Abstract
Objective:
Older persons with HIV (PWH) are particularly susceptible to life-space restrictions. The aims of this study included: 1) using global positioning system (GPS) derived indicators as an assessment of time spent at home among older adults with and without HIV; 2) using ecological momentary assessment (EMA) to examine real-time relationships between life-space, mood (happiness, sadness, anxious), fatigue and pain; and 3) determining if number of daily social interactions moderated the effect of life-space on mood.
Methods:
Eighty-eight older adults (PWH n=54, HIV-negative [HIV−] n=34) completed smartphone-based EMA surveys assessing mood, fatigue, pain, and social interactions four times/day for two weeks. Participants’ smartphones were GPS enabled throughout the study. Mixed-effects regression models analyzed concurrent and lagged associations among life-space and behavioral indicators of health.
Results:
PWH spent more of their time at home (79% vs 70%, z=−2.08; p=.04) and reported lower mean happiness (3.2 vs 3.7; z=2.63; p=.007) compared to HIV− participants. Controlling for covariates, more daily social interactions were associated with higher ratings of real-time happiness (b=0.12; t=5.61; df = 1087.9; p< 0.001). Similar findings were seen in lagged analyses: prior day social interactions (b=0.15; t=7.3; df = 1024.9; p<.0001) and HIV status (b=−0.48; t=−2.56; df= 1026.8; p=0.01) attenuated the effect of prior day time spent at home on happiness.
Conclusions:
Accounting for engagement in social interactions reduced the significant effect of time spent at home and lower happiness. Interventions targeting social isolation within the context of constricted life-space may be beneficial for increasing positive mood in older adults, and especially relevant to older PWH.
Keywords: remote assessment, mobile health, mood, well-being, isolation, aging
Objective
Life-space, defined as the geographical area in which a person lives their daily life, is a gerontological concept most commonly assessed via self-report1–4. A more constricted life-space (i.e. spending more time within the constraints of one’s home or near home) is associated with poorer physical and mental health in older age1,5,6 and higher levels of social isolation7. Social isolation itself is associated with a variety of mental and physical health issues as well an increased risk of morbidity and mortality8,9. As individuals age, medical issues, relocation, and death of people in their networks increase the risk for social isolation. These associations might be particularly problematic for older people living with HIV (PWH).
Medical advancements such as combination antiretroviral therapy (ART)10 have become more widespread, resulting in decreased mortality, increased life expectancy and thus, an increase in the numbers of older PWH in the U.S. Older PWH may be especially vulnerable to age-associated social network changes, which can decrease adaptive coping and increase their risk of depressive symptoms11, especially for heterosexual PWH or those of color12. Emlet (2006) assessed the risk of social isolation based on social network patterns and found that the risk of social isolation in older PWH was greater than 38%, which was greater than a 25% risk for younger PWH (ages 20–39). In terms of objective measures of life-space, few currently exist in the literature for PWH. Prior findings by our group13 showed preliminary evidence that older PWH may have a constricted life-space as they spent the majority of their time at home (74%)13 and alone (63%)14, which was concurrently associated with lower ratings of happiness. This estimate is likely to increase given that millions have been instructed to ‘shelter-in-place’ and to refrain from in-person social interactions, especially for older adults and those with underlying health conditions, due to the spread of COVID-19 around the globe15.
While studies rely heavily on retrospective self-report, valid and reliable methods using mobile technologies can quantify real-time daily activity. One method is ecological momentary assessment (EMA), which has demonstrated higher reliability and greater sensitivity to change compared to laboratory-based based reporting13,16. Furthermore, Global Positioning System (GPS)-indicators are a valid form of life-space assessment compared to other measures of geographical activity18 and have been associated with mood in clinical samples20. Utilizing GPS-indicators in combination with EMA methodology may provide a more objective, time-sensitive, and non-invasive method of obtaining information on how the size of life-space is associated with behavioral indicators of health (e.g., mood, fatigue, pain).
Very little is known about life-space among older PWH and its associations with mood, in comparison to a HIV− group, as compared to other gerontological samples1,2,5–7,17,18. Using GPS indicators, we aimed to compare the percent of time spent at home vs outside the home between older PWH and HIV−. Given previous findings that older PWH spend the majority of their time at home13 and alone14, we hypothesized that PWH would spend more time at home than HIV−. We also aimed to examine concurrent and lagged (i.e., responses from one day to predict mood or life-space the next day) between- and within-persons relationships between life-space and mood, fatigue, and pain among older adults, and if these relationships were moderated by HIV serostatus. We hypothesized that spending more time at home would be associated with lower ratings of real-time positive mood (i.e., happy mood), higher ratings of real-time negative mood (i.e., depressed, anxious) and increased fatigue and pain for the overall sample. We hypothesized the within-person relationships would associations would be stronger for PWH compared to those living without HIV and exist at the concurrent and next day levels of analyses. Finally, we aimed to determine if the relationship between number of social interactions per day and mood moderated the effect of life-space on mood in the overall sample.
Methods
Participants
A total of 54 PWH and 34 HIV− community-dwelling participants were included in this study. Data was collected between 2016 and 2019. Participants were recruited from a participant pool at the University of California San Diego (UCSD) HIV Neurobehavioral Research Program (HNRP) or through the community (HIV clinics, flyers and community centers). Inclusion criteria included: aged 50+, ability to provide written informed consent and English fluency. Participants were excluded if they had a non-HIV neurological disorder (i.e. stroke), head injury with loss of consciousness ≥30 minutes, serious mental illness (e.g., schizophrenia), or indication of a severe learning disability (as indicated by a score of <70 on The Wide Range Achievement Test 4th Edition Reading21. Participants with a positive urine toxicology result on day of testing (any drugs with the exception of marijuana) or alcohol breathalyzer test were rescheduled for a different day. Those with less than seven days of GPS data (N=2) and any day with less than 24 samples were excluded from analyses. UCSD’s Institutional Review Board approved this study, and all participants demonstrated decisional capacity to consent for research and provided written, informed consent22.
Materials and Procedures
All participants completed a comprehensive in-person baseline assessment consisting of neuromedical and neurobehavioral evaluations. Participants were given a Samsung Galaxy S 4.2 YP-GI1 8GB smartphone with 4G Android Operating system equipped with GPS logger (https://gpslogger.app/). They were provided an individualized, face-to-face tutorial with a trained research associate on how to complete EMA surveys and operate/troubleshoot the smartphone, and were sent home with a smartphone operating manual. Participants were compensated for both in-person study visits at a rate of $15/hour (standard rate at the HNRP). Bonus compensation ($1/survey) was provided for each EMA survey participants completed, with an option of $1/survey × 56 surveys = $56. Participants were provided with an additional $15 for returning the study mobile devices.
