fibrous bands | 14/20 (70%) |
| Soft tissue infiltration | 10/20 (50%) |
| Necrosis | 04/20 (20%) |
| Nuclear atypia | |
| Moderate | 06/20 (30%) |
| Marked | 02/20 (10%) |
| Prominent nucleoli | 04/20 (20%) |
| Mitosis | |
| Not seen | 03/20 (15%) |
| < 1/10HPF | 13/20 (65%) |
| > 1/10HPF | 04/20 (20%) |
| Adjuvant radiotherapy | 08/16 (50%) |
| Local recurrence | 01/16 (06%) |
| Metastasis | 02/16 (12%) |
| Outcome | |
| Alive | 14/16 (88%) |
| Dead | 02/16 (12%) |
Serum calcium and serum PTH levels were available in 13 and 12 patients, respectively. Serum calcium was elevated in 12 patients and serum PTH was increased in 11 patients. The mean serum calcium level was 13.51 mg/dL and the mean serum PTH level was 864.50 ng/L (Table 1). In the one non-secretory PC, serum calcium and serum PTH levels were 8.5 mg/dL and 82 ng/L, respectively. The tumor involved inferior parathyroid glands in 12 cases and the right inferior parathyroid (n = 8/12, 67%) was involved more often than the left inferior parathyroid (n = 4/12, 33%). The superior parathyroid (right) was involved in one case and a left-sided ectopic parathyroid gland in the upper mediastinum was involved in another. Overall, right-sided tumors were seen in 67% of cases (n = 10/15) while left-sided glands were involved in 33% cases (n = 5/15). Two patients had a prior history of tumor excision of the ipsilateral parathyroid gland with diagnoses of PA outside our hospital; however, slides were not available for review in either of the cases. Two patients were diagnosed case of multiple endocrine neoplasia type 1 (MEN1) syndrome. Two patients had a history of concurrent contralateral PA; in one of these cases, a known case of MEN1 syndrome, a PA had apparently progressed to PC after 2 years. Both the diagnoses (PA and PC) were made outside our institute. One patient had concurrent contralateral papillary thyroid carcinoma (PTC).
The tumor size was known in 14 cases and ranged from 0.8 to 3.5 cm with a mean (± standard deviation) of 2.4 (± 0.88) cm. Frozen section examination was obtained for the primary diagnosis in 9 cases. The diagnosis of PC was accurately suggested in 8 cases while PA was favored in one case. The specimen received for frozen section examination was an en-block (along with the thyroid gland) parathyroid lesion resection in 5 cases, while the parathyroid lesion excision alone was received in the remaining 4 cases (the resection of thyroid gland followed immediately in the same sitting). The specimen was sliced and the cut surface was grossly examined for foci suggestive of invasion. Tissue from suspicious foci was sampled for microscopic examination. The number of sections studied to arrive at a diagnosis of PC was 1 (n = 3, 37%), 2 (n = 4, 50%), or 3 (n = 1, 13%). Although the diagnosis was based on a combination of histopathologic findings, the essential diagnostic features for PC were vascular invasion (n = 4, 50%) and soft tissue infiltration (n = 3, 37%). In one case, a diagnosis of PC was suggested based on the presence of marked nuclear atypia and necrosis.
