Abstract
Hyalinizing clear cell carcinoma (HCCC) is a rare, low-grade neoplasm accounting for approximately 1% of salivary gland neoplasms. Histologically, it is characterized by a monomorphous population of clear cells arranged in sheets, nests, or cords, lacking ductal structures. Until recently, clear cell carcinoma of the oral cavity (OC) represented a diagnosis of exclusion when other head and neck pathologic entities such as epithelial-myoepithelial carcinoma or mucoepidermoid carcinoma could be ruled out, making definitive diagnosis by light microscopy and immunoprofiling a challenge. As a result, initial biopsies are often misclassified, and could result in under- or overtreatment. More recently, the presence of the EWSR1-ATF1 gene fusion has been adopted to definitively diagnose HCCC. Typically, HCCC demonstrates clinical indolence and responds well to curative surgical excision alone for localized disease, with adjuvant radiotherapy (RT) reserved for high risk features including perineural invasion, lymphovascular invasion, and regional cervical metastasis. The literature, however, lacks consensus regarding the role of adjuvant radiotherapy. In this article, we report a case of HCCC in a rare site involving the ventral tongue, with high risk features of perineural invasion and cervical nodal metastasis. The patient underwent surgical excision alone; declining adjuvant radiotherapy despite the high risk features, and was alive with no evidence of disease at the 42-month mark. Furthermore, we provide an update on the current prognostic indicators for HCCC, and emphasize the need for chromosomal analysis to achieve a definitive diagnosis.
Keywords: Clear cell carcinoma, Hyalinizing clear cell carcinoma, EWSR1 fusion, Salivary gland tumor, Nodal metastasis
Introduction
Hyalinizing clear cell carcinoma (HCCC), formerly known as clear cell carcinoma (CCC), not otherwise specified (NOS), is a rare salivary gland neoplasm most often arising from minor salivary glands in the oral cavity (OC), followed by the oropharynx, and major salivary glands [1–3]. Occurring most commonly in the sixth to eighth decades of life, these indolent neoplasms may not become symptomatic until months to years of painless, non-ulcerated growth [3]. Previously considered a diagnosis of exclusion, the use of immunohistochemistry and fluorescent in situ hybridization (FISH) for the Ewing sarcoma breakpoint region-1 (EWSR1) can now distinguish HCCC from other more aggressive, clear cell-containing malignancies including mucoepidermoid carcinoma, acinic cell carcinoma, epithelial-myoepithelial carcinoma and renal cell carcinoma [4–6]. For the clinician, treatment decisions are obfuscated when the histopathologic diagnosis is equivocal, and, in the case of misdiagnosis as high-grade clear cell variants such as mucoepidermoid carcinoma, leads to unnecessary surgery and/or radiotherapy (RT), and psychological distress for the patient. While the general treatment of HCCC is wide local excision, the addition of a cervical lymph node dissection may be useful if a nodal metastasis is suspected. The role of adjuvant radiotherapy also remains unclear [7]. Because there is currently no histological grading system or specific treatment guidelines for HCCC due to the short follow-up period in the literature, median of 24 months, and wide time range of recurrence (4–288 months), patients diagnosed with HCCC require ongoing long-term surveillance [7, 8].
Based on review of the available English language literature, our case represents one of two of HCCC in the oral tongue and not only presents with nodal metastasis and perineural invasion (PNI), but also responded well to surgical excision only without radiotherapy, with follow-up of 42 months exceeding that seen in the published studies of larger series. This case is the second report in the literature of oral cavity HCCC with high risk features, cured with surgery alone, with adequate follow-up.
Case Report
A 70-year-old white male presented with a slowly-growing, 2 cm painless exophytic ulcerative lesion on the midline ventral tongue which had been present for up to 6 months prior to being noted on a routine dental examination. An outside biopsy demonstrated moderately differentiated invasive squamous cell carcinoma (SCCa) with clear cell features. On oncologic workup per NCCN guidelines, there was no evidence of regional or distant metastasis noted on clinical examination and computed tomography (CT) imaging. The clinical appearance was that of an exophytic and ulcerative, well-circumscribed lesion located at the midline of the ventral tongue. On palpation, the senior author suspected tumor depth of invasion of at least 3–4 mm, and offered the patient elective neck dissections. This is the standard for lesions with up to 30% risk of occult disease, and in this case were performed bilaterally given the midline position of the tumor and chance for bilateral metastasis seen in squamous cell carcinoma. Selective neck dissection with removal of levels I–IV bilaterally and preservation of non-lymphatic structures (sternocleidomastoid muscle, internal jugular vein, spinal accessory nerve) was completed [9–11]. Intraoperative frozen sections demonstrated a low-grade carcinoma with a differential diagnosis including low-grade mucoepidermoid carcinoma, and polymorphous low-grade adenocarcinoma,however squamous cell carcinoma was not ruled out. A midline partial glossectomy was performed with 1.0–1.5 cm margins (Fig. 1a) and primary closure achieved after negative margins were confirmed (Fig. 1b). The neck dissection was completed in standard fashion. The patient had an uneventful postoperative course. At 2-month follow-up both the primary site and neck had healed well without any deficits. The tongue was freely mobile with good anteroposterior projection, maintaining good speech and swallowing function (Fig. 1c).
Fig. 1.
Tumor on ventral surface of the gone (a) treated with wide local excision and primary closure (b). Patient regained excellent speech and swallowing function at 2-months postoperatively (c)
Final pathology results by hematoxylin and eosin staining (H&E) demonstrated hyalinizing clear cell carcinoma of minor salivary gland origin (Fig. 2a) with perineural invasion (Fig. 2b) and metastasis to 1 of 26 cervical lymph nodes. FISH analysis for EWSR1 revealed positive chromosomal anomaly at 22q12 in 91% of cells (Fig. 3). The final pathological staging was pT1N1 hyalinizing clear cell carcinoma.
