Abstract
South Asian individuals are at an increased risk of type 2 diabetes (T2DM). However, the mechanisms behind this increased risk in South Asians in the United States are not well understood. The Mediators of Atherosclerosis in South Asians Living in America (MASALA) study is the only longitudinal cohort of South Asians in the United States and provides key insights as to the epidemiology of T2DM in South Asians. Evidence from MASALA suggests that South Asians experience a disproportionately high burden of prevalent and incident T2DM compared to members of other race/ethnic groups. Higher insulin resistance in South Asians, even with low BMI, more impairment in insulin secretion, and greater deposition of ectopic fat likely play a role in T2DM etiology. Furthermore, South Asian migrants to the United States experience a range of factors related to acculturation, social networks, and religious beliefs, which may impact physical activity and dietary practices. Interventions to prevent T2DM in South Asians should include a focus on cultural factors related to health and should consider the complete mechanistic pathway and the relative contributions of both insulin resistance, β cell dysfunction, and ectopic fat deposition on T2DM development in South Asians, particularly in those with lower BMI.
Keywords: type 2 diabetes, South Asian, ethnicity, the United States
Introduction
South Asians (those who live in or have their roots in India, Pakistan, Sri Lanka, Bangladesh, Nepal, Bhutan, or the Maldives) are one of the fastest-growing race/ethnic groups in the United States.1 According to the most recent United States (U.S.) Census data, between 2000 and 2010, the Asian Indian population grew by 68% and currently totals 5.4 million people.1 South Asians are also a group that is at high risk of cardiometabolic diseases, such as type 2 diabetes (T2DM).2–4 Recent evidence from India indicates an overall diabetes prevalence of 7.3% that varied from 4.3% in the state of Bihar to 10.0% in the state of Punjab.5 Additionally, a previous study from Chennai, India, noted that the incidence rate of T2DM was high at 20.2 per 1000 person-years.6 South Asians in the diaspora also have a higher prevalence and incidence of T2DM compared with other race/ethnicities. A recent study examining the prevalence of T2DM among adults in the United States by race/ethnicity reported that after adjustment for age, sex, and body mass index (BMI), the prevalence of T2DM was 27% in South Asians compared with 8.0% in non-Hispanic White individuals.4 Furthermore, a study from Ontario, Canada, found that after adjusting for differences in baseline BMI, age, sex, and other sociodemographic characteristics, South Asians were 3.4 times more likely than White people to develop T2DM.2 Additionally, South Asians had a higher risk of incident T2DM at younger ages and at lower levels of adiposity. In particular, South Asians developed T2DM an average of 9 years earlier than White participants, and at an average BMI of 24 kg/m2 compared with BMI 30 kg/m2 for an equivalent incidence rate in White people.2 However, the mechanisms behind this increased risk of T2DM in migrant South Asians have not been well elucidated. It is possible that innate susceptibilities for insulin resistance, dysfunction in insulin secretion, and a propensity for ectopic fat deposition may play a role.7 It is also possible that lifestyle practices related to culture and migration, as well as epigenetic and metabolomic mechanisms may be important in determining T2DM risk in this population.
The Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study is the only longitudinal cohort of South Asians in the United States and provides key insights as to the epidemiology of T2DM in migrant South Asians. MASALA was created as a community-based sample of 906 adults aged 40 and over from the San Francisco Bay and greater Chicago areas,8 and was designed to be methodologically similar to the Multi-Ethnic Study of Atherosclerosis (MESA)9 for valid cross-ethnic comparisons with the four largest U.S. race/ethnic groups (White Americans, African Americans, Hispanic Americans, and Chinese Americans).8 Hence, to be eligible for MASALA, participants could not have any existing cardiovascular disease (heart attack, stroke, heart failure, and atrial fibrillation) and could not have had any procedures or surgeries done on their heart or blood vessels.8 Baseline study enrollment for MASALA occurred between 2010 and 2013, and a second follow-up examination of 749 participants was conducted between 2015 and 2018. In addition, the second wave of 258 participants was recruited for MASALA in 2017–2018,10 bringing the total study sample to 1164 South Asians (Fig. 1). Approximately 98% of the MASALA study population were immigrants to the United States: 83% of participants were Indian immigrants (n = 965), 6% were from Pakistan, 0.5% were from Bangladesh, and 8% were born in other diaspora countries. Hence, MASALA represents a majority Asian Indian immigrant population with no existing cardiovascular disease.
Figure 1.
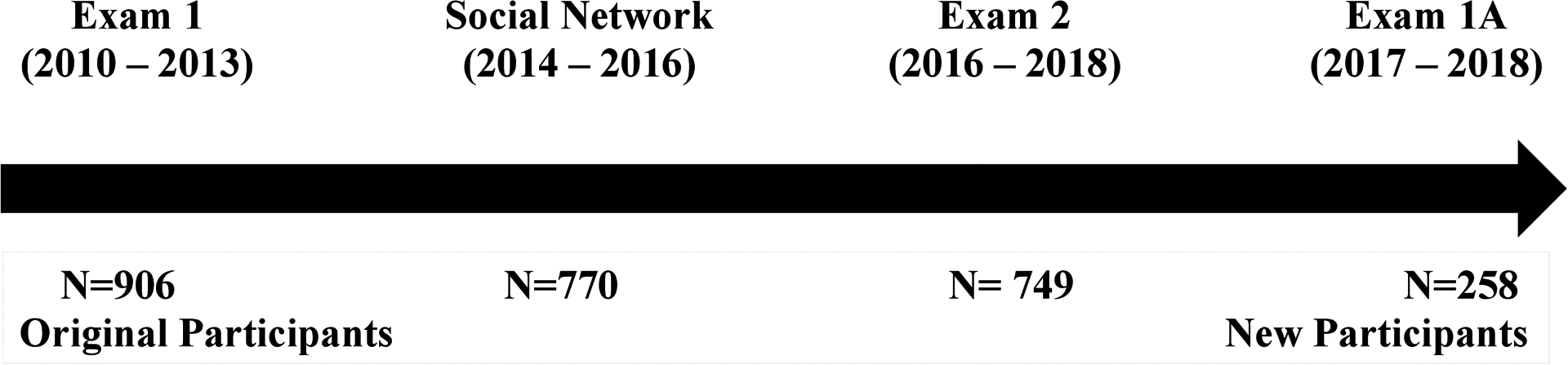
MASALA study flow diagram of enrollment.
Prevalent and incident T2DM
Findings from the MASALA study have also indicated a high prevalence and incidence of T2DM in U.S. South Asians. In a cross-sectional analysis comparing MASALA participants with White, African American, Latino, and Chinese American individuals from the MESA study, South Asians had a significantly higher age-adjusted prevalence of T2DM (23%) compared with MESA participants (6% in White Americans, 18% in African Americans, 17% in Latin Americans, and 13% in Chinese Americans).11 Recent longitudinal data from MASALA also found that South Asians have a high incidence rate of T2DM (18.9 per 1000 person-years),12 which is higher than the rate of 11.1 per 1000 person-years in White individuals found in a comparable study.13 In this review, we will summarize findings from the MASALA study to date regarding the biological, environmental, behavioral, and sociocultural risk factors contributing to the disproportionate risk of T2DM in South Asians in the United States (Table 1).
Table 1.
