Abstract
Background/Objective:
Near infrared spectroscopy (NIRS)-based measures of cerebral autoregulation (CAR) can potentially identify neonates with hypoxic-ischemic encephalopathy (HIE) who are at greatest risk of irreversible brain injury. However, modest predictive abilities have precluded previously described metrics from entering clinical care. We previously validated a novel autoregulation metric in a piglet model of induced hypotension called the hemoglobin volume phase index (HVP). The objective of this study was to evaluate the clinical ability of the HVP to predict adverse outcomes neonates with HIE.
Methods:
This is a prospective study of neonates with HIE who underwent therapeutic hypothermia (TH) at a level 4 NICU. Continuous cerebral NIRS and mean arterial blood pressure (MAP) from indwelling arterial catheters were measured during TH and through rewarming. Multivariate autoregressive process was used to calculate the coherence between MAP and the sum total of the oxy- and deoxygenated Hb densities (HbT), a surrogate measure of cerebral blood volume (CBV). The HVP was calculated as the cosine-transformed phase shift at the frequency of maximal MAP-HbT coherence. Brain injury was assessed by neonatal MRI, and developmental outcomes were assessed by the Bayley Scales of Infant Development (BSID-III) at 15–30 months. The ability of the HVP to predict a) death or severe brain injury by MRI and b) death or significant developmental delay was assessed using logistic regression analyses.
Results:
50 neonates with moderate or severe HIE were monitored. Median HVP was higher, representing more dysfunctional autoregulation, in infants who had adverse outcomes. After adjusting for sex and encephalopathy grade at presentation, HVP at 21–24 and 24–27 hours of life predicted death or brain injury by MRI (21–24h: OR 8.8, p=0.037; 24–27h: OR 31, p=0.011) and death or developmental delay at 15–30 months (21–24h: OR 11.8, p=0.05; 24–27h: OR 15, p=0.035).
Conclusions:
Based on this pilot study of neonates with HIE, HVP merits further study as an indicator of death or severe brain injury on neonatal MRI and neurodevelopmental delay in early childhood. Larger studies are warranted for further clinical validation of the HVP to evaluate cerebral autoregulation following HIE.
Keywords: neonatal encephalopathy, newborn, magnetic resonance imaging, brain injury
INTRODUCTION
Perinatal hypoxic ischemic encephalopathy (HIE) is a major cause of neonatal death [1; 2] and leads to lifelong neuropsychological disability in up to a third of survivors, with enormous personal, familial, and socioeconomic impact.[3; 4; 5; 6] Though therapeutic hypothermia has neuroprotective benefits, approximately 40% of infants treated with hypothermia still suffer death or disability.[7; 8; 9; 10; 11; 12; 13] Methods to individualize therapy are critical in HIE because the therapeutic window differs among patients owing to variable timing and severity of evolving secondary brain injury. While MRI and EEG are useful tools, MRI provides a single point-in-time measure after TH when treatment options are limited, and EEG requires technical expertise for interpretation. The absence of methods to monitor the neonatal brain’s response to treatment is a critical barrier to advancing effective neuroprotection and improving outcomes in babies with HIE.
Cerebrovascular autoregulation maintains cerebral blood flow (CBF) across changes in perfusion pressure in the healthy brain. The relationship between blood pressure and near-infrared spectroscopy (NIRS) – based measures of CBF and cerebral blood volume (CBV) can be used to measure cerebral autoregulation and vasoreactivity. [13–15] The presence of autoregulatory dysfunction can serve as a physiologic biomarker of secondary brain injury after a hypoxic-ischemic insult.[14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25] Dysfunctional autoregulation may also mediate secondary brain injury in HIE, given the potential mechanistic link to cerebral reperfusion injury,[15; 16; 17; 25] and its association with subsequent brain injury severity.[18; 19; 20; 21; 22; 23] Diagnosing ongoing dysfunctional autoregulation could enable clinicians to target the optimal blood pressure to maintain cerebral perfusion pressure on the “autoregulatory plateau”[18; 22] as a therapeutic approach to preventing additional brain injury. The promise of cerebral autoregulation monitoring in HIE is supported by prior work by our groups and others which have demonstrated the link between autoregulatory dysfunction in the neonatal period and subsequent brain injury by MRI and adverse neurodevelopmental outcomes.[14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25]
However, the modest predictive ability of previously tested autoregulation measures has precluded autoregulation monitoring from entering clinical care in neonates. To address these challenges, we recently described the superior sensitivity and specificity compared to other metrics, of a novel algorithm interrogating the spectral phase shift between NIRS total hemoglobin (HbT; a surrogate measure of CBV) and blood pressure at the frequency of maximal coherence.[26] This measure, now referred to as the Hemoglobin Volume Phase index (HVP), was tested against established autoregulation metrics[20; 21; 22; 23; 24; 27; 28; 29; 30; 31] in a neonatal piglet model of HIE[32; 33] and more accurately distinguished functional from dysfunctional autoregulation than existing methods. In this work, we aim to test the HVP in a clinical dataset from babies with HIE. We sought to characterize the temporal evolution of the HVP in babies with HIE undergoing TH, and to determine whether higher HVP, indicating impaired autoregulatory vasoreactivity, was associated with more severe brain injury. We focused on two key time periods in HIE: (1) 24 hours after birth when secondary HIE injury peaks [34] and (2) after the completion of rewarming, when cerebral energy metabolism is no longer inhibited by therapeutic hypothermia.