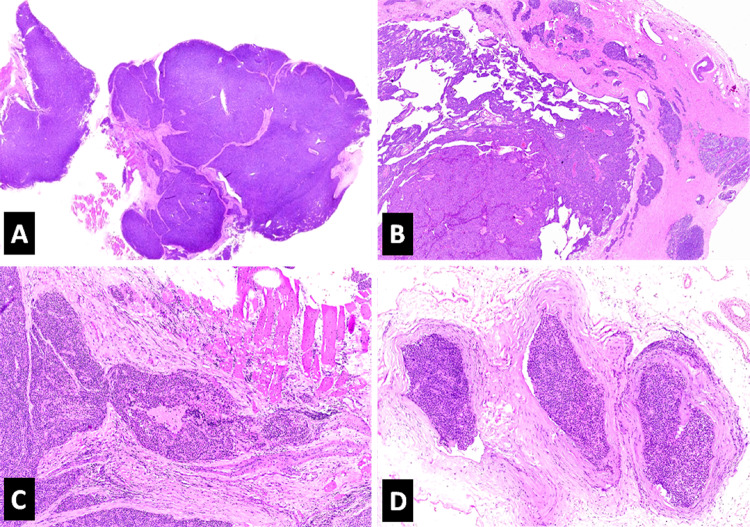
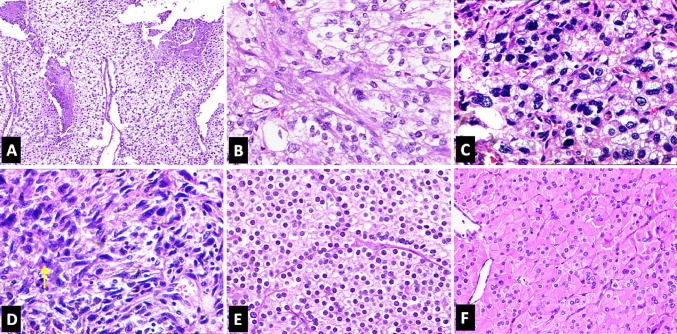
On final histopathologic examination, foci of capsular invasion were identified in all 20 tumors. The next most common diagnostic feature was vascular invasion which was seen in 19 out of the 20 cases (95%) (Fig. 1). Invasion into the adjacent soft tissue was documented in half of the cases (Fig. 1). None showed PNI or distant metastasis at presentation. Histologically, a nodular growth pattern was seen in 14 cases (70%) and a solid growth pattern in 6 (30%) (Fig. 1). Two cases with a solid growth pattern also showed focal trabecular architecture. Intratumoral fibrous bands were noted in 14 tumors (70%) (Fig. 1), and necrosis was seen in 4 (20%) (Fig. 2). Moderate nuclear atypia was noted in 6 (30%) (Fig. 2) and prominent nucleoli in 4 (20%). Two cases showed marked nuclear atypia; one with focal spindling of tumor cells (Fig. 2). Most cases (n = 13, 65%) had occasional mitotic activity (< 1/10HPF). Four (20%) had a mitotic rate of > 1/10HPF (maximum 3–4/10HPF) (Fig. 2), whereas mitotic figures were not identified in tumor sections reviewed from 3 cases (15%). None of the cases showed atypical mitoses. All but one tumor was predominantly composed of chief cells (Fig. 2).
Fig. 1.
Photomicrographs of the histopathologic features of parathyroid carcinoma (H&E*). a Intratumoral fibrous bands imparting a nodular pattern to the tumor (10X). b Capsular invasion (40X). c Soft tissue (muscle) invasion (100X). d Extracapsular vascular invasion (100X). *Hematoxylin and Eosin
Fig. 2.
Photomicrographs showing histopathologic features of parathyroid carcinoma (H&E*). a Necrosis (100X). b Moderate nuclear atypia (200X). c Marked nuclear atypia (200X). d Spindling of tumor cells with the presence of mitosis (yellow arrow) (200X). e Histological features of conventional PC (200X). f Histological features of oncocytic PC (200X). *Hematoxylin and Eosin
Lymph node dissection was performed in 10 cases, including central clearance and one or more levels of lateral neck dissection; however, none of the patients had nodal metastasis. Follow-up was available in 16 cases (80%) with a median follow-up of 21.5 months (mean 24 months, range 6 months to 58 months). Metastasis and/or local recurrence on follow-up was seen in two cases, of which one patient experienced local recurrence followed by esophageal and subcutaneous metastases after 3 years of disease-free survival (DFS). The other patient developed multiple brain and liver metastases after 1 year of DFS. The latter case was a non-secretory PC. Both patients eventually succumbed to the disease. The remaining 14 patients were alive with no evidence of disease at the last follow-up. Adjuvant radiotherapy (RT) was offered in 8 patients (50%).
Discussion
Parathyroid carcinoma is a rare endocrine malignancy, the incidence of which has been relatively increasing with the advent of newer techniques for detecting serum calcium and PTH levels [3, 15]. PC is an uncommon cause of PHPT and accounts for < 1% of cases. After being described by de Quervain in 1904, PC literature comprises mainly of case reports with few larger case series. Most of the larger case series evaluated clinical and surgical outcomes and only a few have attempted to define the histologic features that separate PC from its benign counterparts [6–12, 15–22]. The findings of these major studies evaluating the histologic features of PC have been enlisted and compared with the current study in Table 3. Some of these studies have suggested various diagnostic criteria for PC [6, 7]. These criteria were followed inconsistently until the WHO recommended strict inclusion criteria [23]. These recommendations were further refined in 2017 based on the proposed diagnostic criteria of Rosai et al. [13, 14, 23]. Accordingly, the diagnosis of PC should be restricted to tumors with evidence of invasive growth involving adjacent structures (thyroid or soft tissue), capsular and extracapsular blood vessels, perineural space, and/or to those with documented metastases [13]. We discuss clinicopathologic features of PC in a series of 20 cases diagnosed based on the criteria described by Rosai et al. [14].