HIV disease characteristics
An HIV/HCV antibody point-of-care rapid test (Miriad-MedMira™) was administered to all participants to assess HIV serostatus and confirmed by the Western Blot Test. For PWH, self-report data were obtained regarding HIV characteristics, such as estimated duration of infection, Nadir CD4, and medications. Viral load detectability (<50 copies/mL) and current CD4 count was measured in blood plasma.
Psychiatric/substance use assessments
All participants were administered the Composite International Diagnostic Interview (CIDI version 2.1), a semi-structured clinical interview23,24, which allowed for the assignment of current and lifetime histories of Diagnostic and Statistical Manual-Fourth Edition (DSM-IV) diagnoses for Major Depressive Disorder (MDD), and Substance Use Disorders (SUD; abuse and/or dependence) for any of the following substances: alcohol, cannabis, opioids, methamphetamine, cocaine, sedatives and hallucinogens. Current depressive symptoms were assessed using the Beck-Depression Inventory, 2nd Edition (BDI-II)25. Self-report measures of social support (i.e. Duke Social Support Index26 and Medical Outcomes Survey (MOS) Social Support Survey27) were also administered.
14-Day EMA and GPS Tracking
Following their baseline visit, participants completed 14-days of mobile health data collection. Participants were asked to respond to four smartphone-based EMA surveys per day for 14-days for a maximum possible of 56 total observations per individual; these surveys were spaced to occur in the morning, midday, afternoon, and evening approximately 3–4 hours apart according to each participant’s sleep-wake schedule. The study phone alert sounded to signal participants to complete each survey; alarms could be silenced for 10-minute intervals, and a reminder alarm sounded every two minutes during this 10-minute window. If the phone was lost or stolen, the study phone’s operating system was encrypted, and the study phones were locked so the survey program could not be exited. Data was downloaded when the phone was returned.
EMA surveys asked a variety of questions regarding mood, fatigue, pain, and social interactions. Mood (happy, depressed, anxious, worried) and fatigue questions (e.g., “I feel tired…”) were assessed via a 5-point scale (1=not at all to 5=very much). Responses for anxious and worried were highly correlated and therefore averaged to create a measure of “anxious.” To assess pain, participants were asked What is your pain level right now? (1=minimal or no pain to 10=severe pain). Participants were also asked how many times they socialized with someone else (“spent more than 5 minutes talking/communicating with someone else”) since the last alarm. During the baseline visit, research staff clarified this item included talking via the phone or video but not text communication (e.g., email, texting, social media). Response choices were assessed via a 5-point scale (0=no interactions to 4=four or more interactions), and averaged per study day, per participant. Overall, the 88 participants provided a total of 4,423 EMA responses. Aggregate mood ratings were significantly correlated with baseline measurements: Happiness (Spearmen’s r(86)=−0.43, p<.0001) and depression (r(86)=0.65, p<.0001) with Beck Depression Inventory-II scores, anxiety with Beck Anxiety Inventory-II scores (r(86)=0.38, p=.0003), fatigue with the Profile of Mood States Fatigue subscale (r(83)=0.59, p<.0001).
GPS data collection
The mobile application GPS logger was installed on the study phones, which logged the location information of participants locally on the device and produced one XML-based GPS Exchange Format file for every 24-hour sampling periods. Longitude, latitude, timestamps, speed, direction, and altitude were recorded at a 5-minute interval, producing a maximum of 288 samples per day. All daily XML files were pre-processed with Python scripts for error cleaning and noise filtering.
Distance between coordinate points were measured by the great-circle distance, which applied the Haversine formula28 to each serially sampled GPS coordinate, producing the shortest distance over the earth’s surface from one point to the other. Density-Based Spatial Clustering of Applications with Noise29, was used to estimate participants’ home location by applying it to at least an hour of sleep-time (2–5am) data points with the search radius of 100 feet and minimal of 12 data points (for further details, see Text, Supplemental Digital Content 1).
After the home location computed, for each data point five great-circle distance classes were calculated: (a) at home (within 100 meters from estimated home center), (b) not at home (>100 meters from home), (c) around home (>100 meters and <=800 meters from home), (d) in neighborhood (>100 and <=1,600 meters from home), and (e) away from home (>1,600 meters from home)19.
Despite efforts to encourage regular charging of the devices, review of the raw data demonstrated that in some cases the device recorded less than the anticipated 288 samples per day. Participants with more than 14 days of data were truncated, excluding past the first 14 days the participant had the device. The number of samples per day, per participant, was calculated and used to adjust the average distance calculations in order to limit errors introduced by sample frequency variances. Total GPS points at home were divided by the total GPS points collected per day to create percent at home, both daily and overall average, per person, over the time enrolled in the study. The same set of identifiers for participants were assigned to the GPS data and thus available days of EMA survey data were matched to correspond with available days of GPS data.
Statistical Analyses.
Group differences in demographic and psychiatric characteristics were carried out using independent t-tests, χ2 tests, and Fisher’s exact tests as appropriate. Group differences in average EMA survey results and GPS points across the two-week period were calculated using Mann-Whitney Test, and related non-parametric effect sizes reported30. Associations between mean levels of mood, social interactions, and life-space were conducted using Spearman’s r. Linear mixed-effects models (LMM) with subject-specific random intercepts controlling for study day were conducted for all analyses. First, relationships between concurrent mood and life-space measures were assessed for the overall sample. Pain, anxious, and depressed mood ratings with highly skewed distribution were log10 transformed to improve normality of distribution prior to fitting LMM. In addition, multiple testing for the related outcomes (i.e., EMA mood ratings) was corrected using the Benjamini-Hochberg (BH) procedure (k=5, the number of the multiple tests performed). Next, for the significant concurrent relationships in the overall sample, interactive effects of HIV status and life-space, and social interactions and life-space on mood were investigated with concurrent and lagged analyses. If interactions were p>.05, they were removed from the model and rerun. Estimates and confidence intervals are expressed according to a 25% change in life-space. JMP version 13.0.0 and SPSS version 26.0.0.0, α <.05, were used for analyses, and R version 3.5.0 was used to create figures.