METHODS
Study Population
This is a secondary analysis of data collected from a prospective observational study of newborns with HIE referred for TH at a regional referral level-IV NICU. Inclusion criteria were: 1) greater than 35 weeks gestational age, 2) greater than 1800 grams at birth, 3) physiological evidence of perinatal asphyxia with either a) demonstrated severe metabolic acidosis with pH≤7 or base deficit ≥16, or b) milder acidosis with pH 7.01–7.15 or base deficit 10–16, accompanied by Apgar score ≤5 or need for positive-pressure ventilation at 10 minutes and a sentinel perinatal event, and 4) signs of moderate to severe clinical encephalopathy and/or seizures. Infants were treated with whole-body hypothermia according to the National Institute of Child Health and Human Development Neonatal Research Network protocol [7]. Autoregulation data from a subset of this cohort have been previously reported.[21] The study was approved by the Children’s National Institutional Review Board, informed consent was obtained from a parent of each participant and the study was performed in accordance with the ethical standards consistent with the 1964 Declaration of Helsinki.
Data Collection
Demographic and clinical data were collected from birth hospital and study site medical records. Initial grade of encephalopathy as classified by modified Sarnat criteria [7; 35]. Continuous recordings of NIRS cerebral oximetry (NIRO 200, Hamamatsu Photonics, Hamamatsu, Japan) and MAP from an indwelling arterial catheter (Philips IntelliVue MP70, MA, USA) were recorded in a time-locked manner at a rate of 5Hz and 125 Hz, respectively, and up-sampled to 1 kiloHz using custom software developed in LabView (National Instruments, TX, USA). NIRS optodes were placed over the right and left fronto-temporal regions. After obtaining parental consent, monitoring was initiated within 24 hours of cooling initiation and continued for at least 6 hours after the completion of rewarming.
Physiological Signals Processing and Hemoglobin Phase Index
When blood pressure is on the autoregulatory plateau, functional vasoreactivity is identified when an increase in blood pressure causes a decrease in CBV, thereby generating coherence with a phase shift (blood pressure and CBV are out-of-phase). When blood pressure is below the lower limit of autoregulation with pressure-passive CBV, blood pressure is coherent to CBV without a phase shift (blood pressure and CBF or CBV are in-phase). [36; 37] Thus, the HVP is based on an estimate of phase shift between mean arterial blood pressure (MAP) and CBV (estimated by NIRS Total Hemoglobin; HbT) when these signals are maximally coherent.
NIRS HbT and MAP signals were partitioned into 6-min epochs and inspected to reject artifacts using an automated process.[29; 38] Coherence between HbT and MAP signals was quantified using a multivariate autoregressive (MAR) process with model order determined by Akaike information criterion.[39] We estimated the regression coefficients through a modified Yule-Walker approach,[40; 41] and calculated a covariance matrix from the residual errors. The MAR coefficients and error covariance matrix were used to calculate the cross-spectrum between the two signals and the auto (power) spectra of the two signals.[41] We calculated the spectral coherence as the ratio of the square of the magnitude of the cross-spectrum to the product of the auto-spectra of the two signals. Coherence is a continuous measure that approaches a value of one as two signals become increasingly associated, and equals zero in the case of independence. Coherence does not discern the presence of phase shift between two signals that have power in a common frequency. Thus, within the frequency range 0.003–0.02 Hz (50 to 333 s) where blood pressure autoregulation is detectable,[37; 42] we calculated the phase shift between MAP and HbT at the frequency of maximal coherence. HVP is calculated as the cosine-transform of the phase shift in a given time epoch for ease of interpretation, providing an HVP range between −1 and 1. High HVP indicates dysfunctional autoregulation and low HVP indicates functional autoregulation.[26] HVP was calculated in each 6-min epoch from time of enrollment (within 24 h of life) through 12 hours after rewarming and summarized as the median value within 3 hour windows referenced from time of birth. If more than 50% of data was missing in a given time window, either due to artifact rejection or absent data, then no value was considered for that given window in that particular patient.