Risk factors associated with type 2 diabetes or associated lifestyle factors
| Risk factor | Direction of effect | Reference |
|---|---|---|
| Biological factors | ||
| Insulin resistance | ↑ | Kanaya (2014)11 Gujral (2014)20 |
| Impaired insulin secretion | ↑ | Kanaya (2014)11 Gujral (2014)20 |
| Hepatic fat deposition | ↑ | Shah (2016)39 Gujral (2020)12 |
| Visceral fat area | ↑ | Gujral (2020)12 |
| Low lean muscle mass | ↑ | Shah (2016)39 |
| History of gestational diabetes | ↑ | Gadgil (2017)102 |
| Behavioral factors | ||
| Healthy plant-based diet | ↓ | Gadgil (2015)32 Bhupathiraju (2019)53 |
| Fried sweets, snacks, and high-fat dairy | ↑ | Gadgil (2015)32 Jin (2018)52 |
| Animal protein | ↑ | Gadgil (2015)32 |
| Physical inactivity | ↑ | Kanaya (2015)11 Shah (2014)61 |
| Obstructive sleep apnea or sleep-disordered breathing | ↑ | Deol (2018)66 |
| Sociocultural factors | ||
| Separation assimilation strategy | ↑ | Al-Sofiani (2020)69 |
| Integration assimilation strategy | ↓ | Al-Sofiani (2020)69 |
| Acculturation assimilation strategy | ↓ | Al-Sofiani (2020)69 |
| Psychosocial factors | ||
| Exercise partners | ↓ | Thanawala (2020)81 |
| Social influence of adult children | ↓ | Ram (2020)82 |
| Self-reported discrimination | ↑ | Nadimpalli (2017)85 |
| Environmental factors | ||
| Neighborhood walk score | ↓ | Kelley (2016)90 |
| Persistent organic pollutants | ↑ | La Merrill (2019)95 |
Biological risk factors for T2DM
Pathophysiology
Conventional theories of T2DM pathogenesis posit that adiposity-induced insulin resistance followed by a subsequent decline in pancreatic insulin secretion eventually leads to overt diabetes.14–16 However, some race/ethnic groups may have an innate susceptibility for impaired insulin secretion, which may be the primary defect in T2DM pathogenesis in these populations.17–19 Evolutionary aspects related to low lean muscle mass and poor pancreatic insulin secretion suggest that both the propensity to develop insulin resistance as well as early defects in the compensatory ability of insulin secretion may explain some of the high risk of T2DM in South Asians.7 A comparative analysis between South Asian participants from the MASALA study and White, African American, Hispanic American, and Chinese American participants from the MESA study found that South Asians without diabetes had significantly greater levels of insulin resistance compared with White Americans, African Americans, and Hispanic Americans.11 Furthermore, nondiabetic South Asians had significantly poorer insulin secretion compared with members of all other race/ethnic groups. Therefore, it is possible that South Asians have lower levels of β cell function and an inability to adequately compensate for higher glucose levels due to insulin resistance.11 In addition, data from the MASALA pilot study assessing the relative associations of β cell dysfunction and insulin sensitivity with baseline glycemic status and incident glycemic progression in 150 Asian Indian participants found that after controlling for age, sex, BMI, family history of diabetes, hypertension, and smoking status, both insulin resistance and poor insulin secretion were significantly associated with prediabetes and T2DM. However, after an additional adjustment for visceral fat, insulin resistance was no longer associated with prediabetes and T2DM, while the association with poor insulin secretion remained robust.20 Furthermore, after 2.5 years of follow-up, the change in insulin secretion between baseline and follow-up was significantly associated with incident hyperglycemia, while the change in insulin resistance was not.20 These results suggest that while both insulin resistance and impaired insulin secretion are associated with T2DM and prediabetes in South Asians, it appears that the association with impaired insulin secretion is stronger and independent of visceral adiposity. Therefore, it is important to consider the role of impaired insulin secretion in diabetes pathogenesis in South Asians, as well as contributing factors and possible methods for the prevention of β cell decline in this population.
Epigenetics
Epigenetic mechanisms affecting gene expression, such as microRNAs (miRs), may serve as an explanation of the heightened and unique risk of T2DM in South Asians. Epigenetic regulation of miRs can occur in response to environmental insults, unhealthy diet, and physical inactivity, or may be related to transgenerational in utero exposures.21,22 Recent studies have noted the role of miRs on the regulation of the insulin signaling pathway and insulin resistance.23 However, the specific biological mechanisms underlying the association between miRs and T2DM may vary between groups and/or individuals. Therefore, the identification of circulating miR biomarkers in differing race/ethnic groups may provide insight as to the causal pathways related to T2DM risk in specific populations. Little is known about the association between circulating miRs and glycemic impairment in migrant South Asians. An analysis of 128 participants from the MASALA pilot study evaluated the association between circulating miRs and prevalent glycemic impairment at baseline as well as glycemic progression after 2.5 years of follow-up.24 Results of this study indicated a positive relationship between miR-191 and glycemic impairment. Furthermore, six miRs (i.e., miR-122, miR-15a, miR-197, miR-320a, miR-423, and miR-486) were inversely associated with incident glycemic impairment at follow-up, even after adjustment for clinically relevant covariates.24 These results suggest that circulating miRs may be a tool for identifying the biological mechanisms underlying T2DM risk in South Asians. This has implications for improved treatment approaches and targets as well as early detection of the risk of glycemic impairment, which can be used to inform prevention efforts. However, this study incorporated a priori approach, whereby miRs that were previously thought to be associated with glycemic impairment were selected. Future studies should incorporate a more agnostic approach to miR detection in order to identify novel targets related to the risk of T2DM in South Asians.24
Metabolomics
The development of T2DM is the result of a complex set of interactions between genetic and environmental factors and can involve dysfunction across many different organ systems.25 These complexities create difficulties in understanding the molecular pathways contributing to T2DM development and how they may differ by population subgroups. Circulating metabolites are measurable products of metabolic processes that may offer insights as to these processes and help to provide a more comprehensive understanding of T2DM risk. A recent analysis in MASALA assessed untargeted metabolomic and lipidomic profiles with targeted integration of known signals to identify metabolites most contributory to T2DM. Investigators identified 258 metabolites with a detectable signal in >98% of samples. Of these, 59 metabolites were associated with prevalent T2DM, 32 with fasting glucose, and 45 with HbA1c at follow-up. The most predominant metabolites associated with glycemic measures in migrant South Asians were sphingomyelins, tri- and diacylglycerols, and carnitines.26
Diet and dietary patterns are particularly important when considering metabolomic influences, as diet affects the human metabolome either by a direct contribution of metabolites or by the indirect influence of metabolic pathways that consume or produce specific metabolic intermediates.27 Specific dietary patterns may influence metabolic signatures that can contribute to disease risk. For example, previous research has indicated that a diet high in red meat, potatoes, and sweets is associated with a metabolic signature high in branched-chain amino acids (BCAAs) and short-chain acylcarnitines (AC),28 which have been linked to obesity, insulin resistance, and T2DM.29–31 While South Asians in the United States have a high risk of T2DM, they also have varying dietary patterns that contribute differently to metabolic risk.32 Understanding the metabolomic profiles of these distinct patterns may offer insight as to varying pathways of T2DM development in this population. An analysis of the MASALA pilot study aimed to assess the associations between dietary patterns and serum metabolic profiles as well as the associations between these profiles and biomarkers of cardiometabolic risk.33 Dietary patterns were described as either Western/nonvegetarian or vegetarian. The Western dietary pattern included higher intakes of red meat and was positively associated with a BCAa, aromatic amino acid, and short-chain AC metabolomic pattern. Furthermore, among those without T2DM, intakes of fish and vegetables were additionally associated with this pattern, which was significantly and positively associated with measures of glycemia, insulin resistance, triglycerides, and liver-to-spleen attenuation ratio.33
Newer analyses of the larger MASALA cohort characterized the metabolites associated with three distinct dietary patterns: animal protein; snacks, sweets, and high-fat dairy; or fruits and vegetables. Five acylcarnitines, 10 amino acids, and 34 lipids were associated with greater consumption of animal proteins.34 Of these, the most significant were trigonelline, proline betaine, and fatty acids. Two acylcarnitines and 18 lipids were associated with fried snacks, sweets, and high-fat dairy consumption, of which the top two metabolites were very long-chain unsaturated fatty acids and fatty acids. However, there were no significant associations between circulating metabolites and the dietary consumption pattern of fruits, vegetables, nuts, and legumes.34 Taken together these results suggest that dietary patterns high in red meat and animal proteins are associated with metabolomic signatures known to be related to cardiometabolic disease risk. Furthermore, amongst vegetarian diets, those that were comprised more of fried snacks and sweets indicated a poorer metabolomic profile than those comprised of fruits, vegetables, and legumes. Future longitudinal research is needed to examine whether changes in dietary patterns and resulting metabolite signatures can influence future cardiometabolic risk.