Results
Participant characteristics
Table 1 demonstrates the participants’ clinicodemographic characteristics by HIV group. Groups were matched on age, education, and ethnicity; there were significantly more men in the PWH group. PWH were more likely to: have low-income, never have been married, on disability, and live alone. There were no differences in levels of perceived social support (Table 1). PWH were more likely to have a diagnosis of lifetime MDD and current depressive symptomatology (although average BDI-II scores for the PWH group is within the minimal range [0–13]), lifetime or current psychiatric diagnosis, and lifetime alcohol, cannabis, or other substance use disorder.
Table 1.
Participant characteristics (N = 88) by HIV status: People Living with HIV (PWH) and HIV-individuals.
| Mean (SD), Median [IQR], or Count (%) | |||||
|---|---|---|---|---|---|
| PWH (n = 54) | HIV− (n = 34) | t, z, χ2 | df | p-value | |
| Demographics | |||||
| Age | 60.0 (6.34) | 59.5 (6.74) | −0.31 | 66.9 | 0.75 |
| Education | 14.04 (2.51) | 14.97 (2.58) | 1.55 | 69.0 | 0.12 |
| Male | 44 (82%) | 18 (53%) | 8.16 | 1 | 0.008 |
| Sexual Orientation | <.0001 | ||||
| Gay/Bisexual | 38 (70.4%) | 8 (24.2%) | |||
| Heterosexual | 14 (25.9%) | 25 (75.8%) | |||
| Other | 2 (3.7%) | 0 | |||
| Ethnicity | 0.88 | ||||
| NH White | 35 (65%) | 22 (65%) | |||
| NH Black | 12 (22%) | 6 (18%) | |||
| Hispanic | 5 (8%) | 4 (12%) | |||
| Other | 2 (4%) | 2 (6%) | |||
| Psychosocial Characteristics | |||||
| Income Level | 0.02 | ||||
| Low | 32 (60%) | 10(29%) | |||
| Medium | 16 (30%) | 13 (38%) | |||
| High | 3 (6%) | 8 (24%) | |||
| Don’t Know | 3 (6%) | 3 (9%) | |||
| Source of income | 0.0005 | ||||
| Employment | 14 (26%) | 15 (44%) | |||
| Disability | 23 (43%) | 2 (6%) | |||
| Retirement/Savings/Other | 17 (33%) | 17 (50%) | |||
| Employment Status | 3.4 | 1 | 0.07 | ||
| Currently Employed | 15 (28%) | 16 (47%) | |||
| Currently not Employed | 39 (72%) | 18 (53%) | |||
| Marital Status | 0.002 | ||||
| Married/Marriage-like Relationship | 5 (9%) | 11 (32%) | |||
| Divorced | 10 (19%) | 11 (32%) | |||
| Separated | 1 (2%) | 2 (6%) | |||
| Widowed | 4 (7%) | 2 (6%) | |||
| Never Married | 34 (63%) | 8 (24%) | |||
| Living alone | 32 (59%) | 12 (35%) | 4.8 | 1 | 0.05 |
| Currently on Disability | 31 (57%) | 3 (9%) | <.0001 | ||
| Duke Social Support Scale | 8.5 (1.9) | 8.4 (1.3) | −0.02 | 82.2 | 0.98 |
| MOS Overall Support Scaled Score | 3.6 (1.1) | 3.7 (1.1) | 0.42 | 70.3 | 0.67 |
| Beck Depression Inventory - II | 7.5 [2.14] | 2 [0,4] | 3.92 | 86 | 0.0002 |
| Psychiatric Characteristics | |||||
| LT MDD | 39 (72%) | 7 (21%) | 22.3 | 1 | <.0001 |
| Current Any Psychiatric Dx | 11 (20%) | 1 (3%) | 5 | 1 | 0.03 |
| LT Any Substance Dx | 39 (72%) | 16 (47%) | 5.6 | 1 | 0.02 |
| Current Any Substance Dx | 2 (4%) | 1 (3%) | 1.2 | 1 | 1 |
| HIV Disease Characteristics | |||||
| AIDS Status | 38(44%) | -- | -- | -- | -- |
| Estimated years of infection | 25.2 [18;29] | -- | -- | -- | -- |
| Current CD4 count | 714 [559;899] | -- | -- | -- | -- |
| Nadir CD4 count | 145 [42;275] | -- | -- | -- | -- |
| ARV Status (on ART) (N=54) | 50 (95%) | -- | -- | -- | -- |
| Plasma viral load undetectablea | 45 (96%) | -- | -- | -- | -- |
| Months exposure to ART | |||||
| 231.5 [153;278] | -- | -- | -- | -- | |
Note: ART = Anti-Retroviral Therapy; ARV = Anti-Retroviral; BMI = Body Mass Index; Dx = Diagnoses; HCV=Hepatitis C virus; IQR=interquartile range; LT= Lifetime; MDD = Major Depressive Disorder; MOS = Medical Outcomes Survey; NH = non-Hispanic.
Among those on ART, defined as <50 copies/mLch,
p-values were calculated using independent t-tests, χ2 tests, and Fisher’s exact tests as appropriate. Bolded p-values indicate p < .05.
Adherence to mobile technology protocol
Sixty-six participants (75%) had completely matched days of EMA and GPS data collected for all 14 days of the study, 19 participants (21.6%) had 10–13 days, while the remaining three individuals (3.4%) had 8–9 days of complete data.
Life-space and EMA measures by HIV status
Table 2 compares life-space and EMA mood and social interactions measures averaged across study time (i.e., 2-week maximum) by HIV status. Compared to HIV− participants, PWH spent more of their overall time at home, had significantly lower happiness ratings, higher depressed mood and pain ratings, and fewer daily social interactions, with small effect sizes (Mann-Whitney effect size calculations, |0.16| to |0.28|). Supplemental Table 1 depicts Spearman’s r correlations between life-space and EMA ratings averaged across study time for the entire sample are show.
Table 2:
Comparison of life-space, EMA mood ratings, and social interactions averaged across study time (i.e. 2 weeks maximum) by HIV status.
| Measures Mean (SD) |
PWH (n = 54) | HIV− (n = 34) | z | p-value | Effect Size |
|---|---|---|---|---|---|
| Mean % time at home | 79.0 (15.1) | 70.6 (20.7) | −2.08 | .04 | −0.22 |
| Mean Happy | 3.20 (0.9) | 3.72 (0.8) | 2.63 | 0.017a | 0.28 |
| Mean Depressed | 1.55 (0.8) | 1.21 (0.4) | −2.48 | 0.017a | −0.26 |
| Mean Anxious | 1.53 (0.9) | 1.19 (0.5) | −1.31 | 0.19a | −0.14 |
| Mean Pain Level | 2.96 (2.1) | 1.98 (1.5) | −2.49 | 0.017a | −0.27 |
| Mean Fatigue Level | 2.11 (0.7) | 1.87 (0.6) | −1.49 | 0.16a | −0.16 |
| Mean Number of Social Interactions | 1.69 (0.9) | 2.09 (0.8) | −2.23 | 0.03 | −0.24 |
Note: p-values were calculated using Mann-Whitney Test, Effect Size calculation: z/√(88); EMA: Ecological Momentary Assessment; bolded p-values indicate p < .05;
p-value was corrected for multiple testing of EMA mood ratings with Benjamini-Hochberg (BH) procedure method.