Magnetic Resonance Imaging
Brain MRIs were performed following TH at target age 4–7 days. All scans were performed on a 3 Tesla scanner (Discovery MR750, GE Healthcare, Milwaukee, WI) and included the following sequences: 3D T1-weighted Spoiled Gradient Recalled, T2-weighted double acquisition axial SE proton density, axial T2 propeller (in cases of patient motion), axial T2-Star Weighted Susceptibility Imaging, coronal T1 FLAIR propeller, and axial 30-direction DTI. MRIs were reviewed by an experienced neuroradiologist (G.V.), who was blinded to physiologic data and patient outcomes, and who assessed injury severity according to the National Institute of Child Health and Human Development (NICHD) scoring system.[43] Each level reflects a greater involvement of brain injury: 0, normal; 1A, minimal cerebral lesions only with no involvement of BG or thalamus (T) or anterior limb of the internal capsule (ALIC) or posterior limb of the internal capsule (PLIC) and no area of watershed infarction; 1B, more extensive cerebral lesions without basal ganglia and thalamic (BGT), PLIC or ALIC involvement or infarction; 2A, any BGT, ALIC or PLIC involvement or watershed infarction noted without any other cerebral lesions; 2B, involvement of either BGT, ALIC or PLIC or area of infarction and additional cerebral lesions; and 3, cerebral hemispheric devastation. For each scan, T1, T2, and DWI were reviewed for overall scoring of injury. Severe brain injury by MRI was defined as NICHD score ≥ 2B.
Neurodevelopmental Assessment
Neurodevelopmental outcomes were assessed using the Bayley-III Scales of Infant and Toddler Development by a certified developmental psychologist according to our institutional protocol for follow-up after TH. Infants are routinely assessed at 9, 15, 18 and 30 months to follow developmental progress. The Bayley-III measures cognitive, language, and gross and fine motor domains, providing composite scores where 100 (±15 SD) is the normative mean [16]. Given reports that the Bayley-III overestimates developmental performance, significant neurodevelopmental delay was defined as Bayley-III Cognitive Composite or Motor Composite <85 (1SD). [22–25] The latest available assessment at or beyond the 15 month visit was used to classify outcomes.
Statistical Analysis
Descriptive statistics included means (standard deviations) and medians (ranges) for continuous variables, as well as frequencies (percentages) for categorical data. Adverse outcomes were defined as 1) death or severe brain injury by MRI and 2) death or significant neurodevelopmental delay. Based on our prior work demonstrating that autonomic and autoregulatory dysfunction most differentiated outcome groups around 1) 24 hours of life (peak of secondary injury progression in HIE [34]) and 2) post-rewarming [21; 44], we focused our multivariable analyses on 4 timepoints of interest: two early (21:00–23:59, 24:00–26:59 hours of life) and two post-rewarming (84:00–86:59, 87:00–89:00 hours of life) periods. We performed logistic regression analyses to assess the ability of the HVP at these time periods of interest to predict adverse outcomes. We also performed receiver operating curve analyses to generate clinically relevant cutpoints for the HVP at timepoints where the HVP significantly differentiated outcome groups. Statistical analysis was performed using SPSS version 22 software (IBM Analytics, Armonk, New York, USA).
RESULTS
Study population and outcomes
A total of 52 patients were enrolled and underwent physiological monitoring. Two patients were excluded from the analysis as one was later diagnosed with a genetic syndrome and another patient had poor quality data. Therefore, data from 50 patients with moderate to severe HIE were included in these analyses. The study population had a mean gestational age of 38.6 ± 1.5 weeks and 50% were male. Baseline characteristics were consistent with moderate to severe HIE with median (range) presenting pH of 6.93 (6.5–7.25), base deficit of 19 (6.5–30.9) and Apgar scores at 1 and 5 minutes of 1 (0–9) and 3 (0–9) respectively. Thirteen patients (26%) had severe encephalopathy and ten patients (20%) died during hospitalization following withdrawal of care due to poor neurological prognosis.
Hemoglobin Phase Index and Brain Injury by MRI
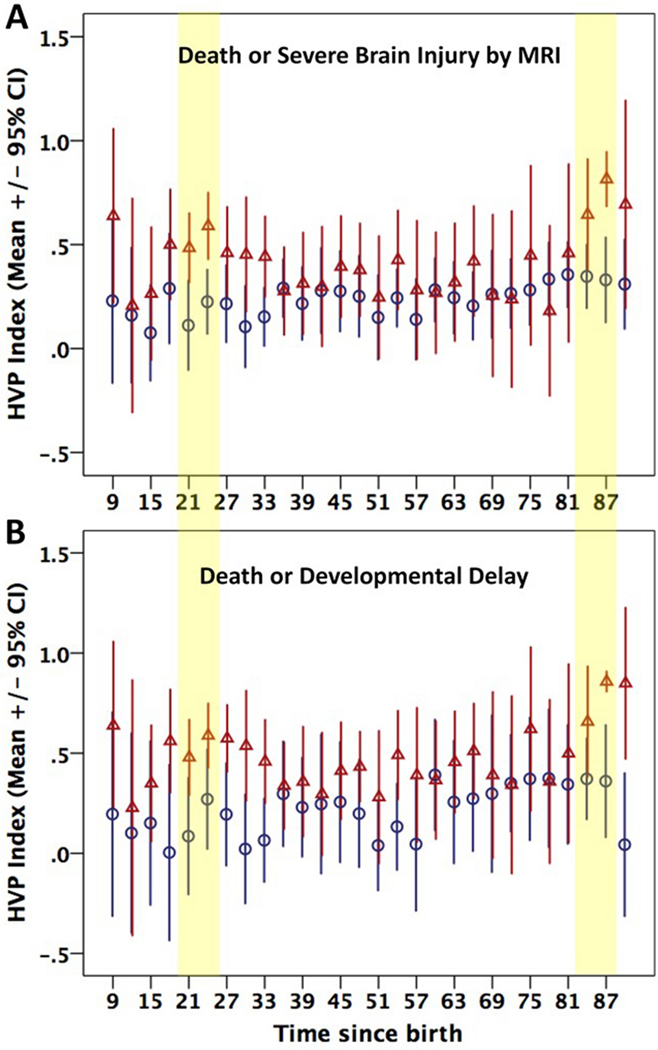