Table 3.
Review of major published series with an emphasis on pathological aspects of PC
| Schantz et al. [6] (1973) | Smith et al. [8] (1984) | Bondeson et al. [7] (1993) | Busaidy et al. [9] (2004) | Erovic et al. [10] (2013) | Quinn et al. [11] (2015) | Ryhanen et al. [12] (2017) | Present study (2020) | Summary | |
|---|---|---|---|---|---|---|---|---|---|
| Study period | 1930–1970 (40 years) | 1950–1981 (31 years) | 1968–1990 (22 years) | 1980–2002 (22 years) | 1976–2005 (30 years) | 2004–2014 (10 years) | 2000–2011 (11 years) | 2004–2018 (13 years) | Average study period: 22-years |
| Institutions involved | Single | Single | Thirty-seven | Single | Single | Single | Nationwide study | Single | – |
| No. of patients | 70 | 20 | 56 (41 pathological diagnosis) | 27 | 16 | 18 | 32 | 20 | Total: 259, Median: 23.5, Mean: 32.75 |
| Mean age | 44.3 years (13–84 years) | 46.7 years (16–75 years) | 54 years (31–72 years) | 59 years | Median 61 years (17–83 years) | 47.8 years (21–73 years) | Around 52 years | ||
| Male/female (ratio) | 36/34 (1.05:1) | – | 16/11 (1.45:1) | 6/10 (0.6:1) | 8/10 (0.8:1) | 14/18 (0.77:1) | 10/10 (1:1) | 90/93 (0.96/1) | |
| Mean serum PTH (ng/L) | none | none | 1238 (131.3 pmol/L) | 489 (489 pg/ml) | 989 | 864.5 | – | ||
| Mean serum calcium (mg/dL) | 15.2 | 13.4 | 19.84 (4.96 mmol/L) | 13 | 7.04 (1.76 mmol/L) | 13.51 | – | ||
| Average tumor size | 3.3 cm | 3 cm | 3.3 cm | 3.5 cm | 2.95 cm | 2.4 cm | 3 cm | ||
| Major histopathological features supporting the diagnosis of PC |
Fibrous bands (90%) Mitoses (81%) Capsular invasion (67%) |
Trabecular architecture (95%) Mitoses (95%) Fibrous trabeculae & capsule (89%) |
Fibrous bands (80%) Mitoses (74%) Macronucleoli (52%) |
Fibrous bands (44%) Mitoses (40%) Vascular Invasion (37%) |
Vascular Invasion (85%) Capsular invasion (69%) Invasion of surrounding tissue (46%) |
Capsular invasion (100%) Fibrous bands (94%) Invasion of surrounding tissue (78%) Vascular invasion (67%) |
Diffuse growth pattern Chief cell type Mitoses Nuclear atypia |
Capsular invasion (100%) Vascular invasion (95%) Invasion of surrounding tissue (50%) Fibrous bands (70%) |
Frequency of major histopathological variables: fibrous bands (~ 79%) Capsular invasion (~ 72%) Vascular invasion (~ 63%) Invasion of surrounding tissue (~ 58%) |
| Other supporting histopathological features |
Trabecular architecture Vascular invasion (12%) |
Nuclear atypia Prominent nucleoli Vascular invasion |
Nuclear atypia (63%) Necrosis (36%) Trabecular architecture (20%) |
Capsular invasion (26%) Trabecular architecture (11%) Lymphatic invasion (11%) |
Nuclear atypia (89%) Macronucleoli (22%) Necrosis (22%) |
Fibrous septae (88%) Capsular invasion (72%) Vascular invasion (72%) |
Nodular pattern (70%) Mitotic activity (85%) Nuclear atypia (40%) Necrosis (20%) |
||
| Local recurrence | 18 (26%) | 10 (50%) | 30 (54%) | 11 (41%) | 5 (31%) | 6 (33%) | 6 (21%) | 1 (6%) | ~ 34% |
| Metastasis | 18 (26%) | 4 (20%) | 21 (37%) | 6 (22%) | 2 (12%) | 2 (11%) | 5 (16%) | 2 (12%) | ~ 23% |
~, approximately
PC occurs a decade earlier than PA with most cases occurring in the 4th to 5th decades of life [24]. Accordingly, the median age in our series was 49 years with an interquartile age range of 41.75–56.5 years and range of 21–73 years. Unlike PA, PC is reported more frequently in males; however, as in other studies, we noted equal distribution among both sexes [3, 14, 25]. While most of our patients (54%) presented with the symptoms related to hypercalcemia such as anorexia, muscle ache, joint pain, vomiting, and renal stones, two patients (15%) were diagnosed while under evaluation for incidentally detected hypercalcemia. On the other hand, only two patients (15%) presented with a neck mass which is suggested to be one of the major clinical findings for PC [26].