Linear mixed model results of concurrent daily percent of time spent at home predicting daily mood ratings
Across the entire sample, daily percent of time spent at home significantly only predicted concurrent daily happiness ratings within persons (b= −0.08, t= −3.3, df= 1096.8, p=0.001, after BH correction, p = 0.005, CI=−0.13 to −0.03), such that controlling for time, a 25% percent increase in time spent at home one day resulted in a 0.08-unit lower concurrent happiness rating. No other mood ratings were significantly associated with percent of time spent at home across the entire sample.
Next, we included HIV status and the interaction effect between HIV status and daily percent of time spent at home for only daily happiness (Table 3). There was no significant interaction of daily percent time spent at home and HIV status, and thus the interaction was removed and the model rerun. Figure 1 depicts the association of time spent at home and happiness by HIV status. More time spent at home was significantly associated with lower happiness (t = 3.20, df = 1097.4, p=0.001, CI=−0.13 to −0.03), such that for every 25% percent increase of time spent at home, concurrent happiness ratings were lower by 0.08 units (Figure 1). Furthermore, PWH had lower happiness ratings than HIV− (t=2.75, df = 85.1, p=0.007, CI=0.15 to 0.91), such that PWH rated their concurrent happiness 0.53-units lower than HIV−.
Table 3.
Linear mixed-effects model results of interaction effect of HIV status and daily percent of time spent at home on daily happiness ratings, controlling for time (study days).
| Daily Happy | Coefficient (95% CI) | t, df | p-value |
|---|---|---|---|
| Daily % at home | −0.08 (−0.15 to −0.008) | −2.18, 1086.8 | 0.03 |
| HIV status [HIV−] | 0.59 (0.99 to 1.08) | 2.37, 216.1 | 0.02 |
| HIV by daily % at home | −0.02 (−0.13 to 0.08) | −0.38, 1099.5 | 0.71 |
Note: Bolded p-values indicate p < .05; estimates and confidence intervals for life-space are expressed according to a 25% increase in daily % at home.
Figure 1:
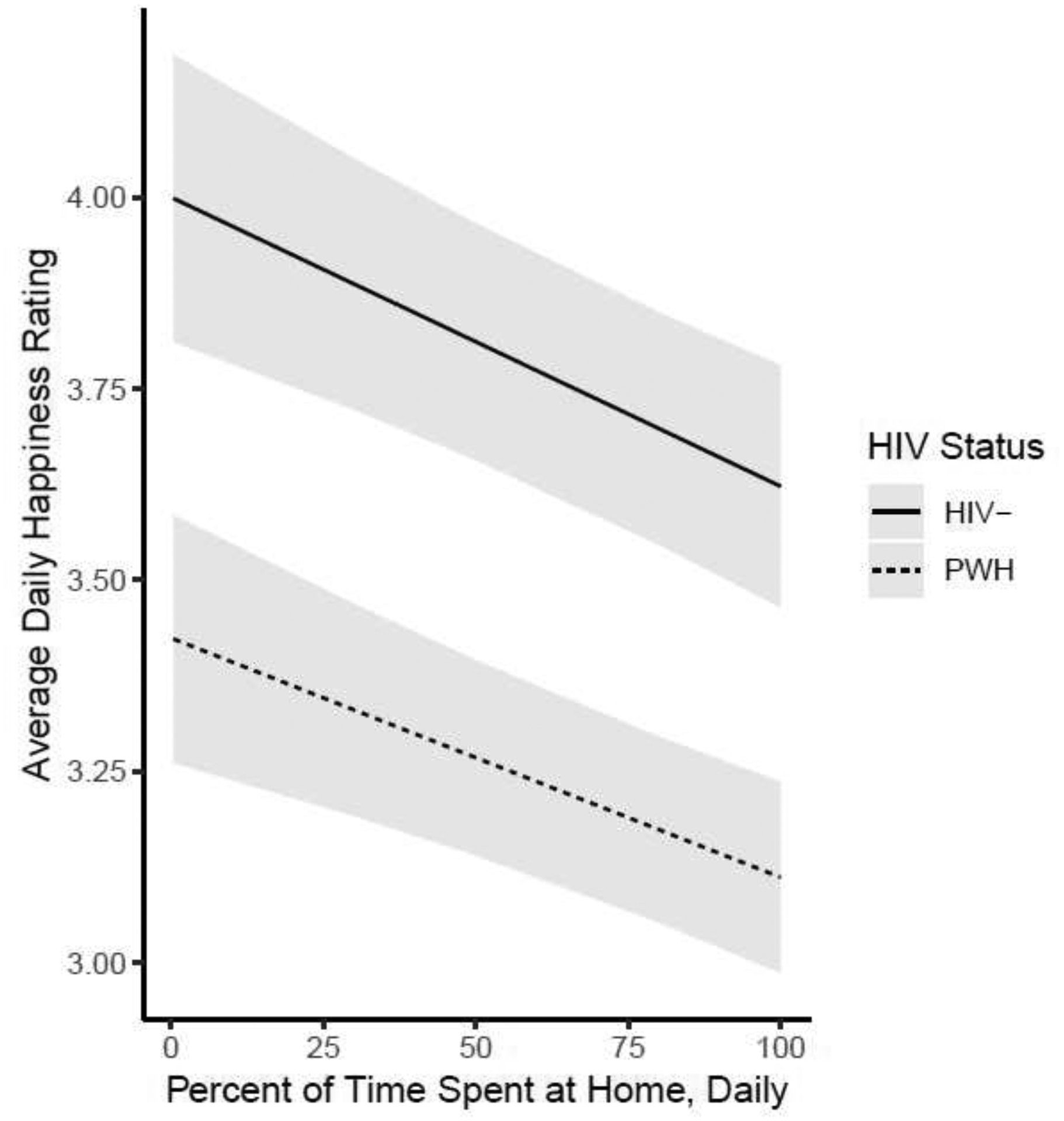
The association of time spent at home and concurrent happiness by HIV status, controlling for study day, shaded areas represent 95% Confidence Intervals.