Fig. 2.
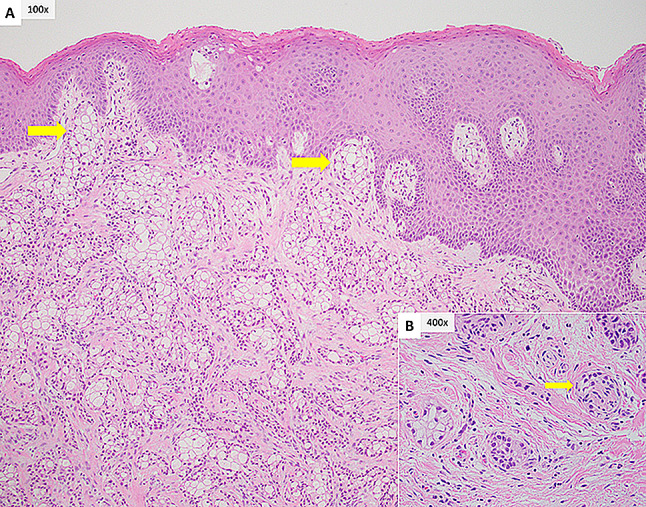
a The tumor is present in cords and nests of epithelial and clear cells with interspersed hyalinized stroma. Focal invasion of the overlying epithelium is seen (yellow arrow). b Perineural invasion of a small nerve (yellow arrow)
Fig. 3.
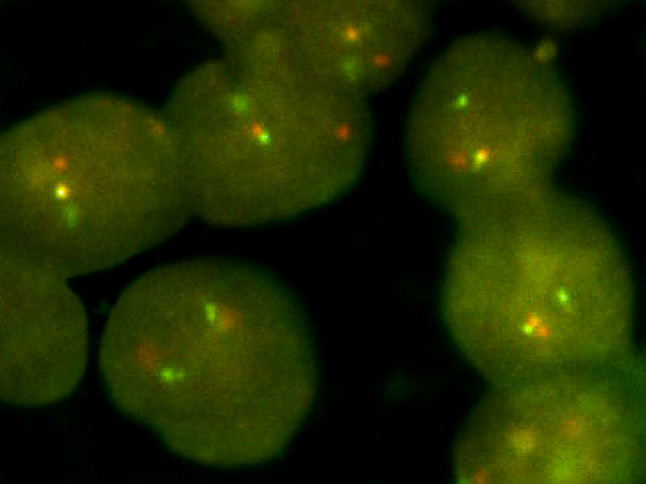
This image is of the EWSR1 break apart probe showing positive gene rearrangement in the tumors cells using FISH
After discussion at the institutional Multispecialty Head and Neck Tumor Board, 60 cGy of postoperative radiation therapy over 30 fractions was recommended to the primary site and bilateral necks due to perineural invasion and a positive nodal status. After reviewing the final histopathology, and expected survival benefit for adjuvant radiotherapy, the patient declined further treatment. The patient continues to be followed intermittently due to social issues, and is alive without clinical evidence of disease at the 42-month mark.
Discussion
Although rare, cases of hyalinizing clear cell carcinoma continue to be reported in the literature due to limited evidence on overall disease-specific survival associated with various treatment modalities. Specifically, although there remains limited evidence on the curative effects of radiotherapy and or chemotherapy as mono- or adjuvant therapy, the recommendation of adjuvant radiotherapy can be reserved for cases with an increased likelihood of disease recurrence, i.e., those with high risk features such as PNI, LVI, or nodal metastasis [7, 8]. While the majority of HCCC have been reported to occur in the oral cavity followed by the oropharynx, there continues to be an increasing number of case reports of HCCC described in the lung, trachea, paranasal sinus, and thymus [12–18]. Within the oral cavity, the palate is the most common site, followed by the maxilla, then floor of mouth [1]. Because oral tongue HCCC is commonly reported together with tongue base (BOT) tumors, the true incidence of oral tongue HCCC is unknown [7, 8]. Abergotti et al. reported 8 cases of HCCC involving the oral tongue [1].
The uniqueness of this case report is the primary location along with its pathological characteristics and response to treatment. Our case presented as a lesion on the ventral surface of the oral tongue. When reviewing the literature, most articles that categorize the primary location of the tongue do not distinguish the BOT (oropharynx) from the oral tongue (oral cavity). This is a key distinction because oral tongue HCCC, although lower in incidence, has been reported with a higher rate of LVI, PNI, and nodal metastasis (Table 1). Therefore, the clinicopathologic behavior of HCCC may vary based on location and respond differently to treatment modalities, as seen in HPV-driven SCCa of the oropharynx. HCCC, an indolent tumor, also lacks radiosensitivity and would be expected to respond poorly to single-modality radiation therapy. Of the more recent review articles, only one distinguished between BOT and oral tongue HCCC [1]. This issue results in a fundamental reporting error, making either major salivary gland the second most common primary location of HCCC reported in the literature [1, 7, 8, 19, 20], however when the BOT and oral tongue are reported as separate entities, oropharynx is the second most common location following the oral cavity [1].
Table 1.