Serum calcium was elevated in 12 out of 13 patients (mean 13.51 mg/dL) while the serum PTH level was elevated in 11 out of 12 patients (mean 864.50 ng/L). Symptomatic patients with serum calcium levels > 14 mg/dL or 3–4 mg/dL above the upper limit of the normal range are suspicious of having PC, while the 3–10 fold rise in serum PTH is highly suggestive of PC [27]. The only patient in this series where these two biomarkers were normal, was a case of non-secretory PC. Patients with non-secretory PC are anticipated to have a worse clinical course as they tend to be missed by the routine screening assays. Therefore, the initial presentation is often a neck mass [28]. This was true in our patient who was one of the two patients who died of disease.
As demonstrated in prior studies, PC has a predilection for the inferior parathyroid glands for unknown reasons [29]. In this study, the tumor involved inferior parathyroid glands in 86% of cases, mostly right-sided (57%), also noted by prior studies [21, 30]. Although there is no documented association of PC with laterality of involvement, the majority of the tumors (67%) in this study involved right-sided parathyroid glands. Cases of PC involving the ectopic parathyroid glands are described in the literature [31]. One of our cases had PC involving an ectopic parathyroid gland in the upper mediastinum. As the inferior parathyroid glands arise from the 3rd pharyngeal pouch embryologically, and PC is more prevalent in the inferior parathyroid glands, it is logical for the mediastinum to be the most common site for ectopic PC [32]. However, the mere ectopic location of a parathyroid gland does not increase the risk for PC per se [32].
Although not scientifically proven, some authors have supported the hyperplasia-adenoma-carcinoma progression hypothesis [5]. Two of our patients were diagnosed with ipsilateral PA outside our institute before being diagnosed as PC on further follow-up. Unfortunately, the prior pathology for these two patients was unavailable for review and this study cannot substantiate the above theory. Furthermore, one of the patients had a synchronous contralateral PA while the other had a synchronous contralateral PTC. This occurrence of concurrent PA and PTC is described in the literature [24, 28]. PC in a setting of MEN1 syndrome, as reported in two of our cases, is extremely rare with only occasional case reports published [33]. In a larger case series of parathyroidectomy in 83 MEN1 patients, no PC were found [34]. However, one of our patients, known to have MEN1 syndrome, had a history of contralateral PA progressing to PC in addition to the existent PC. Both diagnoses of PA and transformation to PC were made outside our institute and the slides were not available for review.
PC usually presents as a larger tumor than PA [5]. A T-size greater than 3 cm in a hypercalcemic patient with hyperparathyroidism is highly suggestive of PC and a size greater than 4 cm is associated with increased risk of death (hazard ratio 1.91, 95%; confidence interval 1.35–2.69) [27, 35]. The mean T-size in this series was 2.4 cm. Surprisingly, the smallest tumor in this series was 0.8 cm, indicating that a high index of clinical suspicion may trump T-size.
Whereas many studies have not found use in frozen sections due to the difficulty in histopathologic diagnosis, our findings challenge this dogma [29, 36]. In 8 out of 9 tumors evaluated by frozen section examination, the diagnosis favored PC. The only case where a diagnosis of PA was favored was a patient with MEN1 syndrome where the probability of PC was unlikely. Four frozen section cases were lesional excisions that were not initially addressed with en-block resection. The frozen section diagnosis changed management in these cases as the thyroid was immediately resected in the same surgery. Distinguishing PC from PA upfront is important as the best chance of achieving cure is at the time of first surgical exploration with en-bloc resection [25, 27, 32]. The reason behind the higher rate of accurate frozen section diagnosis in our cohort could be the availability of experienced head & neck pathologists in a tertiary referral center that solely caters to oncology patients. It also underscores the fact that rare tumors like those of the parathyroid can be dealt with efficiently in specialized centers.