Of the surviving infants, 21 patients had normal MRI, 14 had mild to moderate injury (score 1A-2A) and 5 had severe injury (score 2B-3). Mean and 95% confidence intervals (CI) for HVP in infants who died or had severe MRI injury were plotted over time in comparison to infants who survived with normal-moderate injury by MRI (Figure 1A). Generally, higher HVP (representing more dysfunctional autoregulation) was observed in the adverse outcome group compared to the favorable outcome group, who had more stable and consistently lower HVP values. Multivariable analyses assessing the ability of HVP to predict death or severe brain injury by MRI are summarized in Table 2. HVP at 21 and 24 hours of life remained predictive of death or severe brain injury by MRI after adjusting for sex and clinical grade of encephalopathy at presentation. ROC curves for prediction of death or severe brain injury by MRI are shown in Figure 2A/C. An HVP cutpoint of 0.344 (corresponding to phase shift of 1.2 radians/ 70 degrees) at 24 hours had excellent predictive ability (AUC=0.801) for death or severe brain injury by MRI, with a sensitivity of 86% and specificity of 74%. Most patients who died did not continue on monitoring post rewarming and therefore most infants in the adverse outcome group did not have data available during the post-rewarming periods of interest. Despite the reduced sample size, HVP at 84 and 87 hours of life demonstrated a non-significant trend towards being predictive of death or severe brain injury by MRI.
1.
Hemoglobin Volume Phase (HVP) Index over time stratified by (A) brain injury by MRI and (B) neurodevelopmental outcome. Adverse and favorable outcome groups are represented by triangles and circles respectively. Bars represent mean ± 95% confidence intervals (CI). Shaded regions highlight key timepoints of clinical relevance: peak of secondary injury at around 24 hours of life and post-rewarming.
Table 2.
Prediction of Death or Developmental Delay
| Timepoint | Odds Ratio | 95%CI | P value | Adj P | N (#events) |
|---|---|---|---|---|---|
| 21–24 hours | 11.77 | 0.981–141.32 | 0.052 | 0.050 | 25 (11) |
| 24–27 hours | 14.99 | 1.06–210.88 | 0.045 | 0.035 | 28 (15) |
| 84–87 hours | 15.31 | 0.714–328.55 | 0.081 | 0.111 | 20 (8) |
| 87–90 hours | 1487 | 0.277–8e-4 | 0.096 | 0.218 | 14 (5) |
adjusted for sex, encephalopathy grade (moderate vs severe)
2.
Receiver operating curves (ROC) for ability of HVP at 21 (A-B) and 24 (C-D) hours of life (HOL) to predict outcome. Area under the receiver operative curve (AUC) and optimal HVP cutpoints with their associated sensitivity and specificity values are provided.
Hemoglobin Phase Index and Developmental Outcome
Of the 40 surviving infants, developmental outcomes at median age of 30 (range 15.2–35.9) months were available in 23 (58%) patients. Five patients had significant developmental delay while 18 patients had age-appropriate developmental performance. The patients lost to follow-up were similar with regard to baseline demographic and clinical characteristics compared to the population with available outcome data (p>0.05). HVP was again generally higher in patients who died or had significant developmental delay compared to those with normal outcomes (Figure 1B). After controlling for sex and encephalopathy grade, HVP at 21 and 24 hours of life was significantly predictive of death or developmental delay (Table 2). Optimal cutpoints selected for prediction of death and developmental delay were similar to those identified for adverse outcome based on MRI (Figure 2). The relationship between post rewarming HVP and developmental outcomes was not significant in the limited number of babies with available HVP and outcome data.
DISCUSSION
In this clinical cohort of babies with HIE, we demonstrate significant predictive ability of the HVP to distinguish babies with adverse neurologic outcomes from those with more favorable outcomes. In particular, HVP measured around 24 hours of life, the peak of secondary energy failure injury in HIE [34], reliably predicted death or significant brain injury measured by neonatal MRI and later functional performance. We cannot distinguish whether dysfunctional autoregulation is a consequence of the primary hypoxic-ischemic insult, or a mediator of secondary injury progression. However, that HVP measured at specific timepoints during TH was predictive of outcomes after controlling for the initial severity of encephalopathy suggests that it may be reflective of the variable progression of injury within similar grades of initial clinical severity subtypes. This metric, derived from a preclinical model [26], may provide real-time insight into evolving secondary injury that can help guide treatment for neonates with HIE.