Anthropometry and body composition
South Asians are at an increased risk of T2DM despite having a lower mean BMI compared with other race/ethnic populations.3,35 It is hypothesized that this risk is due in part to increased deposition of central obesity and ectopic fat in South Asian populations.36–38 However, previous studies have not adequately measured multiple body compartments across race/ethnic populations to determine whether elevated T2DM risk is mediated by differences in body composition. A cross-sectional analysis of harmonized data from the MASALA and MESA studies used computed tomography (CT) scans to determine levels of ectopic fat deposition across five different race/ethnic groups. Compared with the four race/ethnic groups in MESA South Asians had greater intermuscular fat and fatty liver, and lower lean muscle mass.39 South Asians also had significantly greater visceral fat compared with Chinese Americans, African Americans, and Hispanics, as well as greater pericardial fat compared with African Americans. Furthermore, South Asians had lower adiponectin levels compared with all other ethnic groups aside from Chinese Americans, as well as and higher levels of resistin than all other groups. It is, therefore, possible that a less favorable body composition or adipokine profile may explain the increased T2DM risk in South Asians.39 However, a subsequent comparative analysis of MASALA and MESA participants examined the association between ectopic fat and prevalent T2DM. Results of this study found that increased ectopic fat deposition in all body compartments was associated with glycemic impairment in all five race/ethnic groups, aside from intermuscular fat area and T2DM. In multivariate logistic regression models, while South Asians had a significantly higher odds for impaired fasting glucose and T2DM compared with all four MESA groups, this increased risk was not greatly attenuated after adjustment for body composition and adipokines.40 These results suggest that other factors besides ectopic fat accumulation account for the increased risk of prevalent T2DM in South Asians. In addition, given that South Asians tend to have lower lean muscle mass compared with individuals of other race/ethnic populations, further research is needed to explore the differential effects of resistance compared with aerobic exercise on muscle mass, mitochondrial function, and insulin resistance in South Asians.
It is possible, however, that increased ectopic fat may play a role in incident T2DM in South Asian populations. A longitudinal analysis of MASALA data estimated the incidence of prediabetes and T2DM after 5 years and the associated risk factors. South Asians had a high incidence rate of glycemic progression, either from normal glucose tolerance to prediabetes or from prediabetes to T2DM.12 In standardized logistic regression models, after controlling for age and sex, only greater hepatic fat or visceral fat area were associated with any glycemic progression.12 Given that ectopic fat is associated with T2DM risk in all race/ethnic populations, prevention and treatment efforts aimed at reducing ectopic fat deposition, particularly hepatic fat, are recommended. However, it is likely that other factors besides ectopic fat deposition alone significantly contribute to the disproportionate risk of T2DM in South Asians, and further research is needed to elucidate the complex interplay of these factors on overall T2DM risk and progression.
Behavioral factors
While there are no large diabetes prevention studies of South Asians in the United States, previous lifestyle interventions conducted in India have demonstrated that changes in diet and physical activity can reduce the risk of diabetes incidence.41,42 The Indian Diabetes Prevention Program randomized 531 individuals with impaired glucose tolerance to four treatment arms: (1) lifestyle modification; (2) metformin; (3) lifestyle plus metformin; or (4) control. After 3 years of follow-up, the relative risk reduction of diabetes incidence was 28.5% in the lifestyle modification group, 26.4% in the metformin group, and 28.2% in the lifestyle plus metformin group compared with controls.41 Similarly, the Diabetes Community Lifestyle Improvement Program (D-CLIP) randomized 531 overweight or obese adults with prediabetes from Chennai, India, to either a control arm or a 6-month culturally tailored lifestyle curriculum plus stepwise addition of metformin for those at the highest risk of conversion to T2DM. After 3 years of follow-up, the overall relative risk reduction was 32% in the lifestyle modification group compared with the control group.42 When assessed by prediabetes subtype, the relative risk reduction was 36% in those with impaired glucose tolerance, 36% in those with impaired glucose tolerance plus impaired fasting glucose, and 12% in those with isolated fasting glucose.42 Together, these results indicate that lifestyle modification and or metformin treatment can successfully reduce the incidence of T2DM in South Asians with impaired glucose tolerance or combined impaired glucose tolerance and impaired fasting glucose. However, more research is needed to understand the most appropriate forms of prevention in those with impaired fasting glucose. Furthermore, in order to design the most appropriate methods of intervention, a deeper understanding of the cultural factors influencing diet and physical activity practices in South Asian communities is necessary.
Diet
Dietary intake has long been associated with an increased risk of T2DM. Higher intakes of refined carbohydrates and saturated and trans fats have been shown to increase T2DM risk by negatively impacting glucose metabolism and insulin resistance. By contrast, low glycemic index foods and foods high in dietary fiber have been shown to improve glycemic and insulinemic responses, thereby reducing the risk of T2DM.43,44 The typical South Asian diet is high in carbohydrates, trans fats, and saturated fat.45 However, it is important to understand the varying patterns of dietary consumption in relation to metabolic parameters in South Asian immigrants in the United States who may eat a variety of both traditional South Asian as well as Western foods. Food group intake amongst participants in the MASALA study was assessed using the Study of Health Assessment and Risk in Ethnic groups South Asian food frequency questionnaire (FFQ), which was developed and validated in South Asians in Canada.46 The FFQ included 163 items, of which 61 items were unique to the South Asian diet, and assessed usual eating habits, frequency, and serving sizes over the past 12 months.46 Principal component analysis of the dietary data identified the following three predominant dietary patterns: (1) animal protein; (2) fried snacks, sweets, and high-fat dairy; and (3) fruits, vegetables, nuts, and legumes.32 The animal protein dietary pattern was associated with higher BMI and waist-to-hip ratio measurements. Furthermore, the fried snacks, sweets, and high-fat dairy pattern, which was predominantly a vegetarian pattern, was associated with greater insulin resistance and lower HDL cholesterol.32 Both the animal protein and fried snacks, sweets, and high-fat dairy dietary patterns were associated with adverse metabolic outcomes, and modification of major components of these dietary patterns may serve to improve cardiometabolic risk factors, including the risk of T2DM.
Furthermore, data from the MASALA pilot study assessing the association between dietary intake and T2DM in 150 Asian Indians found that in a fully adjusted logistic regression model, there was a 70% increase in odds of diabetes per standard deviation in a gram of protein intake/day.47 However, these results should be interpreted with caution, as it could not be determined whether animal sources of protein such as meat and fish were more likely to be associated with T2DM than was plant-based protein.47 The role of dietary protein in T2DM pathogenesis may be mediated through several mechanisms, including increases in saturated fats, nitrates, advanced glycation end products.47 Therefore, protein intake should be focused on foods low in saturated fats and nitrates such as dairy milk and plant proteins.48
In addition, evidence from primarily Western populations has noted that vegetarian diets may be beneficial in reducing T2DM risk.49 Compared with other race/ethnic groups, a higher proportion of South Asians follow a vegetarian diet.50,51 However, a vegetarian diet alone does not necessarily equate to beneficial health outcomes, and vegetarian diets can still be associated with the consumption of foods high in saturated fats, trans fats, and sugars.51 In the MASALA study, approximately 38% of the participants reported consuming a vegetarian diet, and 58% of those were women.52 In adjusted analyses, compared with a nonvegetarian diet, consuming a vegetarian diet was associated with lower BMI, visceral fat, waist circumference, fasting glucose, insulin resistance, total and LDL cholesterol, and odds of fatty liver.52 Vegetarian participants from the MASALA study reported more weekly consumption occasions of beans, legumes, and whole grains compared with nonvegetarians, while nonvegetarians reported greater weekly consumption of desserts.52 After adjustment for the consumption of desserts, visceral fat deposition remained significantly higher in nonvegetarians compared with vegetarians, thereby suggesting a differential association in the type of fat accumulated by higher consumption of sweets and desserts.52 Interestingly, there were no significant differences in fruit and vegetable consumption between vegetarians and nonvegetarians. However, a more recent longitudinal analysis of MASALA data used a healthy plant-based diet index (hDPI) to further assess the association between healthy and unhealthy plant-based diets and cardiometabolic risk. The hPDI was created by assigning healthy plant foods (whole grains, fruits, vegetables, herbs/spices, nuts, legumes, and tea/coffee) positive scores, while less healthy plant foods (juices, sugar-sweetened beverages, refined grains, deep-fried snacks/pickles, potatoes, coconut, and sweets) and animal foods received reverse scores.53 Results of this analysis noted that at baseline, the hPDI was inversely associated with insulin resistance and HbA1c. There were no associations with insulin secretion fasting and 2-h glucose, triglycerides, HDL cholesterol, C-reactive protein, adiponectin, or subclinical atherosclerosis. After 5 years of follow-up, there were no associations between hPDI and measures of glycemia (glucose and HbA1C) and dyslipidemia. However, the hPDI was inversely associated with a lower odds of fatty liver and lower risk of incident T2DM.53 These findings suggest that a healthy plant-based diet has the potential to reduce the risk of incident T2DM in the long term.
