Abstract
Clear cell carcinoma (CCC) is a rare low-grade malignant salivary gland carcinoma. EWSR1-ATF1 fusion has been characterized as a consistent finding in CCC, with breakpoints described between EWSR1 exon 11 and ATF1 exon 3. So far, over 100 cases of CCC harboring EWSR1 rearrangement arising from salivary gland of the oral cavity have been reported. Although EWSR1 involvement in these cases was confirmed by EWSR1 break-apart FISH indicating the translocation, sequence analysis for EWSR1-ATF1 fusion type has been reported only in three cases of CCC so far. Herein, we report a CCC case with novel EWSR1-ATF1 fusion (EWSR1 exon 15 and ATF1 exon 5) arising in minor salivary gland and review the role of the chimeric variants in some malignancies with EWSR1-ATF1 rearrangement. Current tumor was composed of the small nests of clear tumor cells and hyalized fibrous stroma. Immunohistochemically, the tumor was positive for AE1/AE3, CK5/6 and p63, negative for S100, Melan-A, SMA and CD10. After 8 months of follow-up, there are no evidence of recurrence.
Keywords: Clear cell carcinoma, Hyalinizing clear cell carcinoma, EWSR1, ATF1, Salivary gland tumor
Introduction
Clear cell carcinoma (CCC, also referred to as hyalinizing clear cell carcinoma) is a low-grade salivary gland carcinoma composed of tumor cells with clear cytoplasm and with/without hyalinization [1]. In 2011, Antonescu et al. reported that a specific gene fusion in CCC, which was not present in other salivary gland tumor: EWSR1 (Ewing sarcoma breakpoint region 1)-ATF1 (activating transcription factor 1) [2]. Since then, over 100 cases of CCC harboring EWSR1 rearrangement arising in the salivary gland of the oral cavity have been reported in the literature [2–10]. In these cases, EWSR1 involvements were demonstrated by EWSR1 break-apart FISH (fluorescence in situ hybridization), which is a useful diagnostic tool to confirm the EWSR1 rearrangement [2, 5]. In contrast, EWSR1 chimeric types were determined only in three cases by sequence analysis; EWSR1 exon 11 and ATF1 exon 3 fusion was observed in two cases, and EWSR1 exon 14 and CREB1 (cAMP response element binding protein 1) exon 5 fusion was observed in one case (Table 1) [2, 7, 9].
Table 1.
Clinical characters of clear cell carcinoma cases of the salivary gland origin
| Break point | Age(y)/sex | Location | Follow-up | First sign or symptom | |
|---|---|---|---|---|---|
| Antonescu et al. [2] | EWSR1 exon11—ATF1 exon3 | NA | NA | NA | NA |
| Nakano et al. [7] | EWSR1 exon11—ATF1 exon3 | 52/M | Root of tongue | No evidence of disease at 3 months | Painless swelling |
| Chapman et al. [9] | EWSR1 exon14—CREB exon5 | 68/F | Left base of tongue | No evidence of disease at 18 months | NA |
| This case | EWSR1 exon15—ATF1 exon5 | 45/F | Right hard palate | No evidence of disease at 8 months | Painless swelling |
EWSR1 Ewing sarcoma breakpoint region 1, ATF1 activating transcription factor 1, CREB1 cAMP response element binding protein 1, NA date not available
EWSR1-ATF1 and EWSR1-CREB1 gene fusion has been identified in various tumors, such as CCC, CCOC (clear cell odontogenic carcinoma), CCS (clear cell sarcoma), CCLTGT (clear cell sarcoma-like tumor of the gastrointestinal tract) and so on [2, 4, 11–13]. Among these tumors, the variability of EWSR1 and ATF1 intronic break points leads to various EWSR1-ATF1 chimeric variants [11–13]. However, there is some morphological overlaps with clear cell among the tumors harboring the gene fusion [4, 11, 13]. Herein, we report a case of CCC harboring new type of EWSR1-ATF1 fusion (EWSR1 exon 15 and ATF1 exon 5) and review the role of the chimeric variants in tumors harboring EWSR1-ATF1 rearrangement.