Two distinct approaches have been described to quantify autoregulatory function in neonates. The pressure-passivity index (PPI), a frequency domain method,[20; 21; 27; 28; 29] estimates coherence or concordance between NIRS oxy-deoxy hemoglobin difference (HbD, a surrogate measure of CBF) and MAP signals. High coherence reflects pressure-passive CBF where changes in MAP cause similar changes in CBF. While we previously demonstrated that PPI relates to brain injury in HIE, the predictive ability was modest (AUC 0.664–0.719) and variable over time.[21] This may be partially due to using HbD, which is sensitive to the changes in oxygenated hemoglobin[45; 46] that occur with changes in cerebral metabolic rate[47] and oxygen supply[48] during therapeutic hypothermia and clinical care.
In contrast, the Hemoglobin Volume Index (HVx) measures correlation between MAP and HbT (CBV). This time domain method was primarily developed from invasive intracranial monitoring after brain trauma [49; 50; 51] with adaptation to NIRS.[30; 31; 32; 42] This approach relies on the concept that cerebral vasoreactivity mediates autoregulation through local vasoconstriction and vasodilation with resultant fluctuations in CBV. When autoregulation is functional, MAP negatively correlates to HbT. Conversely, when autoregulation is dysfunctional, MAP and HbT positively correlate. This approach can identify the optimal MAP with most robust autoregulation. While previous reports support a relationship between optimal MAP range and reduced brain injury in HIE, [18; 19; 22; 24], optimal MAP could not be identified in all neonates and HVx as a single measure did not predict neurologic injury in HIE. This is often caused when the MAP is too static to reveal the boundaries of the autoregulation curve, or due to autoregulation that is too impaired to show optimization at any MAP. As a standalone measure, HVx may be sensitive to slow changes in MAP and NIRS signals that are unrelated to autoregulation, which reduces accuracy.
The HVP also has several advantages over wavelet coherence analysis, which suffers from singularities and boundary effects that require a smoothing approach which potentially introduces artifacts.[52] Published wavelet coherence analyses in HIE utilize signals at ultra-low frequencies <0.0002 Hz,[45; 53; 54] which is equivalent to intracranial waveforms with less than one cycle per 1.4 hours. This requires long inspection windows that are less practical for clinical bedside use. Importantly, data collected in this ultra-low frequency range may not be associated with blood pressure autoregulatory vasoreactivity. Data collection at frequencies <0.001 Hz incorporates a myriad of physiologic events that are not related to blood pressure autoregulation, including noise from slow oscillations and drifts in blood volume with changes in arterial CO2. Prior studies demonstrated in piglets that a 60-second (0.017 Hz) MAP wave is the optimal frequency to discriminate functional from impaired autoregulation [37; 42] and that vasoreactivity cannot be accurately measured at frequencies >0.03 Hz, because the autoregulation function acts as a high-pass filter, responding only to sustained changes in blood pressure rather than brief transient changes (such as input from pulse and respiration signals).[37] Therefore, we use the frequency range 0.003–0.02 Hz – the range known to capture blood pressure autoregulatory vasoreactivity – to calculate HVP.
The HVP was conceptualized to address these limitations of prior methods. By using a frequency based filter to quantify phase shift when HbT and MAP signals are maximally coherent, the HVP approach can optimize accuracy by reducing noise and focusing on physiologically relevant signals to assess autoregulation. This is supported by the superior performance of the HVP in distinguishing the lower limit of autoregulation in the preclinical piglet model of HIE compared to previously described metrics [26]. This study translates these findings into clinical testing in HIE. That the HVP had good predictive ability for neurologic outcomes in a clinical cohort of babies with HIE supports it validity as a measure of autoregulatory dysfunction. Furthermore, the optimal cutpoint identified for HVP of ~0.3 indicates that during functional autoregulation there is a phase difference of 70° or above between HbT and MAP. This is comparable with preclinical studies that defined a phase angle threshold greater than 95° as the optimal phase shift between blood pressure and intracranial pressure to detect impaired autoregulation [37].
Our study design and need for invasive arterial blood pressure monitoring precluded the inclusion of a control group of healthy newborns. While normative ranges for the HVP would be of interest, the goal of continuous autoregulation monitoring in clinical practice would be to aid in identifying infants with evolving brain injury amongst at-risk newborns. Thus, a control group was not essential to study aims. Our study has additional limitations including small sample size, particularly during latter periods of hypothermia and rewarming due to patient attrition. Data artifact and missing data (due to recording periods being interrupted during clinical care) also prevented continuous data being available for all patients in every time window. Although we presented continuous HVP data over time through rewarming for our study population (Figure 1), our statistical analyses focused on 2 selected time periods of interest based on scientific and pragmatic rationale. While this approach may have introduced selection bias, our small sample size precluded adjusting for multiple comparisons, therefore we defined a priori time periods of interest rather than testing every individual time window. These sample size limitations also affected our ability to account for additional covariates in our analyses. Additional studies are needed to assess the impact of potentially important clinical confounders that are known to affect cerebral autoregulation including PaCO2 [55; 56; 57], sedatives [58], hemoglobin [59], EEG suppression/ seizures [58; 60], and cardiopulmonary injury (inotropes, mechanical ventilation, inhaled nitric oxide, ventricular dysfunction) [61; 62; 63]. We did, however, account for two key variables including encephalopathy grade (known to be a significant determinant of outcome) [7; 35] and sex (given that males may be more vulnerable to neurologic injury from HIE than females) [63]. Given the developmental outcomes were assessed during clinical visits, a high proportion of patients were lost to follow-up (only 23 patients had available outcome with 5 meeting the definition of significant developmental delay). While this may introduce selection bias, it is reassuring that the sample retained in follow-up was representative of the overall cohort with regards to baseline characteristics. Future studies are needed to confirm the predictive ability of the HVP over time during TH, and in the context of clinical confounders, in a larger cohort of infants with long term outcomes.