In 1973, Schantz and Castleman suggested several characteristic histological features for PC diagnosis which comprised capsular or vascular invasion, the presence of fibrous bands, mitotic figures, and a trabecular growth pattern [6]. These were widely used until standard diagnostic criteria were proposed by the WHO and Armed Forces Institute of Pathology (AFIP) [14, 23]. The WHO criteria were refined in the 2017 edition [13]. Among the recently proposed WHO recommendations, capsular/extracapsular vascular invasion was the most common finding in this study with presence in all but one case. This contrasts with data from M. D. Anderson which states that vascular invasion is a rare finding but corroborates with Finish data that showed higher rates of vascular invasion [9, 12].
Concerning the additional WHO criteria, invasion into surrounding soft tissue was seen in 50% of our cases while no cases had PNI or metastases at presentation. Capsular invasion was seen in all 20 cases. According to the 2004 WHO publication, one of the criteria to diagnose PC was the presence of ‘capsular penetration with growth into adjacent tissue’ [23]. The question of whether the mere presence of capsular penetration is itself diagnostic of PC was not clear. Likely for this reason, some of the studies on PC in this era included cases with capsular invasion in their study cohort [4, 12]. The recent edition of the WHO rephrased the criteria from ‘capsular penetration’ to ‘invasive growth involving adjacent structures’ [13]. Furthermore, capsular invasion is not mentioned in the microscopic description. Interestingly, in our study, capsular/extracapsular vascular invasion was accompanied by capsular invasion in all but one case. It was ensured that all cases showing capsular invasion were true invasion and not the entrapment of the tumor cells in the capsule (pseudoinvasion). Many times, tumor invading the capsule induces a fibrotic reaction in the surrounding stroma. This may make it difficult to appreciate the adipose tissue necessary to define ‘invasion of the adjacent soft tissue’. Definite capsular invasion should be considered essential for diagnosis of PC.
Intratumoral fibrous bands, an important feature listed in many studies were seen in 70% of PC cases [6, 7, 9–11]. A nodular growth pattern ascribed to the tumor by the intratumoral fibrous bands was the most common growth pattern noted (n = 14/20, 70%) in this series. The trabecular pattern listed as one of the key histological features by Schultz and Castleman was seen only focally in two cases. The trabecular pattern, a similarly rare finding in the M.D Anderson series, is shared with benign lesions [7, 9]. On the other hand, some studies reported solid growth patterns to be a consistent finding in PC [12, 37].
Necrosis was noted in only 20% of our cases which is congruent with the frequency reported in the literature [7, 11]. Cellular details like nuclear pleomorphism, presence of nucleoli, and mitotic activity may be equivocal findings since these features can also be seen in PA and atypical PA [37]. Some authors suggest a triad of coagulative necrosis, macronucleoli, and mitotic rate of more than 5/50 HPF as an indicator of high-risk malignant behavior [7, 38]. In this study, however, prominent nucleoli were noted in only 20% cases while the mitotic activity was occasional (< 1/10HPF) in most cases (n = 13 cases, 65%). Most tumors in this series predominantly consisted of chief cells; however, one tumor was composed exclusively of oxyphil cells. Despite a different morphologic appearance, the oxyphil variant of PC behaves similarly chief cell tumors [39]. We did not perform an immunohistochemical evaluation for the expression of parafibromin as its role as a diagnostic marker is uncertain [11, 12, 37]. However, a lack of expression is highly suggestive of aggressive behavior and connotes a need for reflex testing for a germline mutation of the HRPT/CDC73 gene [12, 40].
Almost 40% of patients with parathyroid lesions undergo either piecemeal or incomplete tumor resection due to a lack of pre-operative or intraoperative suspicion of PC [28]. An en-block resection is the optimum treatment modality when pre-operative suspicion of PC is high since the recurrence rate for the upfront en-block surgery varies significantly from that of re-exploration [3, 16, 29]. The details of surgical treatment were available in 16 cases in this series and all underwent an en-block resection of the involved parathyroid gland. This highlights the importance of handling rare cancers in specialized institutes with trained personnel and optimum facilities like capabilities for frozen section examination. Dissection of paratracheal lymph nodes is usually performed with a diagnosis of PC along with level VI. Lymph node dissection of the rest of the neck is strongly discouraged unless intraoperative suspicion of involvement is high [25, 26, 41]. Apart from the fact that lymph node status does not influence survival, it has been proven that the lateral compartment nodes are rarely involved [14, 35]. On the contrary, dissecting these nodes rarely offers survival advantage, and the procedure involves a higher risk of operative complications [42]. In this series, the details of nodal dissection were available in 10 patients and all underwent dissection of lateral compartment nodes; none were positive for metastasis.