Results of a multivariable model predicting daily happiness with time spent at home, controlling for demographic differences between HIV groups (i.e., sex, receiving disability, source of income, and whether or not they lived alone) and number of daily social interactions is shown in Table 4 (a model without number of daily social interactions is shown in Supplemental Digital Content, Table 2). The interaction between number of daily social interactions and life-space was not significant, thus removed from the model and re run. There was only a significant main effect within persons, such that an additional social interaction per day was associated with a 0.12 increase in concurrent happiness ratings (Table 4).
Table 4.
Linear mixed-effects model results of daily mobility predicting daily happy ratings, controlling for covariates and time (i.e., study days).
| Coefficient (95% CI) | t, df | p-value | |
|---|---|---|---|
| Daily Happy | |||
| Daily % at home | (t = −1.95, df= 1095.4) | ||
| −0.05 (−0.10 to 0.0003) | 0.05 | ||
| HIV Status [HIV−] | (t = 0.81, df= 79.9) | ||
| 0.18 (−0.27 to 0.63) | 0.42 | ||
| Disability [NO] | (t = 0.24, df= 79.8) | ||
| 0.06 (−0.45 to 0.58) | 0.81 | ||
| Sex [F] | (t =1.73, df= 79.9) | ||
| 0.36 (−0.05 to 0.77) | 0.09 | ||
| Source of Income [Disability] | (t = −0.72, df= 79.8) | ||
| −0.21 (−0.77 to 0.36) | 0.47 | ||
| Source of Income [Employment] | (t =0.37, df= 79.9) | ||
| 0.08 (−0.35 to 0.51) | 0.71 | ||
| Not living alone | (t = 1.82, df= 80.4) | ||
| 0.35 (0.04 to 0.73) | 0.07 | ||
| Number of Daily Interactions | (t = 5.61, df = 1087.9) | ||
| 0.12 (0.08 to 0.16) | <0.001 |
Note: Bolded p-value indicates p < .05; estimates and confidence intervals for life-space are expressed according to a 25% increase in daily % at home.
Lagged relationships between daily time spent at home and happiness ratings
As our concurrent analyses indicated that the only EMA mood variable related to time spent at home was happiness, we only investigated lagged relationships between daily percent of time spent at home and happiness. The relationship between daily happiness ratings and next day percent of time spent at home across the entire sample was not significant (b=−084; CI=−2.55 to 0.87; t=−0.966; df= 921.6; p=0.34). Table 5 Model A illustrates that for every 25% percent increase of time spent at home, happiness ratings the next day were lower by 0.05 units across the entire sample (Table 5, Model A). However, this relationship was no longer significant when controlling for significant main effects of HIV status, study day, and number of social interactions from the previous day (Table 5, Model B). HIV− participants had significantly higher next day happiness ratings than PWH over the duration of the study, and participants significantly rated their happiness lower every day (Table 5, Model B). Additionally, more social interactions from the previous day resulted in significantly higher next day happiness ratings (Table 5, Model B). The significant main effect of number of social interactions on next day happiness ratings by HIV status, controlling for daily percent of time spent at home and study day, is depicted in Figure 2.
Table 5.
Lagged linear mixed-effects model results of A) daily percent of time spent at home predicting next day happiness ratings, B) daily percent of time spent at home across HIV status, controlling for study day and number of interactions the previous day.
| Model A | Coefficient (95% CI) | t, df | p-value |
|---|---|---|---|
| Next day happiness rating | |||
| Daily % time at homea | −0.05 (−0.10 to −0.0002) | (t = −1.97, df= 1029.2) | 0.049 |
| Model B | |||
| Next day happiness rating | |||
| Daily % time at homea | −0.03 (−0.08 to 0.02) | (t = −1.15, df= 1026.8) | 0.25 |
| HIV Status [HIV−] | 0.48 (0.11 to 0.85) | (t = 2.56 df = 85.2) | 0.01 |
| Study Day | −0.02 (−0.02 to −0.009) | (t = −4.33, df = 967.9) | <.0001 |
| Number of Interactions | 0.15 (0.11 to 0.19) | (t = 7.33. df = 1024.9) | <.0001 |
Note: Bolded p-value indicates p < .05;
estimates and confidence intervals for life-space are expressed according to a 25% increase in daily % at home.
Figure 2:
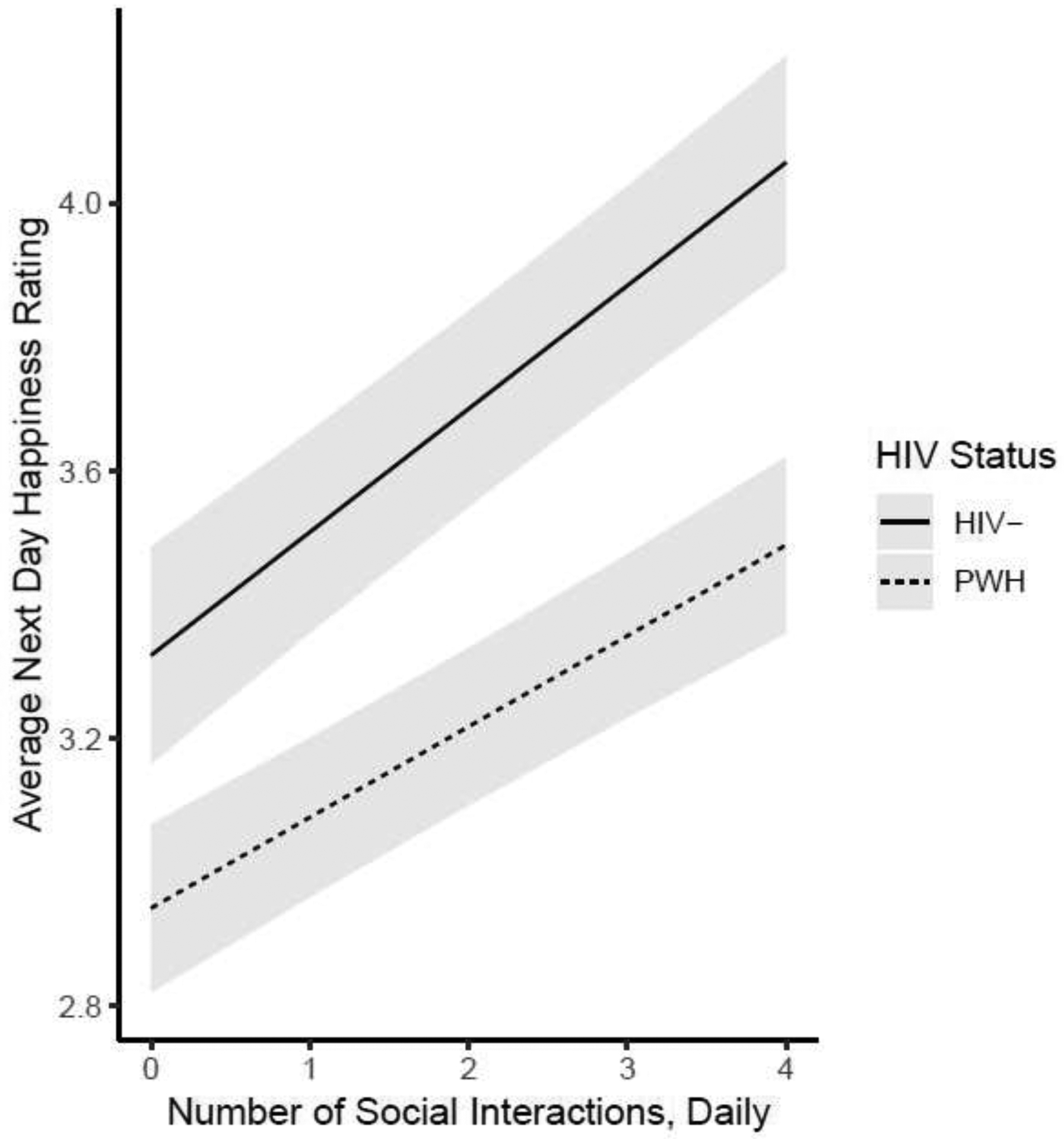
The association of number of daily social interactions and next day happiness by HIV status, controlling for study day, shaded areas represent 95% Confidence Intervals.