CONCLUSIONS
The HVP Index is a novel metric to assess cerebral autoregulation non-invasively in newborns at risk for brain injury. In a small clinical cohort of babies with HIE, the HVP measured around 24 hours of life was predictive of adverse neurological outcomes. Future studies are warranted to test the HVP was a reliable indicator of evolving secondary injury that can help direct care in babies with HIE.
Table 1.
Prediction of Death or Severe Brain Injury by MRI
| Timepoint | Odds Ratio | 95%CI | P value | *Adj P | N (#events) |
|---|---|---|---|---|---|
| 21–24 hours | 8.77 | 1.16–65.97 | 0.035 | 0.037 | 35 (12) |
| 24–27 hours | 31.04 | 2.37–406.46 | 0.009 | 0.011 | 37 (14) |
| 84–87 hours | 12.25 | 0.836–12.25 | 0.067 | 0.166 | 33 (8) |
| 87–90 hours | 211 | 0.447–10e3 | 0.088 | 0.114 | 25 (5) |
adjusted for sex, encephalopathy grade (moderate vs severe)
Acknowledgments
Statement of Financial Support: This study was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075, 1KL2RR031987-01) and the National Institutes of Health Intellectual and Developmental Disabilities Research Consortium (U54 HD090257).
Footnotes
JKL is a paid consultant for Edwards Life Sciences. The other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- [1].Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, and Black RE, Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379 (2012) 2151–61. [DOI] [PubMed] [Google Scholar]
- [2].Lawn J, Shibuya K, and Stein C, No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ 83 (2005) 409–17. [PMC free article] [PubMed] [Google Scholar]
- [3].Shankaran S, Woldt E, Koepke T, Bedard MP, and Nandyal R, Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early human development 25 (1991) 135–48. [DOI] [PubMed] [Google Scholar]
- [4].Robertson CM, Finer NN, and Grace MG, School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. The Journal of pediatrics 114 (1989) 753–60. [DOI] [PubMed] [Google Scholar]
- [5].de Vries LS, and Jongmans MJ, Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Archives of disease in childhood. Fetal and neonatal edition 95 (2010) F220–4. [DOI] [PubMed] [Google Scholar]
- [6].Marlow N, Rose AS, Rands CE, and Draper ES, Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Archives of disease in childhood. Fetal and neonatal edition 90 (2005) F380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, and Jobe AH, Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine 353 (2005) 1574–84. [DOI] [PubMed] [Google Scholar]
- [8].Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, and Higgins RD, Childhood outcomes after hypothermia for neonatal encephalopathy. The New England journal of medicine 366 (2012) 2085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, and Gunn AJ, Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365 (2005) 663–70. [DOI] [PubMed] [Google Scholar]
- [10].Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, and Brocklehurst P, Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England journal of medicine 361 (2009) 1349–58. [DOI] [PubMed] [Google Scholar]
- [11].Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, Wright IM, Kirpalani HM, Darlow BA, and Doyle LW, Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Archives of pediatrics & adolescent medicine 165 (2011) 692–700. [DOI] [PubMed] [Google Scholar]
- [12].Simbruner G, Mittal RA, Rohlmann F, and Muche R, Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 126 (2010) e771–8. [DOI] [PubMed] [Google Scholar]
- [13].Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, and Edwards AD, Effects of hypothermia for perinatal asphyxia on childhood outcomes. The New England journal of medicine 371 (2014) 140–9. [DOI] [PubMed] [Google Scholar]
- [14].van Bel F, Dorrepaal CA, Benders MJ, Zeeuwe PE, van de Bor M, and Berger HM, Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics 92 (1993) 365–72. [PubMed] [Google Scholar]
- [15].Meek JH, Elwell CE, McCormick DC, Edwards AD, Townsend JP, Stewart AL, and Wyatt JS, Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Archives of disease in childhood. Fetal and neonatal edition 81 (1999) F110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pryds O, Greisen G, Lou H, and Friis-Hansen B, Vasoparalysis associated with brain damage in asphyxiated term infants. The Journal of pediatrics 117 (1990) 119–25. [DOI] [PubMed] [Google Scholar]
- [17].Greisen G, Effect of cerebral blood flow and cerebrovascular autoregulation on the distribution, type and extent of cerebral injury. Brain Pathol 2 (1992) 223–8. [DOI] [PubMed] [Google Scholar]