Clinical behavior of published HCCC cases by location
| Location | Freq (%) | Publications | Cases (n) | Size range (cm) | PNI (%) | LVI (%) | Bone invasion (%) | Nodal metastasis (%) | Distant metastasis (%) |
|---|---|---|---|---|---|---|---|---|---|
| BOT | 17.9 | [1, 3–5, 8, 20, 21, 28, 30, 37–48, 50–53] | 40 | 0.8–6 | 18 | 5 | 0 | 18 | 0 |
| Bronchus | 3.6 | [12, 14, 16, 54, 55] | 8 | 1.6–3.5 | 13 | 0 | 0 | 13 | 0 |
| Buccal/cheek | 4.9 | [1, 3, 4, 16, 20, 32, 41, 56–58] | 11 | 1.5–4.9 | 0 | 9 | 0 | 0 | 0 |
| FOM | 6.7 | [4, 8, 20, 28, 35, 41, 51, 53, 58–63] | 15 | 0.5–5 | 33 | 13 | 7 | 13 | 0 |
| Gingiva | 0.9 | [3, 48] | 2 | 0.4 | 0 | 0 | 50 | 0 | 0 |
| Hypopharynx | 0.9 | [8, 64] | 2 | 2–4.8 | 0 | 0 | 0 | 50 | 0 |
| Larynx | 1.3 | [4, 28, 65] | 3 | 1.3–2 | 0 | 0 | 0 | 0 | 0 |
| Lip | 1.3 | [4, 8] | 3 | 1.5 | 0 | 67 | 0 | 0 | 0 |
| Mandible | 3.1 | [4, 8, 20, 28, 45, 66] | 7 | 0.6–4.8 | 29 | 0 | 43 | 14 | 0 |
| Maxillaa | 5.8 | [1, 4, 33, 34, 58, 66–70] | 13 | 0.5–6.5 | 0 | 7 | 38 | 0 | 0 |
| Nasal cavity | 0.9 | [1, 71] | 2 | 3 | 0 | 0 | 0 | 0 | 0 |
| Nasopharynx | 5.5 | [1, 3–5, 30, 72–77] | 12 | 1.7–4.9 | 25 | 0 | 25 | 0 | 0 |
| Neck | 0.4 | [41] | 1 | – | – | – | – | 100 | - |
| Oral | 0.4 | [51] | 1 | 1.2 | – | – | – | - | 0 |
| Orbit/lacrimal gland | 0.4 | [20] | 1 | 4.5 | 0 | 0 | 0 | 0 | 0 |
| Oropharynx | 2.2 | [4, 16, 78, 79] | 5 | 2–3.5 | 0 | 0 | 0 | 0 | 0 |
| Palateb | 26 | [1, 3–5, 7, 8, 20, 21, 28–31, 47, 51, 53, 56–58, 80–92] | 58 | 0.4–5 | 19 | 3 | 10 | 7 | 3 |
| Paranasal sinus | 1.3 | [17, 93] | 3 | 6 | 33 | 33 | 100 | 0 | 0 |
| Parotid | 4.9 | [4, 5, 28, 36, 45, 51, 94, 95] | 11 | 1–10 | 27 | 9 | 0 | 0 | 0 |
| Sinonasal tract | 0.4 | [96] | 1 | 2 | – | – | – | 0 | - |
| Sublingual | 0.4 | [7] | 1 | 2.5 | 0 | 0 | 0 | 0 | 0 |
| Submandibular | 0.4 | [21] | 1 | 4 | 100 | 0 | – | 0 | - |
| Thymus | 0.9 | [18] | 2 | – | – | – | – | - | - |
| Tongue | 5.4 | [4, 20, 28, 49, 51, 82, 97–101] | 12 | 1.5–5 | 25 | 17 | 0 | 25 | 0 |
| Tonsil | 2.2 | [1, 51, 103–105] | 5 | 2.3–3 | 0 | 0 | 0 | 20 | 0 |
| Trachea | 1.3 | [3, 13, 106] | 3 | 1.3–2.5 | 0 | 0 | 0 | - | 0 |
BOT base of tongue, FOM floor of mouth, PNI perineural invasion, LVI lymphovascular invasion
aMaxilla: includes cases in maxillary sinus
bPalate: includes hard and soft palate
With EWSR1-ATF1 gene fusion presenting in the majority of HCCC cases, there is now an increased role for diagnostic molecular markers to differentiate malignant salivary gland neoplasms with clear cell features [4–6, 21]. Additional gene fusions such as MYB-NFIB and CRTC1-MAML2 are specifically associated with adenoid cystic carcinoma and mucoepidermoid carcinoma, respectively [22, 23]. Two additional mutations, CREM and CREB1, genes belonging to the same family of transcription factors as ATF1, were also found translocated with EWSR1 in cases of HCCC, clear cell carcinoma of the soft tissue, and a distinct group of myxoid mesenchymal neoplasms [24–26]. As the list of specific and recurrent gene translocations associated with solid neoplasms continue to evolve, the once homogenous precursor neoplasms will be differentiated by unique and specific genetic alteration(s). By understanding the mechanisms of these genetic translocations in tumorigenesis, new approaches for early diagnosis and targeted therapy can be developed. Overall, the diagnosis of HCCC should take into consideration not only the molecular characteristics of the disease, but also the immunophenotypic, histomorphological, and clinicopathologic features. This is of note, since gene mutations in head and neck cancer overall are rare compared to other body sites [27].
When evaluating nodal metastasis and recurrent disease, a literature review by Daniele et al. showed only 3 of 152 cases of HCCC involving the tongue (excluding BOT) that demonstrated metastatic or recurrent disease. Only one of those 3 cases presented with positive nodal metastasis, did not receive adjuvant radiotherapy, and was alive without disease 11 years postoperatively [28]. We also performed an updated PubMed search using the same keywords as Daniele et al. from the years 2015 to 2020. We specifically screened the new cases involving the oral cavity and salivary glands. Twenty-nine cases were reported with 25 cases involving the oral cavity and 4 involving the major salivary gland, but no new reports associated with the oral tongue [1, 5, 7, 8, 21, 29–36]. Therefore, our case is the second report of oral tongue HCCC in the literature, with the high risk features of perineural invasion and regional metastasis. Interestingly, he was treated with surgery alone and remains without evidence of disease at 42 months postoperatively. A detailed list of reported HCCC cases by location is illustrated in Tables 1 and 2.