The role of radiotherapy in the management of PC is dubious; with few studies supporting its use while others negating it [9, 10, 24]. Fifty percent of cases of this cohort received adjuvant/palliative RT. The most common reason cited for the adjuvant RT was the invasion of adjacent soft tissue.
Adequate follow-up was available in 16 cases with a median follow-up of 21.5 months (mean 24 months, range 6–58 months). One case recurred and subsequently developed esophageal and subcutaneous metastasis. Despite successful primary surgery, the recurrence rate for PC is reported as high as 50–70% [43]. There are multiple explanations for lower recurrence in our series. First, the average time for a recurrence in PC varies between 2 and 5 years whereas the median follow-up in this series was approximately 2 years [32]. This indicates that recurrences may be observed past the time of follow up. Second, the average tumor size in this study was 2.3 cm and most of the patients were treated with upfront en-block resection [27]. Further, almost half of the patients were treated with postoperative RT. Lung, liver, and bones are the most common sites for metastasis for PC [24]. Apart from the above-mentioned patient, another patient also developed a metastasis. This patient had a non-secreting PC and the metastatic sites included liver and brain. Remarkably, these two patients with a history of metastasis were the only mortalities in the cohort. Nonetheless, tumor burden due to the metastasis disease is not the usual cause of mortality in PC; most patients succumb to the hypercalcemic state [27].
In conclusion, we present one of the largest single institutional studies on PC predominantly focusing on the histopathologic characteristics of this rare entity. Vascular invasion (capsular/extracapsular) is the most common WHO recommended histologic feature to be noted in PC. Although capsular invasion is not addressed in the recent WHO microscopic findings of PC, it is the most consistent feature to accompany the other essential diagnostic criteria. The diagnostic criteria of PNI and distant metastasis at presentation are infrequent. The diagnosis of PC on a frozen section can be made with experienced hands and used to guide intraoperative management.
This study does have limitations including its retrospective nature. The restricted sample size and unavailability of thorough clinical details, including follow-up in some cases, are additional limitations. The authors were unable to perform immunohistochemistry for parafibromin expression; neither we were able to do the mutation testing for HRPT/CDC73, as the cost involved preclude their routine use in our country. Nevertheless, this paper accomplishes the primary goal of the study which was to comprehensively evaluate the basic histomorphologic features of this rare tumor.
Acknowledgements
This study was presented by Akash Sali as a poster presentation during the International Academy of Pathology Congress, 2018 held at Amman, Jordan.
Funding
No funding obtained.
Compliance with Ethical Standards
Conflict of interest
No conflict of interest to disclose.
Footnotes
Synopsis
Vascular and soft tissue invasion are the most frequent features used to diagnose parathyroid carcinoma; capsular invasion being an important indicator. The frozen section can clinch an accurate diagnosis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ippolito G, Palazzo FF, Sebag F, De Micco C, Henry JF. Intraoperative diagnosis and treatment of parathyroid cancer and atypical parathyroid adenoma. Br J Surg. 2007;94:566–570. doi: 10.1002/bjs.5570. [DOI] [PubMed] [Google Scholar]
- 2.Ruda JM, Hollenbeak CS, Stack BC., Jr A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–372. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Betea D, Potorac I, Beckers A. Parathyroid carcinoma: challenges in diagnosis and treatment. Ann Endocrinol. 2015;76:169–177. doi: 10.1016/j.ando.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Quaglino F, Manfrino L, Cestino L, Giusti M, Mazza E, Piovesan A, et al. Parathyroid carcinoma: an up-to-date retrospective multicentric analysis. Int J Endocrinol. 2020 doi: 10.1155/2020/7048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan K, Mete O. Parathyroid carcinoma: diagnosis and clinical implications. Turk J Pathol. 2015;31:80–97. doi: 10.5146/tjpath.2015.01316. [DOI] [PubMed] [Google Scholar]
