Abstract
Differentiating adenoid cystic carcinoma (AdCC) from other basaloid neoplasm in a fine needle aspiration (FNA) sample can be challenging. Activation of MYB in AdCC by the fusion transcript MYB-NFIB has been recently demonstrated in salivary gland and other organs. The aim of this study is to evaluate the utility of MYB immunohistochemistry (IHC) in distinguishing AdCCs and other basaloid neoplasm in cytology specimens. Eighteen FNA cases, from salivary gland and other sites, and their subsequent surgical resection specimens were included in the study. Eight cases were confirmed AdCC on resection. MYB IHC was performed on slides made from cytology cell block and surgical resection paraffin blocks. Percentage and intensity of nuclear staining in tumor cells was scored as 0 to 3. The staining results were concordant between cytology specimens and their corresponding surgical resection tumors. Strong diffuse nuclear staining (score 3, N = 5) was exclusively observed in AdCC, both in cytology and surgical specimens. Only one pleomorphic adenoma and one poorly differentiated basaloid carcinoma were positive for MYB staining (score 1 to 2). Any degree of nuclear MYB labeling was seen in 100% AdCC cases (N = 8/8) compared with of 20% (N = 2/10) of all other non-AdCC cases (P = < 0.001). The sensitivity and specificity of any degree MYB positivity for AdCC in cytology specimen is 100% and 78%. The sensitivity and specificity of strong diffuse MYB labeling (score 2 to 3) for AdCC is 83% and 100% in cytology specimen. Strong diffuse nuclear staining of MYB is valuable in supporting a cytologic diagnosis of AdCC. However, weak and focal labeling of MYB should be interpreted with caution as it can be seen in benign and other malignant basaloid lesions.
Keywords: Fine needle aspiration, MYB, Immunohistochemistry, Adenoid cystic carcinoma
Introduction
Adenoid cystic carcinoma (AdCC) is an aggressive malignancy, accounting for approximately 5% of all neoplasm of the head and neck [1]. AdCC arise in various anatomic locations, including breast, tracheobronchial and lung, and morphologically resembles AdCC in salivary gland [2–4]. Although AdCC is characterized by slow growth, the long-term survival is determined by local recurrence rate despite treatment, tendency for perineural invasion, and distant metastasis [1].
Main histological patterns of AdCC include cribriform, tubular and solid [1–4]. The more common cribriform and tubular variants typically show abundant extracellular matrix arranged in globules with well-defined borders partially surrounded by basaloid tumor cells [5–7], which is reflected as characteristic cytology features of scattered balls of mucopolysaccharide matrix material surrounded by cohesive cellular clusters [6, 7]. However, in fine needle aspiration (FNA) specimens with abundant basaloid cells and lacking extracellular matrix materials, a broad differential diagnosis needs to be considered, including AdCC solid variant and other salivary gland tumors with overlapping morphology, such as pleomorphic adenoma (PA), basal cell adenoma/carcinoma, epithelial-myoepithelial carcinoma, and polymorphous low-grade adenocarcinoma [7–10]. An accurate FNA assessment is principal as it guides the level of aggressiveness of the surgical intervention, but due to significant morphologic overlap of basaloid cell neoplasm, a definite diagnosis is not always rendered.
Recently, a growing number of studies have shown the presence of a recurrent translocation in chromosome 6 and 9 in AdCC from different anatomical regions [11, 12]. This characteristic tumor-specific translocation generates a fusion of the MYB oncogene (v-Myb avian MYB viral oncogene homolog) with NFIB (nuclear factor IB) transcription factor gene [13, 14]. The prevalence of fusion is reported variably between 30 and 100% of AdCC in the head and neck region, as well as breast and bronchopulmonary AdCC [15–17]. Intriguingly, MYB overexpression has been observed in cases of AdCC associated both with and without MYB-NFIB fusion by immunohistochemistry (IHC) [18]. The diagnostic value of MYB expression in diagnosing AdCC is still in debate. Furthermore, the correlation between preceding FNA cytology and consequent surgical specimens has not been well validated. Therefore, we evaluated the diagnostic value of IHC expression of MYB in FNA cases initially diagnosed as “basaloid neoplasm” and correlated with subsequent surgical specimens.
Materials and Methods
Case Selection
This study was approved by the institutional review board (IRB). We retrospectively searched our cytopathology archives for FNA interpretations with the terms “adenoid cystic carcinoma”, “basaloid neoplasm” or “neoplasm with basaloid features”. Their subsequent surgical resection pathology was retrieved. Surgical resection cases with a diagnosis of “adenoid cystic carcinoma” and preceding FNA with any diagnostic material were also reviewed. All slides were re-reviewed for confirmation of diagnosis. A detail clinical history and the follow-up in each case were noted, including the age and sex of the patient, the anatomic site and the size of the tumor.