Table 2.
Treatment and outcomes of published HCCC cases by location
| Location | Exc (%) | Exc and LND (%) | Exc, LND, and RT (%) | Exc and RT (%) | RT only (%) | Exc and ChemoRT (%) | Othera (%) | Follow-up range (months) | NED (%) | Recurrence (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| BOT | 58 | 10 | 8 | 5 | 3 | 3 | 3 | 3–124 | 65 | 18 |
| Bronchus | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 10–181 | 100 | 0 |
| Buccal/cheek | 73 | 9 | 0 | 18 | 0 | 0 | 0 | 5–132 | 64 | 27 |
| FOM | 67 | 13 | 20 | 0 | 0 | 0 | 0 | 2.5–108 | 73 | 7 |
| Gingiva | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 12–31 | 100 | 0 |
| Hypopharynx | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 62 | 100 | 0 |
| Larynx | 67 | 0 | 0 | 33 | 0 | 0 | 0 | 24–55 | 100 | 0 |
| Lip | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 2–73 | 100 | 0 |
| Mandible | 71 | 29 | 0 | 0 | 0 | 0 | 0 | 3–78 | 71 | 29 |
| Maxilla | 46 | 7 | 7 | 7 | 0 | 0 | 0 | 3–36 | 38 | 23 |
| Nasal cavity | 50 | 0 | 0 | 50 | 0 | 0 | 0 | 7 | 0 | 50 |
| Nasopharynx | 50 | 0 | 8 | 25 | 0 | 0 | 1 | 2.5–144 | 58 | 8 |
| Neck | – | 100 | 0 | 0 | 0 | 0 | 0 | – | – | – |
| Oral | 100 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | 0 |
| Orbit/lacrimal gland | 100 | 0 | 0 | 0 | 0 | 0 | 0 | – | 100 | 0 |
| Oropharynx | 80 | 0 | 0 | 0 | 0 | 20 | 0 | 12–81 | 60 | 20 |
| Palate | 67 | 2 | 7 | 10 | 0 | 0 | 3 | 6–348 | 60 | 19 |
| Paranasal sinus | 34 | 0 | 0 | 67 | 0 | 0 | 0 | 4–9 | 67 | 0 |
| Parotid | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 1–195 | 55 | 18 |
| Sinonasal tract | – | – | – | – | – | – | – | 48 | 100 | 0 |
| Sublingual | 100 | – | – | – | – | – | – | 12 | 100 | – |
| Submandibular | – | – | – | – | – | – | – | – | 100 | 0 |
| Thymus | – | – | – | – | – | – | – | – | – | – |
| Tongue | 67 | 8 | 0 | 17 | 0 | 0 | 8 | 8–132 | 42 | 8 |
| Tonsil | 80 | 0 | 0 | 20 | 0 | 0 | 0 | 4–72 | 40 | 20 |
| Trachea | 0 | 33 | 33 | 33 | 0 | 0 | 33 | 12–52 | 67 | 0 |
BOT base of tongue, FOM floor of mouth, Exc excision, LND lymph node dissection, RT radiotherapy, ChemoRT chemoradiotherapy, NED no evidence of disease
aOther; includes no treatment or debulking for unresectable tumors
Conclusion
Hyalinizing clear cell carcinoma (HCCC) of the oral cavity remains a relatively uncommon malignant salivary gland neoplasm, but it continues to be frequently presented in the literature due to its unpredictable treatment response and challenging pathological diagnosis. Recent changes in molecular diagnosis have generated a renewed interest in HCCC, as well as clinicians’ demands for updated treatment guidelines. HCCC more commonly presents in white females during the sixth decade of life and is discovered either incidentally or during a routine examination as a painless mass. The differential diagnosis for HCCC varies widely due to the microscopically varied phenotype of clear cell salivary gland tumors, however, the use of FISH analysis for EWSR1 gene rearrangement is shown to be highly specific. As more cases of accurately-diagnosed HCCC are reported with long-term outcomes, clinicians can select treatment with optimal disease-specific survival, individualizing therapy while minimizing morbidity. The current case is one of two in the literature where high-risk features, typically warranting radiotherapy, did not impact survival following single modality surgical treatment. Additional publications are needed for this rare entity, now that molecular diagnosis is widely available, to characterize the benefit of postoperative RT versus observation.
Acknowledgements
The authors of this article would like to thank Dr. Stephen Marbut for generously providing the histology images.
Funding
This study did not receive any funding source.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics Approval
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was deemed exempt from the University of Alabama at Birmingham Institutional Review Board (IRB).