- 6.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–605. doi: 10.1002/1097-0142(197303)31:3<600::AID-CNCR2820310316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol. 1993;17:820–829. doi: 10.1097/00000478-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Smith JF, Coombs RR. Histological diagnosis of carcinoma of the parathyroid gland. J Clin Pathol. 1984;37:1370–1378. doi: 10.1136/jcp.37.12.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26:716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 10.Erovic BM, Goldstein DP, Kim D, Mete O, Brierley J, Tsang R, et al. Parathyroid cancer: outcome analysis of 16 patients treated at the Princess Margaret Hospital. Head Neck. 2013;35:35–39. doi: 10.1002/hed.22908. [DOI] [PubMed] [Google Scholar]
- 11.Quinn CE, Healy J, Lebastchi AH, Brown TC, Stein JE, Prasad ML, et al. Modern experience with aggressive parathyroid tumors in a high-volume New England referral center. J Am Coll Surg. 2015;220:1054–1062. doi: 10.1016/j.jamcollsurg.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Ryhanen EM, Leijon H, Metso S, Eloranta E, Korsoff P, Ahtiainen P, et al. A nationwide study on parathyroid carcinoma. Acta Oncol. 2017;56:991–1003. doi: 10.1080/0284186X.2017.1306103. [DOI] [PubMed] [Google Scholar]
- 13.DeLellis R, Arnold A, Bilezikian J, Eng C, Larsson C, Llyod RV, et al. Parathyroid carcinoma. In: Llyod RV, Osamura RY, Kloppel G, Rosai J, et al., editors. WHO classification of tumours of endocrine organs. 4. IARC: Lyon; 2017. pp. 147–152. [Google Scholar]
- 14.Rosai J, DeLellis R, Carcangiu M, Frable W, Tallini G. Tumor of the thyroid and parathyroid gland (AFIP atlas of tumor pathology) Spring: American Registry of Pathology Press; 2015. [Google Scholar]
- 15.Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–1741. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]
- 16.Hakaim AG, Esselstyn CB., Jr Parathyroid carcinoma: 50-year experience at The Cleveland Clinic Foundation. Cleve Clin J Med. 1993;60:331–335. doi: 10.3949/ccjm.60.4.331. [DOI] [PubMed] [Google Scholar]
- 17.Harari A, Waring A, Fernandez-Ranvier G, Hwang J, Suh I, Mitmaker E, et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab. 2011;96:3679–3686. doi: 10.1210/jc.2011-1571. [DOI] [PubMed] [Google Scholar]
- 18.Healy SJ, Soe KK. Parathyroid cancer cases: a single center’s experience. AACE Clin Case Rep. 2016;2:e221–e227. doi: 10.4158/EP15857.CR. [DOI] [Google Scholar]
- 19.Iacobone M, Lumachi F, Favia G. Up-to-date on parathyroid carcinoma: analysis of an experience of 19 cases. J Surg Oncol. 2004;88:223–228. doi: 10.1002/jso.20152. [DOI] [PubMed] [Google Scholar]
- 20.Kebebew E, Arici C, Duh QY, Clark OH. Localization and reoperation results for persistent and recurrent parathyroid carcinoma. Arch Surg. 2001;136:878–885. doi: 10.1001/archsurg.136.8.878. [DOI] [PubMed] [Google Scholar]
- 21.Lee YS, Hong SW, Jeong JJ, Nam KH, Chung WY, Chang HS, et al. Parathyroid carcinoma: a 16-year experience in a single institution. Endocr J. 2010;57:493–497. doi: 10.1507/endocrj.K09E-365. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman SM, Rigual NR, Hicks WL, Jr, Popat SR, Lore JM, Jr, Douglas WG, et al. Parathyroid carcinoma: a multicenter review of clinicopathologic features and treatment outcomes. Ear Nose Throat J. 2004;83:491–494. doi: 10.1177/014556130408300718. [DOI] [PubMed] [Google Scholar]
- 23.Bondeson L, Grimelius L, DeLellis RA, Lloyd R, Akerstrom G, Larsson C, et al. Parathyroid carcinoma. In: DeLellis RA, Llyod RV, Heitz PU, Eng C, et al., editors. Pathology and genetics of tumours of endocrine organs. Lyon: IARC; 2004. pp. 124–127. [Google Scholar]