Conclusions
Our study found that 1) older PWH were more likely to have lower ratings of happiness, live alone, and spent more time at home than older HIV− individuals, and 2) increased time spent at home based on a GPS derived indicator and living alone were associated with lower concurrent happiness in both PWH and HIV− participants. However, when controlling for the number of social interactions on concurrent and next day ratings of happiness, increased time spent at home was no longer significantly associated with lower happiness ratings. In fact, only social interactions resulted in a significant increase in daily and next day happiness ratings, accounting for other significant demographic factors. Therefore, although spending more time at home was related to lower happiness ratings, this effect appeared to be explained by social interactions with others, for both PWH and HIV− groups.
In prior work from our group using EMA ratings of mood, we found that PWH were happier when they were with others, even though they reported increases in pain and fatigue14. This study uses GPS data to add an important layer of daily time spent at home, which places one at risk for low social interaction and lower happiness. Our findings lend support to empirically-validated interventions such as behavioral activation for depression, aimed to increase engagement in pleasurable daily life activities31,32. Our results suggest mental health interventions for older adults should include components focused on increasing social activity. While we did not have additional data on the type or quality of social interactions, this piece is also critical to investigate. Previous studies have identified quality of social network as more important to mental health than quantity/size33. While in-person interventions for increasing social activity could be made more accessible by utilizing community health workers34, home-based, virtual intervention methods (e.g., via computer) represent another possible and effective way to improve social activity among vulnerable older populations35,36.
Additionally, while our study showed no differences in levels of perceived social support at baseline between groups, PWH were less likely to participate in social interactions per day. Given that studies show social support may moderate the effect of stress on mood37, actively working this pathway by intentionally staying in contact with others may provide daily emotional relief. This is especially important among older PWH, who are more likely to already be staying at home, are more likely to live alone, and are at high risk for social isolation and feelings of loneliness12, 38. Although many interventions to reduce loneliness and social isolation exist for the general population of older adults39, there have been no interventions studies to our knowledge that address social barriers specific to PWH. For example, HIV-related stigma and discrimination40,41, shrinkage of social network due to HIV-related early mortality42, and earlier onset and greater accumulation of physically and emotionally debilitating geriatric syndromes43 each present unique HIV-specific barriers to social engagement and building social support.
Our findings are particularly important for older adults and older PWH during the ongoing COVID-19 pandemic, which has heightened stress and loneliness44. Older PWH are more likely to already be staying at home, who may live alone, and are more vulnerable to physical health consequences of the COVID-1945. Furthermore, housing arrangement, home-care conditions, and substance use may further impact life-space and social interaction patterns of older adults. While we did not have detailed information on these variables, data gathered during the COVID-19 pandemic should address underlying mechanisms that may promote protective effects. While our study informs what types of behaviors may be most beneficial, more research on use of mobile technologies, consideration of confidentiality, ethics, and large-scale feasibility in non-research settings is still needed.
Our study lends additional validity to the use of unobtrusive mobile technologies in research settings for understanding life-space among older adults with and without HIV. We did not find any significant associations between daily percent of time spent at home and concurrent depression, anxiousness, or fatigue, likely due to participants reporting overall lower levels of negative mood and fatigue, with limited variability. Additionally, while increased time spent at home was not associated with lower ratings of pain across the two groups, longitudinal studies have shown significant associations between indicators of pain and social functioning46,47 in the general population. As PWH reported higher levels of pain, future studies should investigate interactive relationships between daily percent of time spent at home and mood on multidimensional, real-time indicators of reported pain (e.g., the McGill Pain Questionnaire) rather than a single dimension assessment (pain intensity)48. Additionally, future studies should assess the role of frailty, as previous studies have shown frailty is associated with decreased social functioning and increased pain49,50. We also did not find a significant association between happiness ratings and next day time spent at home, only the reverse. This may be due to several others factors that likely are better predictors of next-day time spent at home and mood relationships which our study did not measure (e.g., sleep, planned activities, getting good news). Finally, while PWH may be more likely to be living alone, our finding that living alone was marginally associated with lower ratings of happiness when accounting for number of social interactions warrants further research. Understanding the type (e.g., in-person or with remote technologies) and quality of social interactions51 within proven strong and important social networks of PWH52 could potentially highlight the mechanism behind a protective effect for those living alone.