Informed Consent
Informed consent was obtained from all individual participants included in the study. Patients signed informed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albergotti WG, et al. Hyalinizing clear cell carcinoma of the head and neck: case series and update. Head Neck. 2016;38(3):426–433. doi: 10.1002/hed.23902. [DOI] [PubMed] [Google Scholar]
- 2.Seethala RR, Stenman G. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the salivary gland. Head Neck Pathol. 2017;11(1):55–67. doi: 10.1007/s12105-017-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Prera JC, Kwan R, Tripodi J, et al. Reappraising hyalinizing clear cell carcinoma: a population-based study with molecular confirmation. Head Neck. 2017;39(3):503–511. doi: 10.1002/hed.24637. [DOI] [PubMed] [Google Scholar]
- 4.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordoñez B, Have C, Asa SL, Leong IT, Bradley G. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 5.Zhao YN, Wang X, Liang FH, Zhang WJ, Song XT. Hyalinizing clear cell carcinoma of salivary glands: a retrospective study focused on uncommon morphology, immunohistochemistry, and detection of gene fusion using fluorescence in situ hybridization. Pathology. 2018;214(3):380–384. doi: 10.1016/j.prp.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Tanguay J, Weinreb I. What the EWSR1-ATF1 fusion has taught us about hyalinizing clear cell carcinoma. Head Neck Pathol. 2013;7(1):28–34. doi: 10.1007/s12105-013-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniele L, et al. Clear cell carcinoma, not otherwise specified/hyalinising clear cell carcinoma of the salivary gland: the current nomenclature, clinical/pathological characteristics and management. Crit Rev Oncol. 2016;102:55–64. doi: 10.1016/j.critrevonc.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Yang X-H, et al. Hyalinizing clear cell carcinoma of salivary gland origin in the head and neck: clinical and histopathological analysis. Int J Oral Maxillofac Surg. 2018;47(6):692–698. doi: 10.1016/j.ijom.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Yuen APW, et al. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19(7):583–588. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.D’Cruz AK, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. New Engl J Med. 2015;373(6):521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 11.Brockhoff HC, et al. Correlating the depth of invasion at specific anatomic locations with the risk for regional metastatic disease to lymph nodes in the neck for oral squamous cell carcinoma. Head Neck. 2017;39(5):974–979. doi: 10.1002/hed.24724. [DOI] [PubMed] [Google Scholar]
- 12.Jeffus SK, Gardner JM, Steliga MA, Shah AA, Stelow EB, Arnaoutakis K. Hyalinizing clear cell carcinoma of the lung: case report and review of the literature. Am J Clin Pathol. 2017;148(1):73–80. doi: 10.1093/ajcp/aqx048. [DOI] [PubMed] [Google Scholar]
- 13.Icard B, Grider DJ, Aziz S, Rubio E. Primary tracheal hyalinizing clear cell carcinoma. Lung Cancer. 2018;125:100–102. doi: 10.1016/j.lungcan.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Shahi M, Dolan M, Murugan P. Hyalinizing clear cell carcinoma of the bronchus. Head Neck Pathol. 2017;11(4):575–579. doi: 10.1007/s12105-017-0820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471–475. doi: 10.1016/j.humpath.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Takamatsu M, et al. Hyalinizing clear cell carcinoma of the bronchial glands: presentation of three cases and pathological comparisons with salivary gland counterparts and bronchial mucoepidermoid carcinomas. Mod Pathol. 2018;31(6):923–933. doi: 10.1038/s41379-018-0025-7. [DOI] [PubMed] [Google Scholar]
- 17.Al Ali BM, Alyousef MJ, Kamel AS, Al Hamad MA, Al-Bar MH, Algowiez RM. Primary paranasal sinus hyalinizing clear cell carcinoma: a case report. Diagn Pathol. 2017;12(1):70. doi: 10.1186/s13000-017-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porubsky S, Rudolph B, Rückert JC, et al. EWSR1 translocation in primary hyalinising clear cell carcinoma of the thymus. Histopathology. 2019;75(3):431–436. doi: 10.1111/his.13890. [DOI] [PubMed] [Google Scholar]
- 19.Oliver J, et al. Patterns of care and outcome of clear cell carcinoma of the head and neck. Otolaryngology. 2019;161(1):98–104. doi: 10.1177/0194599819835779. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan-Mejia ED, et al. Hyalinizing clear cell carcinoma: report of eight cases and a review of literature. Head Neck Pathol. 2009;3(3):179–185. doi: 10.1007/s12105-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh M-S, et al. Reevaluation of MAML2 fusion–negative mucoepidermoid carcinoma: a subgroup being actually hyalinizing clear cell carcinoma of the salivary gland with EWSR1 translocation. Human Pathol. 2017;61:9–18. doi: 10.1016/j.humpath.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Persson M, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106(44):18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonon G, et al. t (11; 19)(q21; p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 24.Chapman E, et al. Molecular profiling of hyalinizing clear cell carcinomas revealed a subset of tumors harboring a novel EWSR1-CREM fusion: report of 3 cases. Am J Surg Pathol. 2018;42(9):1182–1189. doi: 10.1097/PAS.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 25.Wang W-L, et al. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Mod Pathol. 2009;22(9):1201–1209. doi: 10.1038/modpathol.2009.85. [DOI] [PubMed] [Google Scholar]