Physical activity
Lower physical activity increases the risk of T2DM,54–56 and sedentary time independent of physical activity has been associated with poorer metabolic parameters.57 Comparative data from the United Kingdom indicate that South Asians tend to be less physically active than their White European counterparts.58,59 While most Americans do not meet physical activity guidelines,60 it appears that South Asian Americans fare even worse on physical activity metrics than members of other race/ethnic populations. South Asians in MASALA were the least physically active compared with all race/ethnic groups in MESA and expended approximately half the metabolic equivalents (MET) minutes per week compared with their White counterparts.11 Additionally, low levels of exercise were independently correlated with prediabetes risk, while greater sedentary time spent watching television was significantly associated with odds of prevalent T2DM.61 Given the often-complex interplay between lifestyle practices and culture, interventions aimed at increasing physical activity and improving diet quality in South Asians should also take into account sociocultural factors relevant to the U.S. South Asian community.
Sleep disorders
Disturbances in sleep, including obstructive sleep apnea (OSA) or sleep-disordered breathing (SDB), are associated with T2DM.62–64 A cross-sectional analysis of MASALA participants described the association between SDB and the risk of OSA associated with HbA1c. The Berlin Questionnaire (BQ) was used to screen for SDB and risk of OSA.65 Overall, 24% of participants in the MASALA cohort were at a high risk of OSA, with men having a significantly higher risk compared with women.66 When using the WHO Asian cut-points for BMI to assess risk,67 28% of participants were at risk of OSA. The prevalence of high OSA risk differed significantly by glycemic status, with 18% of those with normal glucose tolerance, 24% of those with prediabetes, and 32% of those with T2DM being classified as high risk. HbA1c was also positively associated with a high risk of OSA.66 In addition, approximately 41% of participants met the criteria for SDB. Hyperglycemia, male sex, hypertension, waist circumference, overweight/obesity, tobacco smoking, and alcohol consumption were all positively associated with SDB.66 These results indicate a high risk of SDB and OSA in middle-aged South Asians, which is associated with hyperglycemia as well as increased body weight. Therefore, clinicians should consider screening for hyperglycemia in South Asian individuals who may need treatment for OSA or SDB. Furthermore, while the BQ uses BMI >30 kg/m2 as an indicator of obesity to identify OSA risk, the Asian cut point of BMI >27.5 kg/m2 to identify obesity should be substituted for use in screening South Asians for the risk of OSA.
Sociocultural factors
Acculturation, religiosity, and migration
Similarly to other immigrant groups in the United States, upon migration, South Asians experience a process of acculturation whereby a degree of adaptation to a new host environment and adoption of new cultural practices occurs. While previous studies have examined the relationship between acculturation and cardiometabolic health, this work has been limited by the reliance on simplified proxy measures of acculturation, such as English fluency or years spent in the United States. Such measures do not account for the complex and multidimensional nature of the overall impact of acculturation on health. The MASALA study, therefore, sought to examine the association between acculturation strategies, using several cultural and behavioral indicators, and cardiovascular risk factors among South Asian immigrants in the United States. Using 12 measures of acculturation in a latent class analysis, South Asian immigrants were characterized into three possible acculturation strategies: (1) separation (preference for South Asian culture over the U.S. culture); (2) integration (similar preference for both South Asian and U.S. cultures); and (3) assimilation (preference for the U.S. over South Asian culture).68 Women in the integration class had the lowest prevalence of T2DM (16.4%) and prediabetes (29.7%) compared with women in the separation class who had the highest prevalence of T2DM (29.3%) and prediabetes (31.5%).69 Women in the integration class also had significantly lower 2-h glucose and insulin compared with women in the separation class. There were no differences, however, amongst men for any of the glycemic indices by acculturation class.69 South Asian women who had assimilation or integration strategy of acculturation had a more favorable glycemic profile and lower T2DM risk compared with those with a separation strategy. Therefore, prevention programs aimed at South Asian populations should take into account differences in acculturation strategy and sex and focus on the adoption and retention of healthy lifestyle practices.
Traditionally, data also indicate that migration to and duration of residence in the United States tends to increase the risk of T2DM in immigrant populations.70–72 However, a cross-sectional analysis comparing participants from MASALA with their counterparts living in Chennai, India, from the Cardiometabolic Risk Reduction Surveillance (CARRS) Study found that the age-adjusted T2DM prevalence was higher among native South Asians in India (38%) than immigrants in the United States (24%), while the age-adjusted prediabetes prevalence was lower in South Asians in India (24%) than in the United States (33%).73 After adjustment for age, sex, waist circumference, and systolic blood pressure, living in the United States was associated with an increased odds for prediabetes (odds ratio 1.2) and a decreased odds for T2DM (odds ratio 0.5).73 Together, these results challenge the paradigm that migration and acculturation are associated with increased T2DM and highlight the notion of possible health benefits upon migration. However, it is important to note that participants from the MASALA study had high levels of educational attainment and socioeconomic status. Therefore, these findings may not be generalizable to other populations, and more research is needed to understand the relationship between acculturation and T2DM risk in immigrant populations. Furthermore, although South Asians in the United States had a lower T2DM prevalence than those living in India, the prevalence in this group was still considerably higher than that in the general U.S. population.74 Therefore, factors besides education attainment may play a large role in T2DM in Asian Indians.
Religion may also play a role in cardiometabolic health amongst South Asians. The South Asian community is heterogeneous and is comprised of members of many different nationalities and religious affiliations, including Hinduism, Sikhism, Islam, Jainism, and Christianity. Several of these religious affiliations have dietary, smoking, and alcohol prohibitions that may influence T2DM risk. A cross-sectional analysis in MASALA examined the associations between religious affiliations and overweight or obese BMI. The risk of overweight and obesity was higher amongst those who identified as Hindu, Sikh, or Muslim compared with those with no religious affiliation.75 This in part may be explained by the notion that religious activities and festivals may involve the consumption of foods high in refined sugars or saturated fats. However, further research is needed to explore the associations between religion and health in South Asian communities. This may help to target interventions tailored around specific religious customs and beliefs to help lower T2DM in this high-risk population.
Psychosocial determinants
Social support and social networks are beneficial for improving lifestyle practices related to T2DM such as diet and physical activity.76–79 However, previously, little was known about the impacts of social support on diet and physical activity in the South Asian American community, who tend to have low levels of physical activity and different dietary practices compared with their White counterparts.80 A cross-sectional analysis evaluating the relationship between self-reported physical activity and egocentric network data of MASALA participants found that having network members who exercised or having exercise partners increased physical activity minutes in men.81 However, in women, the association was only significant if the exercise partner was a spouse.81 Therefore, prevention efforts to improve physical activity in South Asians, particularly in women, should adopt a family-centered approach, whereby spouses serve as exercise partners for each other to help mitigate T2DM risk.