- 24.Al-Kurd A, Mekel M, Mazeh H. Parathyroid carcinoma. Surg Oncol. 2014;23:107–114. doi: 10.1016/j.suronc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Shane E. Clinical review 122: parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 26.Owen RP, Silver CE, Pellitteri PK, Shaha AR, Devaney KO, Werner JA, Rinaldo A, Ferlito A. Parathyroid carcinoma: a review. Head Neck. 2011;33:429–436. doi: 10.1002/hed.21376. [DOI] [PubMed] [Google Scholar]
- 27.Salcuni AS, Cetani F, Guarnieri V, Nicastro V, Romagnoli E, de Martino D, et al. Parathyroid carcinoma. Best Pract Res Clin Endocrinol Metab. 2018;32:877–889. doi: 10.1016/j.beem.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Goswamy J, Lei M, Simo R. Parathyroid carcinoma. CurrOpinOtolaryngol Head Neck Surg. 2016;24:155–162. doi: 10.1097/MOO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Xue S, Wang S, Lv Z, Meng X, Wang G, et al. Clinical characteristics and treatment outcomes of parathyroid carcinoma: a retrospective review of 234 cases. Oncol Lett. 2017;14:7276–7282. doi: 10.3892/ol.2017.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flye MW, Brennan MF. Surgical resection of metastatic parathyroid carcinoma. Ann Surg. 1981;193:425–435. doi: 10.1097/00000658-198104000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao C, Dou C, Chen F, Wang Y, Zhang X, Lai H. An unusual mediastinal parathyroid carcinoma coproducing PTH and PTHrP: a case report. Oncol Lett. 2016;11:4113–4116. doi: 10.3892/ol.2016.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Givi B, Shah J. Parathyroid carcinoma. Clin Oncol. 2010;22:498–507. doi: 10.1016/j.clon.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haven CJ, van Puijenbroek M, Tan MH, Teh BT, Fleuren GJ, van Wezel T, et al. Identification of MEN1 and HRPT2 somatic mutations in paraffin-embedded (sporadic) parathyroid carcinomas. Clin Endocrinol. 2007;67:370–376. doi: 10.1111/j.1365-2265.2007.02894.x. [DOI] [PubMed] [Google Scholar]
- 34.Montenegro FL, Lourenco DM, Jr, Tavares MR, Arap SS, Nascimento CP, Jr, Massoni Neto LM, et al. Total parathyroidectomy in a large cohort of cases with hyperparathyroidism associated with multiple endocrine neoplasia type 1: experience from a single academic center. Clinics. 2012;67:131–139. doi: 10.6061/clinics/2012(Sup01)22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asare EA, Sturgeon C, Winchester DJ, Liu L, Palis B, Perrier ND, et al. Parathyroid carcinoma: an update on treatment outcomes and prognostic factors from the national cancer data base (NCDB) Ann Surg Oncol. 2015;22:3990–3995. doi: 10.1245/s10434-015-4672-3. [DOI] [PubMed] [Google Scholar]
- 36.Delellis RA. Challenging lesions in the differential diagnosis of endocrine tumors: parathyroid carcinoma. Endocr Pathol. 2008;19:221–225. doi: 10.1007/s12022-008-9050-2. [DOI] [PubMed] [Google Scholar]
- 37.Do Cao C, Aubert S, Trinel C, Odou MF, Bayaram M, Patey M. Parathyroid carcinoma: diagnostic criteria, classification, evaluation. Ann Endocrinol. 2015;76:165–168. doi: 10.1016/j.ando.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 38.DeLellis RA. Parathyroid tumors and related disorders. Mod Pathol. 2011;24:S78–S93. doi: 10.1038/modpathol.2010.132. [DOI] [PubMed] [Google Scholar]
- 39.Erickson LA, Jin L, Papotti M, Lloyd RV. Oxyphil parathyroid carcinomas: a clinicopathologic and immunohistochemical study of 10 cases. Am J Surg Pathol. 2002;26:344–349. doi: 10.1097/00000478-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Gill AJ, Clarkson A, Gimm O, Keil J, Dralle H, Howell VM, et al. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT-JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol. 2006;30:1140–1149. doi: 10.1097/01.pas.0000209827.39477.4f. [DOI] [PubMed] [Google Scholar]
- 41.Genden EM. Parathyroid carcinoma. Oper Tech in Otolaryngol Head Neck Surg. 2009;20:79–81. doi: 10.1016/j.otot.2009.02.007. [DOI] [Google Scholar]
- 42.Kebebew E. Parathyroid carcinoma. Curr Treat Options Oncol. 2001;2:347–354. doi: 10.1007/s11864-001-0028-2. [DOI] [PubMed] [Google Scholar]
- 43.Obara T, Okamoto T, Kanbe M, Iihara M. Functioning parathyroid carcinoma: clinicopathologic features and rational treatment. Semin Surg Oncol. 1997;13:134–141. doi: 10.1002/(SICI)1098-2388(199703/04)13:2<134::AID-SSU9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]