Case Report
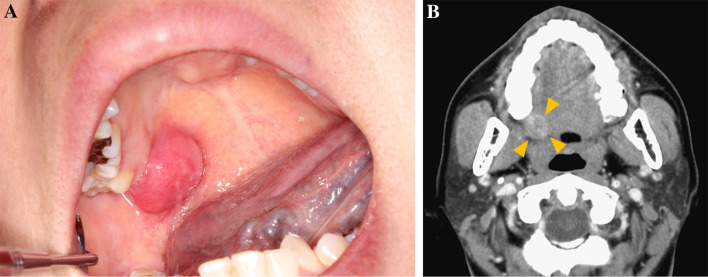
A 45-year-old woman was referred to the Osaka university dental hospital with a chief complaint of painless swelling in the right palate. The patient have noticed an increasing mass for approximately 4 years. The patient had no significant medical history. Intraoral examination revealed a solitary dome-shaped swelling on the right side of palate (Fig. 1a). Swelling was approximately 12 × 6 mm in size. An enhanced CT (computed tomography) revealed an ill-defined heterogeneous lesion without bone destruction (Fig. 1b, arrows). Under the provisional diagnosis of malignant salivary gland tumor, incisional biopsy was performed.
Fig. 1.
Clinical presentation. a Intraoral examination revealed a solitary dome-shaped swelling. b Computed tomography image in horizontal view revealed an ill-defined heterogenous lesion (arrows)
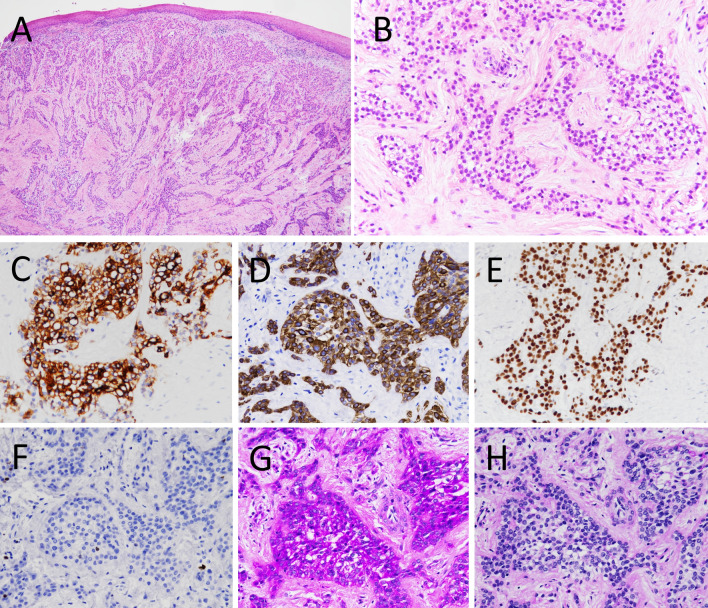
Histological examination revealed the proliferation of neoplastic epithelial cells with clear to eosinophilic cytoplasm. The tumor cells were arranged in nests and islands, which were surrounded by fibrous stroma (Fig. 2a, b). The tumor cells had round to oval nuclei and few mitosis. Immunohistochemistry showed that the tumor cells were focally positive for AE1/AE3 and diffuse positive for both CK (cytokeratin) 5/6 and p63 (Fig. 2c–e). MIB1 (Ki67) index was approximately 2% (Fig. 2f). In contrast, the tumor cells were negative for S100, Melan-A, SMA (smooth muscle actin) or CD10. Additionally, the tumor cells were positive for PAS (Periodic acid-Schiff) staining with diastase sensitivity (Fig. 2g, h). Based on these observations, CCC was highly suspected and then segmental maxillectomy was performed.
Fig. 2.