Another mixed-methods analysis from MASALA found that the adult children of South Asian immigrants who were raised in the United States can provide a positive influence on their parents’ diet and exercise practices, which, in turn, can improve their risk of T2DM.82 Such forms of influence included social support, introducing Western concepts of health, holding their parents accountable for lifestyle changes, and acting as role models for healthy behavior.82 These findings support the notion that adult South Asian children may serve as a trusted source of health information for their parents and that prevention efforts that target a family-centered approach involving adult children may be beneficial. These findings also imply that U.S. born South Asians may have healthier diet and physical activity practices and thereby lower T2DM risk compared with their parents. This could have implications for improved prevention strategies in South Asian communities. However, more research is needed among second- and third-generation South Asians to test this hypothesis.
Negative social interactions can also have a large influence on cardiometabolic health. Previous data indicate that perceived discrimination can have adverse impacts on mental and physical health as well as health behaviors.83 However, few studies have reported associations between self-reported discrimination (SRD) and poorer physical and mental health outcomes among South Asians in the United States. In order to assess the associations between SRD and dietary intake, the MASALA study measured SRD using the Everyday Discrimination Scale.84 While SRD was not related to the consumption of fruits and vegetables, SRD was independently associated with weekly consumption of desserts.85 It is, therefore, possible that the consumption of sweets may be used as a coping mechanism during times of stress amongst South Asians. Additional findings from MASALA also indicate that a high chronic burden score was also significantly associated with T2DM risk.61 Therefore, efforts to prevent T2DM in South Asians should also focus on healthy methods of stress reduction.
Environmental determinants
Built environment
Increasing evidence indicates that characteristics of the built environment, such as the presence of sidewalks and access to parks, can impact physical activity.60,86,87 However, very little data exists as to the impact of the built environment on physical activity in South Asian Americans. A cross-sectional analysis from the MASALA study sought to understand the association between neighborhood walkability and walking for transport among South Asian American men and women. A Neighborhood Walkability Walk Score,88 which included measures of population density, distance to amenities, distance to parks and schools, and characteristics such as intersection density and block length, was used to determine neighborhood walkability. Weekly minutes spent walking for transport was assessed using a detailed, semi-qualitative questionnaire adapted from the Cross-Cultural Activity Participation Study.89 In both unadjusted and adjusted models, a positive association was found between the Walk Score and weekly minutes spent walking for transportation in men. After adjusting for age, BMI, study site, income, and education, each 10-point increase in the Walk Score was associated with 13.2 additional minutes per week of walking for transport for men.90 However, there was no significant association between the Walk Score and walking for transport in women.90 Additional research is needed to better understand the reasons why walking for transport may differ between South Asian American men and women. This would better help to inform interventions and increase physical activity in this population.
Environmental pollutants
Persistent organic pollutants (POPs) encompass a wide variety of manmade chemicals and are of recent concern regarding T2DM risk. Several studies have noted the association between POP exposure and T2DM.91–93 It is possible that some of the increased risk of T2DM in South Asian migrants may be due to previous exposures to POPs, as high levels of human and environmental levels of dichlorodiphenyltrichloroethane (DDT) contamination have been found in India.94 Results of an analysis examining the association between high plasma concentrations of POPs and various diabetes pathologies in MASALA pilot study participants found that despite an average length of duration in the United States of 26 years, MASALA participants had plasma p,p′-DDE and p,p′-DDT levels that more closely resemble those reported in the South Asian diaspora compared with those reported in the general U.S. population.95 Plasma DDT was associated with 2.4-fold increased odds of fatty liver. Plasma DDT was also associated with decreased insulin sensitivity and was further associated with 1.55- and 1.72-fold increased odds of prediabetes and diabetes, respectively, among MASALA participants in confounder-adjusted models.95 Therefore, it is possible that the mechanism by which DDT increases T2DM risk is by increases in ectopic fat and insulin resistance. These results have implications for South Asian migrants throughout the diaspora as well as those still living in South Asia who may have been exposed to high levels of POPs at some point along the life course. Interventions that target ectopic fat metabolism may be useful in reducing T2DM risk in populations with high POP exposures.
T2DM and prediabetes screening
BMI as a screening criterion
Overweight and obesity are well-established risk factors for T2DM. However, evidence suggests that the relationship between body weight and T2DM is not uniform, and that T2DM at low BMI is considerably more prevalent in Asian populations.2,96,97 However, few studies have evaluated BMI cut points for T2DM screening among Asian Americans with a rigorous assessment of test characteristics. A cross-sectional analysis of data from 1663 Asian Americans enrolled in four different U.S. cohort studies, aged 45 years and older, without a prior diagnosis of T2DM aimed to ascertain the most appropriate BMI cut-point for T2DM risk identification in middle to older aged Asian Americans. The analysis included data on South Asian, Japanese, Chinese, Korean, or mixed Asian ethnicity Americans from MASALA, the North Kohala Study, the Seattle Japanese American Community Diabetes Study (JACDS), and the University of California San Diego (UCSD) Filipino Health Study.98 Sensitivity, specificity, and positive predictive values for T2DM were computed at 1-unit increments of BMI, ranging from 22 to 27.5 kg/m2, and area under the receiver operating characteristic curve (AUROC) for logistic regression models was computed. Results of the analysis indicated that current recommendations to screen individuals with BMI ≥ 25 kg/m2 would have low sensitivity and specificity for identifying T2DM risk, and would fail to identify over 30% of Asian Americans with T2DM. However, screening for T2DM at BMI ≥ 23 kg/m2 in Asian Americans had a sensitivity of 84.7% and would fail to identify approximately 15% of Asian Americans with T2DM.98 Therefore, a BMI cut-point of 23 kg/m2 for T2DM screening should be considered as it improves sensitivity and would enable early diagnosis and treatment of T2DM in Asian Americans, including South Asians. Hence, the current American Diabetes Association (ADA) guidelines recommend that T2DM screening should be considered in individuals who are overweight as defined by BMI ≥ 25 kg/m2 or BMI ≥ 23 kg/m2 for Asian Americans and have one more known T2DM risk factor, including Asian ethnicity.99
However, even in normal weight (BMI <23 kg/m2), South Asians may have a high prevalence of cardiometabolic abnormality. The analysis of pooled data from the MASALA and MESA defined cardiometabolic abnormality as having two or more of the following; HDL-C lower than 1.03 mmol/L (<40 mg/dL) in men or 1.29 mmol/L (<50 mg/dL) in women, or any use of lipid-lowering medication; fasting triglyceride level ≥ 1.7 mmol/L (150 mg/dL); fasting plasma glucose ≥ 5.6 mmol/L (100 mg/dL) or any use of glucose-lowering medication; and blood pressure ≥ 130/85 mm Hg or any use of antihypertensive medication.100 The prevalence of cardiometabolic abnormality in normal weight varied significantly by race/ethnicity: 21.0% in White Americans, 32.2% in Chinese Americans, 31.1% in African Americans, 38.5% in Hispanic Americans, and 43.6% in South Asian Americans.100 The increased risk of cardiometabolic abnormality in normal weight in all other race/ethnic groups compared with White individuals remained significant, even after adjustment for relevant covariates, including ectopic fat. For the equivalent number of cardiometabolic abnormalities at a BMI of 25.0 kg/m2 in white participants, the corresponding BMI values were 22.9 kg/m2 in African Americans, 21.5 kg/m2 in Hispanics, 20.9 kg/m2 in Chinese Americans, and 19.6 kg/m2 in South Asians.100 Therefore, screening for cardiometabolic abnormalities such as T2DM or prediabetes, even in normal or underweight may be an important consideration. Furthermore, healthy lifestyle practices should be implemented for the prevention of cardiometabolic diseases at all levels of BMI.