- 26.Kao Y-C, et al. EWSR1 fusions with CREB family transcription factors define a novel myxoid mesenchymal tumor with predilection for intracranial location. Am J Surg Pathol. 2017;41(4):482. doi: 10.1097/PAS.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota T. Is biomarker research advancing in the era of personalized medicine for head and neck cancer? Int J Clin Oncol. 2014;19(2):211–219. doi: 10.1007/s10147-013-0660-4. [DOI] [PubMed] [Google Scholar]
- 28.Milchgrub S, et al. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18(1):74–82. doi: 10.1097/00000478-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Khan TS, et al. Clear cell carcinoma of soft palate-a case report. J Oral Maxillofacial Pathol. 2019;23(2):310. doi: 10.4103/jomfp.JOMFP_101_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano T, et al. Hyalinizing clear cell carcinoma with EWSR1-ATF1 fusion gene: report of three cases with molecular analyses. Virchows Arch. 2015;466(1):37–43. doi: 10.1007/s00428-014-1676-5. [DOI] [PubMed] [Google Scholar]
- 31.Arpaci RB, et al. Two rare entities in the same palate lesion: hyalinizing-type clear cell carcinoma and necrotizing sialometaplasia. J Craniofac Surg. 2014;25(3):e235–e237. doi: 10.1097/SCS.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 32.Yamanishi T, Kutsuma K, Masuyama K. A case of hyalinizing clear cell carcinoma, so-called clear cell carcinoma, not otherwise specified, of the minor salivary glands of the buccal mucosa. Case Rep Otolaryngol. 2015 doi: 10.1155/2015/471693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bostanci A, Ozbudak IH, Turhan M. Hyalinizing clear cell carcinoma of the maxilla. J Maxillofac Oral Surg. 2019;18(3):391–394. doi: 10.1007/s12663-018-1163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gushi E, Seki U, Orhan K. Hyalinizing clear cell carcinoma of maxilla. J Clin Diagn Res. 2017;11(7):ZL01. doi: 10.7860/JCDR/2017/29193.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donohue-Cornejo A, et al. Hyalinizing clear cell carcinoma-a rare entity in the oral cavity: a case report. World J Clin Cases. 2020;8(1):133. doi: 10.12998/wjcc.v8.i1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulciniti F, et al. Hyalinizing clear cell carcinoma of the parotid gland: report of a recurrent case with aggressive cytomorphology and behavior diagnosed on fine-needle cytology sample. Diagn Cytopathol. 2014;42(1):63–68. doi: 10.1002/dc.22956. [DOI] [PubMed] [Google Scholar]
- 37.Adil E, et al. Hyalinizing clear cell adenocarcinoma of the oropharynx. Head Neck. 2013;35(6):E184–E186. doi: 10.1002/hed.22940. [DOI] [PubMed] [Google Scholar]
- 38.Angiero F, Stefani M. Hyalinizing clear cell carcinoma arising on the anterior palatoglossal arch. Anticancer Res. 2007;27(6C):4271–4277. [PubMed] [Google Scholar]
- 39.Baghirath PV, Kumar JV, Vinay BH. Hyalinizing clear cell carcinoma: a rare entity. J Oral Maxillofac Pathol. 2011;15(3):335. doi: 10.4103/0973-029X.86714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balakrishnan R, et al. Hyalinizing clear cell carcinoma of the base of the tongue. J Laryngol Otol. 2002;116(10):851–853. doi: 10.1258/00222150260293718. [DOI] [PubMed] [Google Scholar]
- 41.Barber B, Côté D, Seikaly H. Clearing up clear cell tumours of the head and neck: differentiation of hyalinizing and odontogenic varieties. J Otolaryngol Head Neck Surg. 2010;39(5):E56. [PubMed] [Google Scholar]
- 42.Berho M, Huvos AG. Central hyalinizing clear cell carcinoma of the mandible and the maxilla a clinicopathologic study of two cases with an analysis of the literature. Hum Pathol. 1999;30(1):101–105. doi: 10.1016/s0046-8177(99)90308-8. [DOI] [PubMed] [Google Scholar]
- 43.Boccato P, Rinaldo A, McLaren KM. A rare tumor of salivary gland origin: hyalinizing clear cell carcinoma. ORL. 2001;63(2):119–120. doi: 10.1159/000055723. [DOI] [PubMed] [Google Scholar]
- 44.Browne JD, Holland BW. Combined intraoral and lateral temporal approach for palatal malignancies with temporalis muscle reconstruction. Arch Otolaryngol. 2002;128(5):531–537. doi: 10.1001/archotol.128.5.531. [DOI] [PubMed] [Google Scholar]
- 45.Burgess B, et al. Hyalinizing clear cell carcinoma of the tonsil: a case report. Head Neck Pathol. 2017;11(4):580–583. doi: 10.1007/s12105-017-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cashman EC, Woo SB. Clear cell carcinoma of the major salivary gland: time for a different approach to management? Int J Oral Maxillofac Surg. 2012;41(3):408–409. doi: 10.1016/j.ijom.2011.07.735. [DOI] [PubMed] [Google Scholar]
- 47.Ceballos Sáenz C, et al. Nasopharyngeal hyalinizing clear cell carcinoma: report of the histopathologic features of a case showing EWSR1 rearrangements by FISH and literature review. Int J Surg Pathol. 2014;22(7):667–672. doi: 10.1177/1066896914526778. [DOI] [PubMed] [Google Scholar]
- 48.Chao T-K, et al. Hyalinizing clear cell carcinoma of the hard palate. J Laryngol Otol. 2004;118(5):382–384. doi: 10.1258/002221504323086624. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry AP, et al. Glycogen-rich tumor of the oral minor salivary glands a histochemical and ultrastructural study. Cancer. 1983;52(1):105–111. doi: 10.1002/1097-0142(19830701)52:1<105::aid-cncr2820520120>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 50.Cheng LH, Lin YS, Lee JC. Primary clear cell carcinoma of the nasopharynx. Otolaryngology. 2008;139(4):592–593. doi: 10.1016/j.otohns.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Doxtader E, et al. Primary salivary gland-type tumors of the tracheobronchial tree diagnosed by transbronchial fine needle aspiration: clinical and cytomorphologic features with histopathologic correlation. Diagn Cytopathol. 2019;47(11):1168–1176. doi: 10.1002/dc.24285. [DOI] [PubMed] [Google Scholar]