Histological and immunohistochemical findings in the biopsy specimen. a The tumor did not involve the overlying oral epithelium and showed typical histologic appearance of clear cell carcinoma (CCC). b High power view showed proliferation of epithelial cells with clear to eosinophilic cytoplasm. c AE1/AE3, d CK5/6 and e p63 were immunopositive. f MIB1 index was approximately 2%. g PAS was positive and h PAS diastase was negative
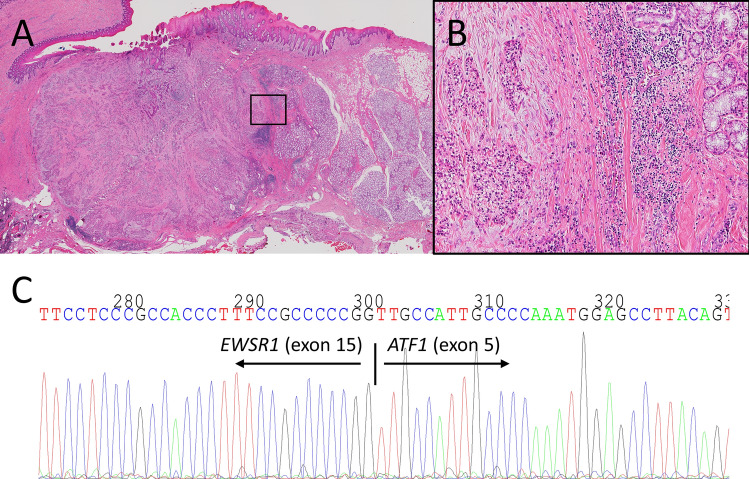
The surgical specimen showed the same histology of the biopsy. The tumor located adjacent to the salivary glands with the infiltrative margin (Fig. 3a, b). Angiolymphatic invasion, perineural invasion or necrosis were not seen. RT-PCR (reverse transcription-polymerase chain reaction) using cDNA obtained from the surgical specimen confirmed the presence of the EWSR1-ATF1 fusion gene. The direct sequence analysis revealed that exon 15 of EWSR1 was fused to exon 5 of ATF1 (Fig. 3c). This fusion type in our case is different from in previous reports of CCC (EWSR1 exon 11 and ATF1 exon 3) (Table 1) [2, 7, 9]. Then we made a final diagnosis of CCC of minor salivary gland. After 8 months of follow-up, there are no evidence of recurrence.
Fig. 3.
Histology and genetic analysis in the surgical specimen. a, b Tumor located adjacent to the salivary glands with the infiltrative margin (b was boxed area in a). c Direct gene sequencing showed exon 15 of EWSR1 and exon 5 of ATF1 fusion
Discussion
CCC is a low-grade salivary gland tumor and has EWSR1-ATF1 fusion in most cases [1, 2]. The EWSR1 fusion type by sequence analysis have been reported only in four cases (including our case) (Table 1) [2, 7, 9]. Because the fusion type of EWSR1-ATF1 in our case is a previously unreported type in CCC, it is necessary to distinguish CCC from other tumors including salivary gland tumor (such as mucoepidermoid carcinoma and myoepithelial carcinoma), malignant melanoma and CCOC (clear cell odontogenic carcinoma). The systemic inspection prior to operation showed no signs of tumor in the other organs, which suggested current tumor was not a metastatic lesion. Mucoepidermoid carcinoma can be ruled out because the tumor was lack of EWSR1 rearrangement [2]. The lack of myoepithelial makers (such as SMA and S100) excluded clear cell myoepithelial carcinoma, which has EWSR1 translocation [14]. The lack of S100 and Melan-A excluded malignant melanoma. It is often difficult to distinguish CCC from CCOC, because the two lesions have histologically and genetically similar features. Previous study reported that tumor location (in the oral mucosa versus in the jaws) is important to differentiate between the two [15]. Thus, we concluded that the current tumor was CCC with new EWSR1-ATF1 fusion type.
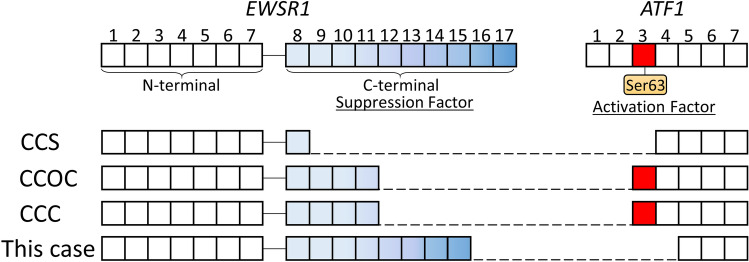
The determination of chimeric transcription types of the tumor is clinically important, because clinical prognosis depends on chimeric transcription [13, 16]. Compare between tumors harboring EWSR1-ATF1 fusion, clinical prognosis was significant different. CCC (EWSR1 exon 11 and ATF1 exon 3) is considered to be a low-grade malignancy, while CCS (EWSR1 exon 8 and ATF1 exon 4 in the majority case) is aggressive clinical behavior with a high propensity for local recurrence and metastasis (Fig. 4) [1, 2, 11, 13]. The clinical behavior of CCOC which harbors same EWSR1 exon 11 and ATF1 exon 3 fusion is similar to one of CCC (Fig. 4) [1, 12, 15]. Therefore, EWSR1-ATF1 chimeric variants are associated with clinical features.