Diabetes and prediabetes diagnostic criteria
Current ADA criteria recommend the use of HbA1c as a screening and diagnostic test for T2DM and prediabetes.99 However, its accuracy may differ in certain race/ethnic populations. The prevalence of T2DM by HbA1c, fasting glucose, and 2-h glucose was compared in 3016 participants from Chennai and Delhi, India, from the CARRS-2 Study to 757 Asian Indian immigrants in the MASALA study. T2DM was defined as fasting glucose ≥ 7.0 mmol/L, 2-h glucose ≥ 11.1 mmol/L, or HbA1c ≥ 6.5%. Isolated HbA1c diabetes was defined as HbA1c ≥ 6.5% with fasting glucose <7.0 mmol/L and 2-h glucose <11.1 mmol/L.101 Results of the study found that a high proportion of T2DM cases were identified by using the HbA1c criteria alone in the absence of elevated fasting or 2-h glucose. Namely, in CARRS-2 Chennai, 19.4% of T2DM cases were diagnosed by isolated HbA1c, while 26.8% of cases were diagnosed by isolated HbA1c in CARRS-2 Delhi, and 10.8% of the new cases were diagnosed by isolated elevated HbA1c in MASALA.101 Among participants from MASALA, those with T2DM by isolated HbA1c had a significantly lower mean fasting glucose, 30-min post-challenge glucose, 2-h glucose, lower cholesterol, and greater mean HOMA-β and resistin than those diagnosed by fasting or 2-h measures. HOMA-IR was also significantly lower in those with T2DM by isolated HbA1c compared with those diagnosed by fasting glucose, while fasting insulin was significantly higher. Similar results were also seen amongst participants from the CARRS-2 study. Therefore, while HbA1c may have high specificity for T2DM diagnosis in other race/ethnic populations, this may not be the case in South Asians. It is also possible that in South Asian populations, the isolated HbA1c criteria may identify individuals with milder glucose intolerance compared with those diagnosed with fasting or 2-h measures, and HbA1c may either capture those who are in earlier stages of the natural history of T2DM development or may serve to misdiagnose individuals. Therefore, the use of HbA1c alone as a diagnostic test for T2DM may not be an appropriate strategy. The combination of both an HbA1c and a 2-h glucose test would likely capture the highest number of people with T2DM. However, this strategy may not always be practical given the burdensome nature of the oral glucose tolerance test. Given that the use of HbA1c as a clinical diagnostic assessment for T2DM is increasingly widespread, there is a need for comprehensive studies to understand the diagnostic accuracy and outcomes of various glycemic measures, particularly in South Asian populations.
Conclusions
The MASALA study is currently the only longitudinal cohort assessing the determinants of cardiometabolic diseases in South Asians in the United States. Evidence from MASALA suggests that South Asians experience a disproportionately high burden of prevalent and incident T2DM compared with members of other race/ethic groups. This notion is highlighted by evidence indicating that South Asians (1) are more insulin-resistant than White Americans, African Americans, and Hispanic Americans, even at lower BMI; (2) demonstrate early impairments in insulin secretion; and (3) demonstrate increased deposition of ectopic fat.
In addition to possible innate predisposition, South Asian migrants to the United States experience a myriad of factors related to acculturation, religious beliefs, migration, and psychosocial stressors, all of which may impact physical activity and dietary practices. Evidence from the MASALA study indicates that individuals who have a mixture of traditional South Asian as well as Western cultural practices have a lower risk of T2DM compared with their counterparts on either extreme. Furthermore, a healthy plant-based diet was found to be more beneficial than both an animal protein–based diet, as well as a vegetarian diet comprised of fried foods, snacks, and desserts. Given the important role of the family in South Asian culture, interventions to improve dietary health and physical activity practices should incorporate family-based approaches and adult children may be important influencers for healthier behaviors for their South Asian parents. More research is needed to understand the cardiometabolic health of second- and third-generation South Asians in the United States who may have different dietary and physical activity practices compared with their migrant counterparts. Furthermore, the high prevalence of T2DM at lower levels of BMI in South Asians suggest that using overweight and obesity as the main criteria for T2DM screening may fail to identify risk in a substantial proportion of individuals. Healthy dietary and physical activity practices should be recommended for all individuals, regardless of BMI or weight status.
Given the high risk of T2DM in South Asians independent of BMI, it is possible that South Asians develop T2DM through differing pathophysiological pathways aside from obesity-driven insulin resistance. Additional research is needed to better understand the etiology and pathophysiology of disease in South Asians, compared with other ethnic groups. Specifically, attention should be paid to the complete mechanistic pathway and the relative contributions of insulin resistance, β cell function, and ectopic fat deposition on T2DM development, particularly in those with lower BMI. In addition, South Asians tend to have lower lean muscle mass than individuals of other race/ethnic populations and more research investigating the differences between resistance and aerobic exercise on muscle mass, mitochondrial function, and the subsequent effects on insulin resistance in South Asian populations is needed. Studies focusing on the role of epigenetic and metabolomic biomarkers of early disease are also warranted, as are studies focusing on the contributions of tobacco and alcohol use, sleep duration and quality, and environmental pollutants. Lastly, there are very few behavioral intervention studies among U.S. South Asians to determine the optimal culturally adapted lifestyle changes that are most effective in reducing ectopic fat and insulin resistance, promoting β cell function, and delaying the onset of T2DM. Such studies would serve to mitigate T2DM development in this high-risk population.
Acknowledgements
U.P.G. was supported by supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant number P30-DK-111024. The MASALA study was supported by Grants R01HL093009 and R01HL120725 from the National Heart, Lung, and Blood Institute, the National Center for Research Resources, and the National Center for Advancing Translational Sciences, the National Institutes of Health (NIH); and through the UCSF-CTSI Grants UL1RR024131 and UL1TR001872. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH. The authors thank the other investigators, the staff, and the participants of the MASALA study for their valuable contributions.
Footnotes
Competing interests:
The authors declare no competing interests.
References
- 1.The Asian Population: 2010. 24.
- 2.Chiu M, Austin PC, Manuel DG, et al. 2011. Deriving Ethnic-Specific BMI Cutoff Points for Assessing Diabetes Risk. Diabetes Care 34: 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oza-Frank R & Narayan KMV. 2010. Overweight and Diabetes Prevalence Among US Immigrants. Am. J. Public Health 100: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng YJ, Kanaya AM, Araneta MRG, et al. 2019. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 322: 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjana RM, Deepa M, Pradeepa R, et al. 2017. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 5: 585–596. [DOI] [PubMed] [Google Scholar]
- 6.Anjana RM, Rani CSS, Deepa M, et al. 2015. Incidence of Diabetes and Prediabetes and Predictors of Progression Among Asian Indians: 10-Year Follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care 38: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 7.Narayan KMV & Kanaya AM. 2020. Why are South Asians prone to type 2 diabetes? A hypothesis based on underexplored pathways. Diabetologia 63: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaya AM, Kandula N, Herrington D, et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study: Objectives, Methods, and Cohort Description. Clin. Cardiol 36: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. 2002. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am. J. Epidemiol 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 10.Kanaya AM, Chang A, Schembri M, et al. 2019. Recruitment and retention of US South Asians for an epidemiologic cohort: Experience from the MASALA study. J. Clin. Transl. Sci 3: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanaya AM, Herrington D, Vittinghoff E, et al. 2014. Understanding the High Prevalence of Diabetes in U.S. South Asians Compared With Four Racial/Ethnic Groups: The MASALA and MESA Studies. Diabetes Care 37: 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gujral UP, Narayan KMV, Kandula NR, et al. 2020. Incidence of diabetes and prediabetes and predictors of glycemic change among South Asians in the USA: the MASALA study. BMJ Open Diabetes Res. Care 8: e001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertoni AG, Burke GL, Owusu JA, et al. 2010. Inflammation and the Incidence of Type 2 Diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 33: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasuga M 2006. Insulin resistance and pancreatic β cell failure. J. Clin. Invest 116: 1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SE 2003. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46: 3–19. [DOI] [PubMed] [Google Scholar]