- 52.Ereno C, et al. Hyalinizing clear cell carcinoma of the hypopharynx metastasizing to the lung: a case report. Histopathology. 2000;37(1):85–95. doi: 10.1046/j.1365-2559.2000.00955-6.x. [DOI] [PubMed] [Google Scholar]
- 53.Félix A, et al. Hyalinizing clear cell carcinoma of salivary glands: a study of extracellular matrix. Oral Oncol. 2002;38(4):364–368. doi: 10.1016/s1368-8375(01)00072-0. [DOI] [PubMed] [Google Scholar]
- 54.Fujita H, et al. Clear cell adenocarcinoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2004;98(5):579–582. doi: 10.1016/j.tripleo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda A, et al. Nasopharyngeal hyalinizing clear cell carcinoma with EWSR1 rearrangements diagnosed by fluorescence in situ hybridization. Auris Nasus Larynx. 2015;42(5):412–415. doi: 10.1016/j.anl.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 56.García J, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Human Pathol. 2015;46(3):471–475. doi: 10.1016/j.humpath.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Gon S, et al. Post-radiotherapy locoregional recurrence of hyalinizing clear cell carcinoma of palate. J Cancer Res Ther. 2013;9(2):281. doi: 10.4103/0973-1482.113386. [DOI] [PubMed] [Google Scholar]
- 58.Grenevicki LF, et al. Clear cell carcinoma of the palate. Int J Oral Maxillofac Surg. 2001;30(5):452–454. doi: 10.1054/ijom.2001.0116. [DOI] [PubMed] [Google Scholar]
- 59.Hadi UM, et al. Low grade primary clear cell carcinoma of the sinonasal tract. Rhinology. 2002;40:44–47. [PubMed] [Google Scholar]
- 60.Hayashi K, et al. Glycogen-rich clear cell carcinoma arising from minor salivary glands of the uvula. A case report. Pathol Int. 1988;38(9):1227–1234. doi: 10.1111/j.1440-1827.1988.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 61.Hijjawi SB, et al. Hyalinizing clear cell carcinoma of the tonsil and its treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):e32–e36. doi: 10.1016/j.oooo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Jin R, et al. Recurrent hyalinizing clear cell carcinoma of the base of tongue with high-grade transformation and EWSR1 gene rearrangement by FISH. Head Neck Pathol. 2012;6(3):389–394. doi: 10.1007/s12105-012-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kauzman A, Tabet J-C, Stiharu T-I. Hyalinizing clear cell carcinoma: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2011;112(1):e26–e34. doi: 10.1016/j.tripleo.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 64.Kim DW, et al. An atypical case of rare salivary malignancy, hyalinizing clear cell carcinoma. J Korean Assoc Oral Maxillofac Surg. 2013;39(6):283–288. doi: 10.5125/jkaoms.2013.39.6.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lai G, et al. The role of immunohistochemistry in the diagnosis of hyalinizing clear cell carcinoma of the minor salivary gland: a case report. Eur J Histochem. 2008;52:251–254. doi: 10.4081/1224. [DOI] [PubMed] [Google Scholar]
- 66.Lan J, et al. Primary paranasal sinus clear cell carcinoma with EWSR1-ATF1 fusion: report of 2 molecularly confirmed cases exhibiting unique histopathology. Hum Pathol. 2017;63:139–143. doi: 10.1016/j.humpath.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 67.Lattanzi DA, Polverini P, Chin DC. Glycogen-rich adenocarcinoma of a minor salivary gland. J Oral Maxillofac Surg. 1985;43(2):122–124. doi: 10.1016/0278-2391(85)90059-x. [DOI] [PubMed] [Google Scholar]
- 68.López-Quiles J, et al. Clear cell carcinoma of the major salivary glands in an HIV-infected patient. Int J Oral Maxillofac Surg. 2011;40(7):760–763. doi: 10.1016/j.ijom.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Malfitano MJ, et al. Nasopharyngeal hyalinizing clear cell carcinoma: a case report and review of the literature. Allergy Rhinol. 2019 doi: 10.1177/2152656719889030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manoharan M, Othman NH. Hyalinizing clear cell carcinoma of minor salivary gland: case report. Braz Dental J. 2002;13(1):66–70. [PubMed] [Google Scholar]
- 71.Masilamani S, et al. Hyalinizing clear cell carcinoma of the base of tongue: a distinct and rare entity. Indian J Pathol Microbiol. 2011;54(1):167. doi: 10.4103/0377-4929.77393. [DOI] [PubMed] [Google Scholar]
- 72.Milchgrub S, et al. Hyalinizing clear-cell carcinoma of salivary glands in fine-needle aspiration. Diagn Cytopathol. 2000;23(5):333–337. doi: 10.1002/1097-0339(200011)23:5<333::aid-dc10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 73.Nakashima T, et al. Hyalinizing clear cell carcinoma of the nasopharynx operated by trans-oral and trans-palatal approach. J Laryngol Otol. 2015;129(Suppl 2):S95–S97. doi: 10.1017/S0022215114002485. [DOI] [PubMed] [Google Scholar]
- 74.Nayak DR, et al. Clear cell carcinoma of the larynx—a case report. Int J Pediatr Otorhinolaryngol. 2001;57(2):149–153. doi: 10.1016/s0165-5876(00)00422-5. [DOI] [PubMed] [Google Scholar]
- 75.Newman JP, Funkhouser WK. Pathologic quiz case 1. Clear cell carcinoma of the nasal cavity. Arch Otolaryngol. 1993;119(9):1046–1049. [PubMed] [Google Scholar]
- 76.Ogawa I, et al. Clear cell tumors of minor salivary gland origin: an immunohistochemical and ultrastructural analysis. Oral Surg Oral Med Oral Pathol. 1991;72(2):200–207. doi: 10.1016/0030-4220(91)90164-8. [DOI] [PubMed] [Google Scholar]
- 77.Okon MM, Strflk P. Hyalinizing clear CeU carcinoma of salivary gland. A report. Pol. J. Pathol. 1996;47(1):33–36. [PubMed] [Google Scholar]