Fig. 4.
Diagram showing the EWSR1-ATF1 fusion variants. CCS clear cell sarcoma, CCOC clear cell odontogenic carcinoma, CCC clear cell carcinoma
The EWSR1 gene spans about 40 kb of DNA with 17 exons and provides instructions for making the EWS (Ewing sarcoma protein) [17]. The protein includes a N-terminal transcriptional activation domain (encoded by exons 1–7) and a C-terminal RNA-binding domain (encoded by exons 8–17) [17]. Chromosomal translocations between this gene and various genes encoding transcription factors result in the production of chimeric proteins that are involved in tumorigenesis [11, 17]. These chimeric proteins usually consist of the N-terminal transcriptional activation domain of EWS fused to the C-terminal DNA-binding domain of the transcription factor protein [17]. However, the C-terminal domain of EWS, especially exon 15–17, can efficiently repress its N-terminal activation domain (Fig. 4) [18]. In the cases of CCS, the absence of EWSR1 C-terminal domain may be associated with its aggressive behavior (Fig. 4) [13].
On the other hand, exon 3 is an activation domain of ATF1. Phosphorylation at Ser63 located in exon 3 have an important roles in cell survival and proliferation (Fig. 4) [19]. CCC showed perineural invasion in 43.8–47.1% of the cases, angiolymphatic invasion in about 13% of the cases and necrosis in about 20% of the cases, which was significantly associated with recurrence [6, 8, 10, 15]. Our case had no perinural invasion, angiolymphatic invasion or necrosis, which may be associated with the absence of ATF1 exon 3 (Fig. 4). Considering these reports, the tumor in our case which do not have exon 3 of ATF1 but have the C-terminal domain of EWSR1, are considered to be a good prognosis. Thus EWSR1/ATF1 sequence analysis is important to elucidate the significance of the fusion type in their prognosis.
In summary, we have identified a novel EWSR1-ATF1 fusion gene in CCC arising in minor salivary gland of the oral cavity. Our case have shown clinical behavior in keeping with the low-grade classification.
Author Contributions
All authors contributed to this work. KH, and YU designed this study. KH, YU, SS, KO, YF, EM and ST interpreted the H&E and immunohistochemical findings. KH and MK performed the experiments and assembled data. KH, YU, YH and ST contributed to the writing of the manuscript. YI, SM and TU reviewed the radiological and clinical data. All authors reviewed and approved the manuscript for submission.
Funding
The authors have no funding, financial relationships.
Compliance with Ethical Standards
Conflict of interest
All authors state that they have no conflicts of interest.
Ethical Approval
This case report was approved by the Ethical Review Board of the Graduate School of Dentistry, Osaka University (No. R1-E46) and was performed in accordance with the Committee guidelines and regulations.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Katsutoshi Hirose, Email: hirose@dent.osaka-u.ac.jp.
Yu Usami, Email: yusami@dent.osaka-u.ac.jp.
Masaharu Kohara, Email: KOHARA@molpath.med.osaka-u.ac.jp.
Sunao Sato, Email: sunao@dent.osaka-u.ac.jp.
Yuri Iwamoto, Email: iwamotoyuri@dent.osaka-u.ac.jp.
Shumei Murakami, Email: shumei@dent.osaka-u.ac.jp.
Toshihiro Uchihashi, Email: utihasi@dent.osaka-u.ac.jp.
Kaori Oya, Email: kaori-oya@dent.osaka-u.ac.jp.
Yasuo Fukuda, Email: yfukuda@dent.osaka-u.ac.jp.
Yumiko Hori, Email: yumiko-hori@molpath.med.osaka-u.ac.jp.
Eiichi Morii, Email: morii@molpath.med.osaka-u.ac.jp.
Satoru Toyosawa, Email: toyosawa@dent.osaka-u.ac.jp.