Our study’s strengths include the use of objective indicators of movement and real-time measures of daily mood for demographically matched HIV− and PWH older adults in research settings. Several limitations of our study should be considered. Our HIV− sample was relatively healthy and we were underpowered to investigate differences in mood or time spent at home by medical or psychiatric comorbidities. In terms of GPS indicators, there may be further linear or non-linear associations between mood, percent of time spent at home, and increased distance one travels, especially for individuals who have longer commutes to work53. Additionally, future studies would benefit from understanding reasons for being alone (e.g., preferences, access to social activities) as they may moderate the relationship between time spent at home and daily happiness. Lastly, as our sample of PWH was majority male, further exploration of sex differences is needed, as well as among race/ethnicity and the intersectionality of demographic factors on mood, life-space, and social networks12. Future research should focus on how the built and societal environment may influence daily fluctuating mood, restrict life-space and increased virtual social gatherings are impacting the overall health of our aging population.
Supplementary Material
Highlights.
1). What is the primary question addressed by this study?
Our study elucidated the relationship between constricted life-space, mood, fatigue, and pain, and social engagement among older adults living with and without HIV.
2). What is the main finding of this study?
Older adults living with HIV are more likely to spend more time at home, have lower ratings of daily happiness, and have fewer social interactions per day than HIV−. For older adults, regardless of HIV status, while spending less time at home is associated with higher ratings of happiness, participating in social interactions reduces this relationship (i.e. having more social interactions is associated with being happier, same day and next day).
3). What is the meaning of the finding?
In this time of COVID-19 precautions, staying in contact with others and maintaining social interactions may provide daily emotional support, particularly important for people living with HIV.
Conflicts of Interest and Source of Funding:
This work was supported by the National Institutes of Health: NIMH K23 MH107260 and NIMH K23MH107260 S1 to R.C.M., NIMH P30MH62512 (The HIV Neurobehavioral Research Center (HNRC)), NIA R01AG062387 to R.C.M., NIMH R01116902 to C.A.D, NIMH R21MH116104 to R.C.M., NIDA T32DA031098 to L.M.C., NIAAA F31AA027198 to E.W.P., NIAAA T32AA013525 to L.K. and R25MH108389 to C.N.P. R.C.M. is a co-founder of KeyWise, Inc. and a consultant for NeuroUX. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. For the remaining authors no conflicts of interest were declared.
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Jennifer Iudicello, Ph.D.; Donald Franklin, Jr.; Melanie Sherman; NeuroAssessment Core: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., Thomas D. Marcotte, Ph.D, Christine Fennema-Notestine, Ph.D., Debra Rosario, M.P.H., Matthew Dawson; NeuroBiology Core: Cristian Achim, M.D., Ph.D. (P.I.), Ana Sanchez, Ph.D., Adam Fields, Ph.D.; NeuroGerm Core: Sara Gianella Weibel, M.D. (P.I.), David M. Smith, M.D., Rob Knight, Ph.D., Scott Peterson, Ph.D.; Developmental Core: Scott Letendre, M.D. (P.I.), J. Allen McCutchan; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.) Susan Little, M.D., Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Lucila Ohno-Machado, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Anya Umlauf, M.S., Bin Tang, Ph.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker PS, Bodner EV, Allman RM: Measuring life-space mobility in community-dwelling older adults. Journal of the American Geriatrics Society. 2003; 51:1610–4. [DOI] [PubMed] [Google Scholar]
- 2.May D, Nayak US, Isaacs B: The life-space diary: a measure of mobility in old people at home. International Rehabilitation Medicine. 1985; 7:182–6. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann R, Lohmann N, Rowles GD, Ohta RJ: Aging and the social policy milieu. New York: Academic Press; 1983. [Google Scholar]
- 4.Stalvey BT, Owsley C, Sloane ME, Ball K: The Life Space Questionnaire: A measure of the extent of mobility of older adults. Journal of Applied Gerontology. 1999; 18:460–78. [Google Scholar]
- 5.Byles JE, Leigh L, Vo K, Forder P, Curryer C: Life space and mental health: a study of older community-dwelling persons in Australia. Aging & mental health. 2015; 19:98–106. [DOI] [PubMed] [Google Scholar]
- 6.Xue QL, Fried LP, Glass TA, Laffan A, Chaves PH: Life-space constriction, development of frailty, and the competing risk of mortality: the Women’s Health And Aging Study I. American journal of epidemiology. 2008; 167:240–8. [DOI] [PubMed] [Google Scholar]
- 7.Barnes LL, Wilson RS, Bienias JL, et al. : Correlates of life space in a volunteer cohort of older adults. Experimental aging research. 2007; 33:77–93. [DOI] [PubMed] [Google Scholar]
- 8.Waite L, Cornwell E: Social disconnectedness, perceived isolation, and health among older adults. Journal of Health and Social Behavior. 2009; 50(:31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steptoe A, Shankar A, Demakakos P, Wardle J: Social isolation, loneliness, and all-cause mortality in older men and women. PNAS. 2013; 110:5797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundermann EE, Erlandson KM, Pope CN, et al. : Current challenges and solutions in research and clinical care of older persons living with HIV: Findings presented at the 9th international workshop on HIV and aging. AIDS research and human retroviruses. 2019; 35:985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia H, Uphold CR, Wu S, et al. : Health-related quality of life among men with HIV infection: effects of social support, coping, and depression. AIDS Patient Care & STDs. 2004; 18:594–603. [DOI] [PubMed] [Google Scholar]
- 12.Emlet CA: An examination of the social networks and social isolation in older and younger adults living with HIV/AIDS. Health & social work. 2006; 31:299–308. [DOI] [PubMed] [Google Scholar]
- 13.Moore RC, Kaufmann CN, Rooney AS, et al. : Feasibility and acceptability of ecological momentary assessment of daily functioning among older adults with HIV. The American Journal of Geriatric Psychiatry. 2017; 25:829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paolillo EW, Tang B, Depp CA, et al. : Temporal associations between social activity and mood, fatigue, and pain in older adults with HIV: an ecological momentary assessment study. JMIR mental health. 2018; 5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coronavirus Disease (COVID-19) - events as they happen. [WHO website] March 16, 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed March 27, 2020.