- 16.Saad MF, Knowler WC, Pettitt DJ, et al. 1991. A two-step model for development of non-insulin-dependent diabetes. Am. J. Med 90: 229–235. [PubMed] [Google Scholar]
- 17.Coleman NJ, Miernik J, Philipson L, et al. 2014. Lean versus obese diabetes mellitus patients in the United States minority population. J. Diabetes Complications 28: 500–505. [DOI] [PubMed] [Google Scholar]
- 18.Chan WB, Tong PCY, Chow CC, et al. 2004. The associations of body mass index, C-peptide and metabolic status in Chinese Type 2 diabetic patients. Diabet. Med 21: 349–353. [DOI] [PubMed] [Google Scholar]
- 19.Kodama K, Tojjar D, Yamada S, et al. 2013. Ethnic Differences in the Relationship Between Insulin Sensitivity and Insulin Response: A systematic review and meta-analysis. Diabetes Care 36: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gujral UP, Narayan KMV, Kahn SE, et al. 2014. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: The MASALA study. J. Diabetes Complications 28: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling C & Groop L. 2009. Epigenetics: A Molecular Link Between Environmental Factors and Type 2 Diabetes. Diabetes 58: 2718–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluckman PD, Hanson MA, Buklijas T, et al. 2009. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol 5: 401–408. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty C, Doss CGP, Bandyopadhyay S, et al. 2014. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. WIREs RNA 5: 697–712. [DOI] [PubMed] [Google Scholar]
- 24.Flowers E, Gadgil M, Aouizerat BE, et al. 2015. Circulating micrornas associated with glycemic impairment and progression in Asian Indians. Biomark. Res 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bain JR, Stevens RD, Wenner BR, et al. 2009. Metabolomics Applied to Diabetes Research: Moving From Information to Knowledge. Diabetes 58: 2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadgil MD, Sands C, Lewis MR, et al. 2020. 1581-P: Circulating Metabolites Are Associated with Glycemic Measures in South Asians. Diabetes 69:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Gorman A & Brennan L. 2015. Metabolomic applications in nutritional research: a perspective. J. Sci. Food Agric 95: 2567–2570. [DOI] [PubMed] [Google Scholar]
- 28.Bouchard-Mercier A, Rudkowska I, Lemieux S, et al. 2013. The metabolic signature associated with the Western dietary pattern: a cross-sectional study. Nutr. J 12: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newgard CB, An J, Bain JR, et al. 2009. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 9: 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huffman KM, Shah SH, Stevens RD, et al. 2009. Relationships Between Circulating Metabolic Intermediates and Insulin Action in Overweight to Obese, Inactive Men and Women. Diabetes Care 32: 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Vasan RS, et al. 2011. Metabolite profiles and the risk of developing diabetes. Nat. Med 17: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadgil MD, Anderson CA, Kandula NR, et al. 2015. Dietary Patterns Are Associated with Metabolic Risk Factors in South Asians Living in the United States. J. Nutr 145: 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhupathiraju SN, Guasch-Ferré M, Gadgil MD, et al. 2018. Dietary Patterns among Asian Indians Living in the United States Have Distinct Metabolomic Profiles That Are Associated with Cardiometabolic Risk. J. Nutr 148: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadgil M, Sands C, Lewis M, et al. 2020. Circulating Metabolite Profiles of Diet Patterns in South Asians in the United States. Curr. Dev. Nutr 4: 1402–1402. [Google Scholar]
- 35.Gupta LS, Wu CC, Young S, et al. 2011. Prevalence of Diabetes in New York City, 2002–2008: Comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care 34: 1791–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand SS, Tarnopolsky MA, Rashid S, et al. 2011. Adipocyte Hypertrophy, Fatty Liver and Metabolic Risk Factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS ONE 6: e22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu WC, Araneta MRG, Kanaya AM, et al. 2015. BMI Cut Points to Identify At-Risk Asian Americans for Type 2 Diabetes Screening. Diabetes Care 38: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sattar N & Gill JM. 2014. Type 2 diabetes as a disease of ectopic fat? BMC Med. 12: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah AD, Kandula NR, Lin F, et al. 2016. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int. J. Obes 2005 40: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flowers E, Lin F, Kandula NR, et al. 2019. Body Composition and Diabetes Risk in South Asians: Findings From the MASALA and MESA Studies. Diabetes Care 42: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran A, Snehalatha C, Mary S, et al. 2006. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49: 289–297. [DOI] [PubMed] [Google Scholar]
- 42.Weber MB, Ranjani H, Staimez LR, et al. 2016. The Stepwise Approach to Diabetes Prevention: Results From the D-CLIP Randomized Controlled Trial. Diabetes Care 39: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu: Diet and risk of type II diabetes: the role of… - Google Scholar. Accessed July 27, 2020. https://scholar.google.com/scholar_lookup?journal=Diabetologia&title=Diet+and+risk+of+Type+II+diabetes:+the+role+of+types+of+fat+and+carbohydrate&author=FB+Hu&volume=44&publication_year=2001&pages=805-817&pmid=11508264&.
- 44.Mohan V, Radhika G, Sathya RM, et al. 2009. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br. J. Nutr 102: 1498–1506. [DOI] [PubMed] [Google Scholar]
- 45.Misra A, Khurana L, Isharwal S, et al. 2008. South Asian diets and insulin resistance. Br. J. Nutr 101: 465–473. [DOI] [PubMed] [Google Scholar]
- 46.Kelemen LE, Anand SS, Vuksan V, et al. 2003. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J. Am. Diet. Assoc 103: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 47.Wang ET, de Koning L & Kanaya AM. 2010. Higher Protein Intake Is Associated with Diabetes Risk in South Asian Indians: The Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) Study. J. Am. Coll. Nutr 29: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian S, Xu Q, Jiang R, et al. 2017. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 9: 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenkins DJ, Kendall CW, Marchie A, et al. 2003. Type 2 diabetes and the vegetarian diet. Am. J. Clin. Nutr 78: 610S–616S. [DOI] [PubMed] [Google Scholar]
- 50.Singh PN, Arthur KN, Orlich MJ, et al. 2014. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am. J. Clin. Nutr 100: 359S–364S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaacks LM, Kapoor D, Singh K, et al. 2016. Vegetarianism and cardiometabolic disease risk factors: Differences between South Asian and American adults. Nutr. Burbank Los Angel. Cty. Calif 32: 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Y, Kanaya AM, Kandula NR, et al. 2018. Vegetarian Diets Are Associated with Selected Cardiometabolic Risk Factors among Middle-Older Aged South Asians in the United States. J. Nutr 148: 1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhupathiraju Shilpa N, Shan Zhilei, Hu Frank B, et al. 2019. Abstract P235: A Healthy Plant-Based Diet Index is Favorably Associated With Cardiometabolic Risk Factors in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study. Circulation 139: AP235–AP235. [Google Scholar]
- 54.Jeon CY, Lokken RP, Hu FB, et al. 2007. Physical Activity of Moderate Intensity and Risk of Type 2 Diabetes: A systematic review. Diabetes Care 30: 744–752. [DOI] [PubMed] [Google Scholar]
- 55.Helmrich SP, Ragland DR, Leung RW, et al. 1991. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N. Engl. J. Med 325: 147–152. [DOI] [PubMed] [Google Scholar]
- 56.Laaksonen DE 2005. Physical Activity in the Prevention of Type 2 Diabetes. 54: 8. [DOI] [PubMed] [Google Scholar]
- 57.Bankoski A, Harris TB, McClain JJ, et al. 2011. Sedentary Activity Associated With Metabolic Syndrome Independent of Physical Activity. Diabetes Care 34: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams ED, Stamatakis E, Chandola T, et al. 2011. Assessment of physical activity levels in South Asians in the UK: findings from the Health Survey for England. J. Epidemiol. Community Health 65: 517–521. [DOI] [PubMed] [Google Scholar]