- 78.Orden A, Sciubba JJ, Misiek DJ. Painless mass of the hard palate. J Oral Maxillofac Surg. 1994;52(5):489–493. doi: 10.1016/0278-2391(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 79.O'Regan E, et al. Hyalinizing clear cell carcinoma of salivary gland: an aggressive variant. Oral Oncol. 2004;40(3):348–352. doi: 10.1016/j.oraloncology.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 80.Pillai N, et al. Hyalinizing clear cell carcinoma: base of tongue. Indian J Otolaryngol Head Neck Surg. 2019;71:239–242. doi: 10.1007/s12070-018-01573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ponniah I, SureshKumar P, Karunakaran K. Clear cell carcinoma of minor salivary gland-case report. Ann Acad Med. 2007;36(10):857. [PubMed] [Google Scholar]
- 82.Pujary K, et al. Hyalinizing clear cell carcinoma of the base of tongue. Int J Oral Maxillofac Surg. 2008;37(1):93–96. doi: 10.1016/j.ijom.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 83.Rajab E, Akmal SN, Nasir AM. Glycogen-rich clear cell carcinoma in the tongue. J Laryngol Otol. 1994;108(8):716–718. doi: 10.1017/s0022215100127938. [DOI] [PubMed] [Google Scholar]
- 84.Rezende RB, et al. Differential diagnosis between monomorphic clear cell adenocarcinoma of salivary glands and renal (clear) cell carcinoma. Am J Surg Pathol. 1999;23(12):1532. doi: 10.1097/00000478-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Rinaldo A, et al. Hyalinizing clear cell carcinoma of the oral cavity and of the parotid gland. ORL. 1999;61(1):48–51. doi: 10.1159/000027639. [DOI] [PubMed] [Google Scholar]
- 86.Roby BB, et al. Pathology quiz case 2. Hyalinizing clear cell carcinoma. Arch Otolaryngol. 2012;138(2):207. doi: 10.1001/archoto.2011.1245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sahasrabudhe NS, Puranik SC, Holla VV. Clear cell adenocarcinoma of the maxillary sinus: a case report. Indian J Pathol Microbiol. 2003;46(1):93–95. [PubMed] [Google Scholar]
- 88.Saleh KA, et al. Primary clear cell carcinoma of minor salivary gland of the soft palate: a case report. Med J Malaysia. 2012;67(3):335. [PubMed] [Google Scholar]
- 89.Shah AA, et al. An uncommon primary lung tumour: hyalinizing clear cell carcinoma, salivary gland-type. Histopathology. 2015;67(2):274–276. doi: 10.1111/his.12636. [DOI] [PubMed] [Google Scholar]
- 90.Shah AA, et al. EWSR1 genetic rearrangements in salivary gland tumors: a specific and very common feature of hyalinizing clear cell carcinoma. Am J Surg Pathol. 2013;37(4):571–578. doi: 10.1097/PAS.0b013e3182772a15. [DOI] [PubMed] [Google Scholar]
- 91.Sicurella F, et al. Clear cell carcinoma of minor salivary gland of the tongue. Acta Otorhinolaryngol Ital. 2004;24:157–160. [PubMed] [Google Scholar]
- 92.Simpson RHW, et al. Clear cell carcinoma of minor salivary glands. Histopathology. 1990;17(5):433–438. doi: 10.1111/j.1365-2559.1990.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 93.Solar AA, Schmidt BL, Jordan RCK. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115(1):75–83. doi: 10.1002/cncr.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su HK, et al. Very delayed cervical lymph node metastases from hyalinizing clear cell carcinoma: report of 2 cases. Head Neck. 2015;37(2):E19–E21. doi: 10.1002/hed.23764. [DOI] [PubMed] [Google Scholar]
- 95.Sun Z-J, et al. Hyalinizing clear cell carcinoma in minor salivary glands of maxillary tuberosity. Oral Oncol Extra. 2005;41(10):306–310. [Google Scholar]
- 96.Suzuki H, et al. Hyalinizing clear cell carcinoma arising from the base of the tongue. Acta Otolaryngol. 2006;126(6):653–656. doi: 10.1080/00016480500452509. [DOI] [PubMed] [Google Scholar]
- 97.Tang SK, Wan SK, Chan JKC. Hyalinizing clear cell carcinoma of salivary gland: report of a case with multiple recurrences over 12 years. Am J Surg Pathol. 1995;19(2):240. doi: 10.1097/00000478-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 98.Triantafillidou E, Dimitrakopoulos I, Skordalaki A. Clear cell carcinoma of the minor salivary glands. Report of a case. Aust Dent J. 1997;42(1):8–10. doi: 10.1111/j.1834-7819.1997.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 99.Urban SD, Keith DA, Goodman M. Hyalinizing clear cell carcinoma: report of a case. J Oral Pathol Med. 1996;25(10):562–564. doi: 10.1111/j.1600-0714.1996.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 100.Uri AK, Wetmore RF, Iozzo RV. Glycogen-rich clear cell carcinoma in the tongue. A cytochemical and ultrastructural study. Cancer. 1986;57(9):1803–1809. doi: 10.1002/1097-0142(19860501)57:9<1803::aid-cncr2820570916>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 101.Uzochukwu NO, Shrier DA, Lapoint RJ. Clear cell carcinoma of the base of the tongue: MR imaging findings. Am J Neuroradiol. 2007;28(1):127–128. [PMC free article] [PubMed] [Google Scholar]
- 102.Wang B, et al. Primary salivary clear cell tumors—a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126(6):676–685. doi: 10.5858/2002-126-0676-PSCCTA. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe K, et al. Clear cell carcinoma of the base of the tongue: case report and literature review. Ann Otol Rhinol Laryngol. 2015;124(1):55–61. doi: 10.1177/0003489414542094. [DOI] [PubMed] [Google Scholar]
- 104.Yamashita K, et al. Clear cell carcinoma of the minor salivary gland: an autopsy case with multiple metastases 29 years after the initial surgery and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;107(6):819–825. doi: 10.1016/j.tripleo.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 105.Yang S, et al. Clear cell carcinoma, not otherwise specified, of salivary glands: a clinicopathologic study of 4 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;106(5):712–720. doi: 10.1016/j.tripleo.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 106.Yuan C-T, Hsieh M-S. Tigroid background in cytology of hyalinizing clear cell carcinoma of the salivary gland. Diagn Cytopathol. 2016;44(4):338–341. doi: 10.1002/dc.23423. [DOI] [PubMed] [Google Scholar]