- 16.Cain AE, Depp CA, Jeste DV: Ecological momentary assessment in aging research: A critical review. Journal of psychiatric research. 2009; 43:987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore RC, Depp CA, Wetherell JL, Lenze EJ: Ecological momentary assessment versus standard assessment instruments for measuring mindfulness, depressed mood, and anxiety among older adults. Journal of psychiatric research. 2016; 75:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenk AK, Witbrodt BC, Hoarty CA, et al. : Cellular Telephones Measure Activity and Lifespace in Community-Dwelling Adults: Proof of Principle. Journal of the American Geriatrics Society. 2011; 59:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan N, Lin G: Life-space characterization from cellular telephone collected GPS data. Computers, Environment and Urban Systems. 2013; 39:63–70. [Google Scholar]
- 20.Depp CA, Bashem J, Moore RC, et al. : GPS mobility as a digital biomarker of negative symptoms in schizophrenia: a case control study. NPJ digital medicine. 2019; 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson GS, Robertson GJ (eds): Wide range achievement test (WRAT4). Lutz, FL: Psychological Assessment Resources, 2006. [Google Scholar]
- 22.Jeste DV, Palmer BW, Appelbaum PS, et al. : A new brief instrument for assessing decisional capacity for clinical research. Archives of general psychiatry. 2007; 64:966–74. [DOI] [PubMed] [Google Scholar]
- 23.Wittchen HU, Robins LN, Cottler LB, et al. : Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The British Journal of Psychiatry. 1991; 159:645–53. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Üstün TB: The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI). International journal of methods in psychiatric research. 2004; 13:93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK: Beck depression inventory-II. San Antonio. 1996; 78:490–8. [Google Scholar]
- 26.Koenig HG, Westlund RE, George LK, et al. : Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993; 34:61–9. [DOI] [PubMed] [Google Scholar]
- 27.Sherbourne CD, Stewart AL: The MOS social support survey. Social science & medicine. 1991; 32:705–14. [DOI] [PubMed] [Google Scholar]
- 28.Chopde NR, Nichat MK: Landmark based shortest path detection by using A* and Haversine formula. International Journal of Innovative Research in Computer and Communication Engineering. 2013; 1:298–302. [Google Scholar]
- 29.Ester M, Kriegel HP, Sander J, Xu X: A density-based algorithm for discovering clusters in large spatial databases with noise. InKdd. 1996; 96:226–231. [Google Scholar]
- 30.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. Journal of experimental psychology: General. 2012; 141(1):2. [DOI] [PubMed] [Google Scholar]
- 31.Polenick CA, Flora SR: Behavioral activation for depression in older adults: Theoretical and practical considerations. The Behavior Analyst. 2013; 36:35–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CT, Yeh CJ, Lee MC, Lin HS, Chen VC, Hsieh MH, Yen CH, Lai TJ. Leisure activity, mobility limitation and stress as modifiable risk factors for depressive symptoms in the elderly: results of a national longitudinal study. Archives of Gerontology and Geriatrics. 2012; 54(2):e221–9. [DOI] [PubMed] [Google Scholar]
- 33.Pinquart M, Sorensen S. Influences on loneliness in older adults: A meta-analysis. Basic and applied social psychology. 2001; 23(4):245–66. [Google Scholar]
- 34.Blazer D Social Isolation and Loneliness in Older Adults—A Mental Health/Public Health Challenge. JAMA Psychiatry. 2020. [DOI] [PubMed] [Google Scholar]
- 35.Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthcare informatics research. 2012; 18(3):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibarra F, Baez M, Cernuzzi L, Casati F. A Systematic Review on Technology-Supported Interventions to Improve Old-Age Social Wellbeing: Loneliness, Social Isolation, and Connectedness. Journal of Healthcare Engineering. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause N Social support, stress, and well-being among older adults. Journal of gerontology. 1986; 41:512–9. [DOI] [PubMed] [Google Scholar]
- 38.Greene M, Hessol NA, Perissinotto C, Zepf R, Parrott AH, Foreman C, Whirry R, Gandhi M, John M. Loneliness in older adults living with HIV. AIDS and Behavior. 2018; 22(5):1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Rourke HM, Collins L, Sidani S. Interventions to address social connectedness and loneliness for older adults: a scoping review. BMC geriatrics. 2018; 18(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emlet CA. “You’re awfully old to have this disease”: Experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. The Gerontologist. 2006; 46(6):781–90. [DOI] [PubMed] [Google Scholar]
- 41.Yoo-Jeong M, Hepburn K, Holstad M, Haardörfer R, Waldrop-Valverde D. Correlates of loneliness in older persons living with HIV. AIDS care. 2020; 32(7):869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, Delpech V, Phillips AN. Projected life expectancy of people with HIV according to timing of diagnosis. Aids. 2012; 26(3):335–43. [DOI] [PubMed] [Google Scholar]
- 43.Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Current hiv/aids Reports. 2014; 11(3):279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leyba E: Behavioral Activation to Prevent Depression During COVID-19. [Psychology Today website]. March 29, 2020. Available at https://www.psychologytoday.com/us/blog/joyful-parenting/202003/behavioral-activation-prevent-depression-during-covid-19. Accessed April 14, 2020.
- 45.What to Know About HIV and COVID-19. [CDC website]. March 18, 2020. Available at https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/hiv.html. Accessed April 14, 2020.
- 46.Wolf LD, Davis MC. Loneliness, daily pain, and perceptions of interpersonal events in adults with fibromyalgia. Health Psychology. 2014; 33(9):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boggero IA, Sturgeon JA, Arewasikporn A, Castro SA, King CD, Segerstrom SC. Associations of Pain Intensity and Frequency With Loneliness, Hostility, and Social Functioning: Cross-Sectional, Longitudinal, and Within-Person Relationships. International journal of behavioral medicine. 2019; 26(2):217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute of Medicine (US) Committee on Pain, Disability, and Chronic Illness Behavior; Osterweis M, Kleinman A, Mechanic D, editors. Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives. Washington (DC): National Academies Press (US); 1987. 11, Measuring Pain And Dysfunction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK219245/ [PubMed] [Google Scholar]
- 49.Hoogendijk EO, Suanet B, Dent E, Deeg DJ, Aartsen MJ. Adverse effects of frailty on social functioning in older adults: Results from the Longitudinal Aging Study Amsterdam. Maturitas. 2016; 83:45–50. [DOI] [PubMed] [Google Scholar]
- 50.Nakai Y, Makizako H, Kiyama R, Tomioka K, Taniguchi Y, Kubozono T, Takenaka T, Ohishi M. Association between chronic pain and physical frailty in community-dwelling older adults. International journal of environmental research and public health. 2019; 16(8):1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingram KM, Jones DA, Fass RJ, et al. : Social support and unsupportive social interactions: Their association with depression among people living with HIV. AIDS care. 1999; 11:313–29. [DOI] [PubMed] [Google Scholar]
- 52.Collins RL: Social Support Provision to HIV-Infected Gay Men 1. Journal of Applied Social Psychology. 1994; 24:1848–69. [Google Scholar]
- 53.Olsson LE, Gärling T, Ettema D, et al. : Happiness and satisfaction with work commute. Social indicators research. 2013; 111:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