References
- 1.El-Naggar AK, Chang JKC, Grandis JR, Takada T, Slootweg PJ. World Health Organization classification of head and neck tumours. 4. Lyon: IARC; 2017. pp. 168–169. [Google Scholar]
- 2.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 3.Jin R, Craddock KJ, Irish JC, Perez-Ordonez B, Weinreb I. Recurrent hyalinizing clear cell carcinoma of the base of tongue with high-grade transformation and EWSR1 gene rearrangement by FISH. Head Neck Pathol. 2012;6:389–394. doi: 10.1007/s12105-012-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilodeau EA, Weinreb I, Antonescu CR, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37:1001–1005. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 5.Shah AA, Legallo RD, van Zante A, et al. EWSR1 genetic rearrangements in salivary gland tumors: a specific and very common feature of hyalinizing clear cell carcinoma. Am J Surg Pathol. 2013;37:571–578. doi: 10.1097/PAS.0b013e3182772a15. [DOI] [PubMed] [Google Scholar]
- 6.Albergotti WG, Bilodeau EA, Byrd JK, Mims MM, Lee S, Kim S. Hyalinizing clear cell carcinoma of the head and neck: case series and update. Head Neck. 2016;38:426–433. doi: 10.1002/hed.23902. [DOI] [PubMed] [Google Scholar]
- 7.Nakano T, Yamamoto H, Nishijima T, et al. Hyalinizing clear cell carcinoma with EWSR1-ATF1 fusion gene: report of three cases with molecular analyses. Virchows Arch. 2015;466:37–43. doi: 10.1007/s00428-014-1676-5. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Prera JC, Kwan R, Tripodi J, et al. Reappraising hyalinizing clear cell carcinoma: a population-based study with molecular confirmation. Head Neck. 2017;39:503–511. doi: 10.1002/hed.24637. [DOI] [PubMed] [Google Scholar]
- 9.Chapman E, Skalova A, Ptakova N, et al. Molecular profiling of hyalinizing clear cell carcinomas revealed a subset of tumors harboring a novel EWSR1-CREM fusion: report of 3 cases. Am J Surg Pathol. 2018;42:1182–1189. doi: 10.1097/PAS.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 10.Yang XH, Liu L, Shi YY, Hu YJ, Hu QG, Zhang P. Hyalinizing clear cell carcinoma of salivary gland origin in the head and neck: clinical and histopathological analysis. Int J Oral Maxillofac Surg. 2018;47:692–698. doi: 10.1016/j.ijom.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36:e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 12.Kujiraoka S, Tsunematsu T, Sato Y, et al. Establishment and characterization of a clear cell odontogenic carcinoma cell line with EWSR1-ATF1 fusion gene. Oral Oncol. 2017;69:46–55. doi: 10.1016/j.oraloncology.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang WL, Mayordomo E, Zhang W, et al. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Mod Pathol. 2009;22:1201–1209. doi: 10.1038/modpathol.2009.85. [DOI] [PubMed] [Google Scholar]
- 14.Skálová A, Weinreb I, Hyrcza M, et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol. 2015;39:338–348. doi: 10.1097/PAS.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 15.Bilodeau EA, Hoschar AP, Barnes LL, Hunt JL, Seethala RR. Clear cell carcinoma and clear cell odontogenic carcinoma: a comparative clinicopathologic and immunohistochemical study. Head Neck Pathol. 2011;5:101–107. doi: 10.1007/s12105-011-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–557. doi: 10.1038/nrc3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantile M, Marra L, Franco R, et al. Molecular detection and targeting of EWSR1 fusion transcripts in soft tissue tumors. Med Oncol. 2013;30:412. doi: 10.1007/s12032-012-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossow KL, Janknecht R. The Ewing’s sarcoma gene product functions as a transcriptional activator. Cancer Res. 2001;61:2690–2695. [PubMed] [Google Scholar]
- 19.Hailemariam K, Iwasaki K, Huang BW, Sakamoto K, Tsuji Y. Transcriptional regulation of ferritin and antioxidant genes by HIPK2 under genotoxic stress. J Cell Sci. 2010;123:3863–3871. doi: 10.1242/jcs.073627. [DOI] [PMC free article] [PubMed] [Google Scholar]