- [18].Burton VJ, Gerner G, Cristofalo E, Chung SE, Jennings JM, Parkinson C, Koehler RC, Chavez-Valdez R, Johnston MV, Northington FJ, and Lee JK, A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC neurology 15 (2015) 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tekes A, Poretti A, Scheurkogel MM, Huisman TA, Howlett JA, Alqahtani E, Lee JH, Parkinson C, Shapiro K, Chung SE, Jennings JM, Gilmore MM, Hogue CW, Martin LJ, Koehler RC, Northington FJ, and Lee JK, Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. AJNR. American journal of neuroradiology 36 (2015) 188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Govindan RB, Massaro AN, Andescavage NN, Chang T, and du Plessis A, Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Frontiers in human neuroscience 8 (2014) 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Massaro AN, Govindan RB, Vezina G, Chang T, Andescavage NN, Wang Y, Al-Shargabi T, Metzler M, Harris K, and du Plessis AJ, Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Journal of neurophysiology 114 (2015) 818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TA, Parkinson C, Chung SE, Jennings JM, Jamrogowicz JJ, Larson AC, Lehmann CU, Jackson E, Brady KM, Koehler RC, and Lee JK, Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatric research 74 (2013) 525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee JK, Brady KM, Chung SE, Jennings JM, Whitaker EE, Aganga D, Easley RB, Heitmiller K, Jamrogowicz JL, Larson AC, Lee JH, Jordan LC, Hogue CW, Lehmann CU, Bembea MM, Hunt EA, Koehler RC, and Shaffner DH, A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation 85 (2014) 1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee JK, Poretti A, Perin J, Huisman TA, Parkinson C, Chavez-Valdez R, O’Connor M, Reyes M, Armstrong J, Jennings JM, Gilmore MM, Koehler RC, Northington FJ, and Tekes A, Optimizing Cerebral Autoregulation May Decrease Neonatal Regional Hypoxic-Ischemic Brain Injury. Developmental neuroscience (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fellman V, and Raivio KO, Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatric research 41 (1997) 599–606. [DOI] [PubMed] [Google Scholar]
- [26].Govindan RB, Brady KM, Massaro AN, Perin J, Jennings JM, DuPlessis AJ, Koehler RC, and Lee JK, Comparison of Frequency- and Time-Domain Autoregulation and Vasoreactivity Indices in a Piglet Model of Hypoxia-Ischemia and Hypothermia. Developmental neuroscience (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, and du Plessis AJ, Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatric research 61 (2007) 467–73. [DOI] [PubMed] [Google Scholar]
- [28].Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, Walter G, Laussen PC, Jonas RA, and du Plessis AJ, Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatric research 57 (2005) 35–41. [DOI] [PubMed] [Google Scholar]
- [29].Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, and Volpe JJ, Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106 (2000) 625–32. [DOI] [PubMed] [Google Scholar]
- [30].Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, Koehler RC, and Shaffner DH, Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 38 (2007) 2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, and Shaffner DH, Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39 (2008) 2531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Larson AC, Jamrogowicz JL, Kulikowicz E, Wang B, Yang ZJ, Shaffner DH, Koehler RC, and Lee JK, Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J Appl Physiol (1985) 115 (2013) 1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee JK, Brady KM, Mytar JO, Kibler KK, Carter EL, Hirsch KG, Hogue CW, Easley RB, Jordan LC, Smielewski P, Czosnyka M, Shaffner DH, and Koehler RC, Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Critical care medicine 39 (2011) 2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnston MV, Fatemi A, Wilson MA, and Northington F, Treatment advances in neonatal neuroprotection and neurointensive care. The Lancet. Neurology 10 (2011) 372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sarnat HB, and Sarnat MS, Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of neurology 33 (1976) 696–705. [DOI] [PubMed] [Google Scholar]
- [36].Brady KM, Easley RB, Kibler K, Kaczka DW, Andropoulos D, Fraser CD 3rd, Smielewski P, Czosnyka M, Adams GJ, Rhee CJ, and Rusin CG, Positive end-expiratory pressure oscillation facilitates brain vascular reactivity monitoring. J Appl Physiol (1985) 113 (2012) 1362–8. [DOI] [PubMed] [Google Scholar]
- [37].Fraser CD 3rd, Brady KM, Rhee CJ, Easley RB, Kibler K, Smielewski P, Czosnyka M, Kaczka DW, Andropoulos DB, and Rusin C, The frequency response of cerebral autoregulation. J Appl Physiol (1985) 115 (2013) 52–6. [DOI] [PubMed] [Google Scholar]
- [38].Govindan RB, Massaro AN, and du Plessis A, Ensuring signal quality of cerebral near infrared spectroscopy during continuous longterm monitoring. Journal of neuroscience methods 309 (2018) 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Akaike H, A new look at the statistical model identification. IEEE Trans Auto Contr AC-19 (1974) 716–723. [Google Scholar]
- [40].Barbieri R, Triedman JK, and Saul JP, Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis. American journal of physiology. Regulatory, integrative and comparative physiology 283 (2002) R1210–20. [DOI] [PubMed] [Google Scholar]
- [41].Kay SM, Modern spectral estimation theory and application., Prentice Hall, New Jersey, 1998. [Google Scholar]
- [42].Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler RC, Shaffner DH, and Brady KM, Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40 (2009) 1820–6. [DOI] [PubMed] [Google Scholar]