- 59.MRCP GYHLM, MBChB CL, MBChB MM, et al. 1996. Ethnic differences in public health awareness, health perceptions and physical exercise: Implications for heart disease prevention. Ethn. Health 1: 47–53. [DOI] [PubMed] [Google Scholar]
- 60.Haselwandter EM, Corcoran MP, Folta SC, et al. 2015. The Built Environment, Physical Activity, and Aging in the United States: A State of the Science Review. J. Aging Phys. Act 23: 323–329. [DOI] [PubMed] [Google Scholar]
- 61.Shah AD, Vittinghoff E, Kandula NR, et al. 2015. Correlates of prediabetes and type II diabetes in US South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Ann. Epidemiol 25: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tasali E, Mokhlesi B & Van Cauter E. 2008. Obstructive Sleep Apnea and Type 2 Diabetes: Interacting Epidemics. Chest 133: 496–506. [DOI] [PubMed] [Google Scholar]
- 63.Botros N, Concato J, Mohsenin V, et al. 2009. Obstructive Sleep Apnea as a Risk Factor for Type 2 Diabetes. Am. J. Med 122: 1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reichmuth KJ, Austin D, Skatrud JB, et al. 2005. Association of Sleep Apnea and Type II Diabetes. Am. J. Respir. Crit. Care Med 172: 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Netzer NC., Stoohs RA, Netzer CM, et al. 1999. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med [DOI] [PubMed] [Google Scholar]
- 66.Deol R, Lee KA, Kandula NR, et al. 2018. Risk of Obstructive Sleep Apnoea is Associated with Glycaemia Status in South Asian Men and Women in the United States. Obes. Med 9: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. 2004. The Lancet 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 68.Needham BL, Mukherjee B, Bagchi P, et al. 2017. Acculturation Strategies Among South Asian Immigrants: The Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study. J. Immigr. Minor. Health 19: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Sofiani ME, Langan S, Kanaya AM, et al. 2020. The relationship of acculturation to cardiovascular disease risk factors among U.S. South Asians: Findings from the MASALA study. Diabetes Res. Clin. Pract 161: 108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Afable-Munsuz A, Mayeda ER, Pérez-Stable EJ, et al. 2014. Immigrant Generation and Diabetes Risk Among Mexican Americans: The Sacramento Area Latino Study on Aging. Am. J. Public Health 104: S243–S250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oza-Frank R, Stephenson R & Venkat Narayan KM. 2011. Diabetes Prevalence by Length of Residence Among US Immigrants. J. Immigr. Minor. Health 13: 1–8. [DOI] [PubMed] [Google Scholar]
- 72.Nakanishi S, Okubo M, Yoneda M, et al. 2004. A comparison between Japanese-Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomed. Pharmacother 58: 571–577. [DOI] [PubMed] [Google Scholar]
- 73.Gujral UP, Narayan KMV, Pradeepa RG, et al. 2015. Comparing Type 2 Diabetes, Prediabetes, and Their Associated Risk Factors in Asian Indians in India and in the U.S.: The CARRS and MASALA Studies. Diabetes Care 38: 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caspard H, Jabbour S, Hammar N, et al. 2018. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes. Metab 20: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bharmal NH, McCarthy WJ, Gadgil MD, et al. 2018. The Association of Religious Affiliation with Overweight/Obesity Among South Asians: The Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study. J. Relig. Health 57: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukherjea A, Underwood KC, Stewart AL, et al. 2013. Asian Indian Views on Diet and Health in the United States. Fam. Community Health 36: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eyler AA, Brownson RC, Donatelle RJ, et al. 1999. Physical activity social support and middle- and older-aged minority women: results from a US survey. Soc. Sci. Med 49: 781–789. [DOI] [PubMed] [Google Scholar]
- 78.Lindsay Smith G, Banting L, Eime R, et al. 2017. The association between social support and physical activity in older adults: a systematic review. Int. J. Behav. Nutr. Phys. Act 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen LK, Shepherd MD & Parchman ML. 2004. Family Support, Diet, and Exercise Among Older Mexican Americans With Type 2 Diabetes. Diabetes Educ. 30: 980–993. [DOI] [PubMed] [Google Scholar]
- 80.Gujral UP, Pradeepa R, Weber MB, et al. 2013. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci 1281: 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thanawala MS, Siddique J, Schneider JA, et al. 2020. Association of Social Networks and Physical Activity in South Asians: The Mediators of Atherosclerosis in South Asians Living in America Cohort Study. J. Phys. Act. Health 17: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ram A, Dave SS, Lancki N, et al. 2020. Social influence of adult children on parental health behavior among South Asian immigrants: findings from the MASALA (Mediators of Atherosclerosis in South Asians Living in America) study. Ethn. Health 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascoe EA & Richman LS. 2009. Perceived Discrimination and Health: A Meta-Analytic Review. Psychol. Bull 135: 531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams DR, Yan Yu J.S. Jackson, et al. 1997. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J. Health Psychol 2: 335–351. [DOI] [PubMed] [Google Scholar]
- 85.Nadimpalli SB, Wang J, Kanaya AM, et al. 2017. Are Experiences of Discrimination Related to Poorer Dietary Intakes Among South Asians in the MASALA Study? J. Nutr. Educ. Behav 49: 872–876.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li F, Harmer PA, Cardinal BJ, et al. 2008. Built Environment, Adiposity, and Physical Activity in Adults Aged 50–75. Am. J. Prev. Med 35: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rutt CD & Coleman KJ. 2005. Examining the relationships among built environment, physical activity, and body mass index in El Paso, TX. Prev. Med 40: 831–841. [DOI] [PubMed] [Google Scholar]
- 88.Hirsch JA, Moore KA, Evenson KR, et al. 2013. Walk Score® and Transit Score® and Walking in the Multi-Ethnic Study of Atherosclerosis. Am. J. Prev. Med 45: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ainsworth BE, Irwin ML, Addy CL, et al. 1999. Moderate Physical Activity Patterns of Minority Women: The Cross-Cultural Activity Participation Study. J. Womens Health Gend. Based Med 8: 805–813. [DOI] [PubMed] [Google Scholar]
- 90.Kelley EA, Kandula NR, Kanaya AM, et al. 2016. Neighborhood Walkability and Walking for Transport Among South Asians in the MASALA Study. J. Phys. Act. Health 13: 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lind PM & Lind L. 2018. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia 61: 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carpenter DO 2008. Environmental Contaminants as Risk Factors for Developing Diabetes. Rev. Environ. Health 23:. [DOI] [PubMed] [Google Scholar]
- 93.Taylor KW, Novak RF, Anderson HA, et al. 2013. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ. Health Perspect 121: 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma BM, Bharat GK, Tayal S, et al. 2014. Environment and human exposure to persistent organic pollutants (POPs) in India: A systematic review of recent and historical data. Environ. Int 66: 48–64. [DOI] [PubMed] [Google Scholar]
- 95.La Merrill MA, Johnson CL, Smith MT, et al. 2019. Exposure to Persistent Organic Pollutants (POPs) and Their Relationship to Hepatic Fat and Insulin Insensitivity among Asian Indian Immigrants in the United States. Environ. Sci. Technol 53: 13906–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The DECODE-DECODA Study Group, on behalf of the European Diabetes Epidemiology Group & and the International Diabetes Epidemiology Group. 2003. Age, body mass index and Type 2 diabetes—associations modified by ethnicity. Diabetologia 46: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 97.Diaz VA, Mainous AG, Baker R, et al. 2007. How does ethnicity affect the association between obesity and diabetes? Diabet. Med 24: 1199–1204. [DOI] [PubMed] [Google Scholar]
- 98.Araneta MRG, Kanaya AM, Hsu WC, et al. 2015. Optimum BMI Cut Points to Screen Asian Americans for Type 2 Diabetes. Diabetes Care 38: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Association AD 2018. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 41: S13–S27. [DOI] [PubMed] [Google Scholar]
- 100.Gujral UP, Vittinghoff E, Mongraw-Chaffin M, et al. 2017. Cardiometabolic Abnormalities Among Normal-Weight Persons From Five Racial/Ethnic Groups in the United States. Ann. Intern. Med 166: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gujral U, Prabhakaran D, Pradeepa R, et al. 2019. Isolated HbA1c identifies a different subgroup of individuals with type 2 diabetes compared to fasting or post-challenge glucose in Asian Indians: The CARRS and MASALA Studies. Diabetes Res. Clin. Pract 153: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gadgil MD, Oza‐Frank R, Kandula NR, et al. 2017. Type 2 diabetes after gestational diabetes mellitus in South Asian women in the United States. Diabetes Metab. Res. Rev 33: e2891. [DOI] [PMC free article] [PubMed] [Google Scholar]