- [43].Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, Ehrenkranz RA, Walsh MC, Tyson JE, Donovan EF, Goldberg RN, Bara R, Das A, Finer NN, Sanchez PJ, Poindexter BB, Van Meurs KP, Carlo WA, Stoll BJ, Duara S, Guillet R, and Higgins RD, Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Archives of disease in childhood. Fetal and neonatal edition 97 (2012) F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Massaro AN, Govindan RB, Al-Shargabi T, Andescavage NN, Metzler M, Chang T, Glass P, and du Plessis AJ, Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. Journal of perinatology : official journal of the California Perinatal Association (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tian F, Tarumi T, Liu H, Zhang R, and Chalak L, Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. NeuroImage. Clinical 11 (2016) 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shellhaas RA, Thelen BJ, Bapuraj JR, Burns JW, Swenson AW, Christensen MK, Wiggins SA, and Barks JD, Limited short-term prognostic utility of cerebral NIRS during neonatal therapeutic hypothermia. Neurology 81 (2013) 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kunihara T, Sasaki S, Shiiya N, Murashita T, Matsui Y, and Yasuda K, Near infrared spectrophotometry reflects cerebral metabolism during hypothermic circulatory arrest in adults. ASAIO J 47 (2001) 417–21. [DOI] [PubMed] [Google Scholar]
- [48].Samraj RS, and Nicolas L, Near infrared spectroscopy (NIRS) derived tissue oxygenation in critical illness. Clinical and investigative medicine. Medecine clinique et experimentale 38 (2015) E285–95. [DOI] [PubMed] [Google Scholar]
- [49].Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, Hiler M, Balestreri M, Kirkpatrick PJ, Pickard JD, Hutchinson P, and Czosnyka M, Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurgical focus 25 (2008) E2. [DOI] [PubMed] [Google Scholar]
- [50].Czosnyka M, Smielewski P, Piechnik S, Schmidt EA, Seeley H, al-Rawi P, Matta BF, Kirkpatrick PJ, and Pickard JD, Continuous assessment of cerebral autoregulation--clinical verification of the method in head injured patients. Acta neurochirurgica. Supplement 76 (2000) 483–4. [DOI] [PubMed] [Google Scholar]
- [51].Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, Jallo GI, and Guerguerian AM, Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics 124 (2009) e1205–12. [DOI] [PubMed] [Google Scholar]
- [52].Torrence C, and Webster PJ, Interdecadal changes in the ENSO-monsoon system. J Clim 12 (1999) 2679–2690. [Google Scholar]
- [53].Chalak LF, Tian F, Adams-Huet B, Vasil D, Laptook A, Tarumi T, and Zhang R, Novel Wavelet Real Time Analysis of Neurovascular Coupling in Neonatal Encephalopathy. Scientific reports 7 (2017) 45958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chalak LF, and Zhang R, New Wavelet Neurovascular Bundle for Bedside Evaluation of Cerebral Autoregulation and Neurovascular Coupling in Newborns with Hypoxic-Ischemic Encephalopathy. Developmental neuroscience 39 (2017) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kaiser JR, Gauss CH, and Williams DK, The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatric research 58 (2005) 931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nusbaum DM, Brady KM, Kibler KK, and Blaine Easley R, Acute hypercarbia increases the lower limit of cerebral blood flow autoregulation in a porcine model. Neurological research 38 (2016) 196–204. [DOI] [PubMed] [Google Scholar]
- [57].Lingappan K, Kaiser JR, Srinivasan C, and Gunn AJ, Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatric research 80 (2016) 204–8. [DOI] [PubMed] [Google Scholar]
- [58].Sokoloff MD, Plegue MA, Chervin RD, Barks JD, and Shellhaas RA, Phenobarbital and neonatal seizures affect cerebral oxygen metabolism: a near-infrared spectroscopy study. Pediatric research 78 (2015) 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hoiland RL, Bain AR, Rieger MG, Bailey DM, and Ainslie PN, Hypoxemia, oxygen content, and the regulation of cerebral blood flow. American journal of physiology. Regulatory, integrative and comparative physiology 310 (2016) R398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, and Mathur A, Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. The Journal of pediatrics 163 (2013) 465–70. [DOI] [PubMed] [Google Scholar]
- [61].Armstead WM, Kiessling JW, Kofke WA, and Vavilala MS, Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Critical care medicine 38 (2010) 1868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Armstead WM, Riley J, and Vavilala MS, Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of Up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 14 (2013) e103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chavez-Valdez R, O’Connor M, Perin J, Reyes M, Armstrong J, Parkinson C, Gilmore M, Jennings J, Northington FJ, and Lee JK, Sex-specific associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury in neonatal encephalopathy and therapeutic hypothermia. Pediatric research 81 (2017) 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]