Cytology Specimen Preparation
All cytology samples were collected in BD CytoRich Red fixative (an ethanol-based fixative, Becton, Dickinson and Company, Franklin Lakes, NJ). Cell blocks were made by Hologic Cellient automated cell block system (Hologic, Mississauga, ON). Immunostaining was performed after formalin fixation of cell blocks.
MYB Immunohistochemsitry
Immunohistochemical studies were performed on 5-um sections of formalin-fixed, paraffin-embedded tissue. Slides were first deparaffinized, and rehydrated. Antigen retrieval was carried out with 0.01 M citrate buffer at pH 6.0. Slides were placed in the retrieval solution and were heated in a 770-W microwave oven for 14 min. Afterward, they were cooled to room temperature, and rinsed in distilled water before staining. All the slides were stained on the Dako Autostainer (Agilent/Dako, Santa Clara CA, USA).
The sections were first blocked for endogenous peroxidase activity with an application of Dual Endogenous Block (Agilent/Dako) for 10 min, followed by a brief buffer wash. The slides were then incubated with anti-c-MYB-phospho S11 antibody (rabbit monoclonal, clone EP769Y, Abcam cat. #45150) at 1:400 for 30 min. Following a buffer rinse, sections were incubated with detection reagent Ventana Ultraview Universal HRP Multimer. The sections were washed, and treated with a solution of diaminobenzidine (DAB) and hydrogen peroxide (Agilent/Dako) for 10 min, to produce the visible brown pigment. After rinsing, a toning solution (DAB Enhancer, Agilent/Dako) was used for 2 min to enrich the color. Following rinsing, the sections were counterstained with hematoxylin, dehydrated, and coverslipped with permanent media.
Sections of tonsil, with known positivity for the target proteins were used as positive controls for staining. The positive staining was defined as dark brown staining pattern, generally confined to the expected nuclear region of the cell. Scant or fine granular background staining, or no staining at all was considered negative.
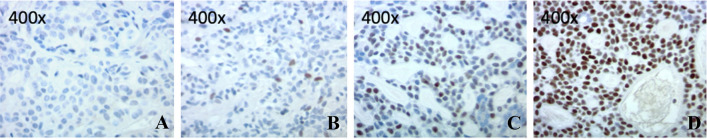
Nuclear MYB labeling was scored by percentage and intensity of labeling. Scattered to no nuclear staining in tumor cells is scored as “0”. Pale and focal nuclear staining of < 50% tumor cells is assigned as score “1”. Moderate nuclear staining of > 50% tumor cells is given to score of “2”. Diffuse strong staining in tumor cells which easily visible with a low power objective is consider as score “3” (Fig. 1).
Fig. 1.
Example cases with assigned MYB nuclear staining intensity score. a 0—scattered to no nuclear staining in tumor cells. b 1—pale and focal nuclear staining of < 50% tumor cells. c 2—moderate nuclear staining of > 50% tumor cells. d 3—diffuse strong staining in tumor cells which easily visible with a low power objective
Statistical Analysis
The data was analyzed using GraphPad Prism 8.0 (San Diego, CA). Fisher's exact test or the chi-square test were used to calculate the relative risk with confidence intervals. Results with a p-value less than 0.05 is considered as statistically significant.
Results
Total 18 FNA cases with paired surgical resections were included in the study, including 8 confirmed AdCC cases from submandibular glands (N = 2), parotid (N = 2), breast (N = 1) or lung (N = 1) and 2 metastatic AdCCs (bone and lung). One case with final diagnosis of “poorly differentiated carcinoma with basaloid features” in parotid gland was also included. Nine parotid gland cases with final diagnoses of “pleomorphic adenoma” (N = 4), “basal cell adenocarcinoma” (N = 2), “mammary analogue secretory carcinoma” (N = 1), “acinar cell carcinoma” (N = 1) and “salivary gland adenocarcinoma” (N = 1) were incorporated as controls (Table 1).
Table 1.
Comparison of MYB expression and pathologic diagnoses in paired FNA cytology samples and surgical resection from 18 patients
| Case | Gender | Age | FNA site | Cytology diagnosis | MYB * | Resection | Surgical histologic diagnosis | MYB |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 78 | Cervical lymph node | Metastatic salivary gland carcinoma with basaloid features | 3 | Lung | Metastatic adenoid cystic carcinoma | 3 |
| 2 | M | 47 | Submandibular gland | Basaloid salivary gland neoplasm | 3 | Submandibular gland | Adenoid cystic carcinoma | 3 |
| 3 | M | 39 | Parotid | Basaloid salivary gland neoplasm | 3 | Partoid | Adenoid cystic carcinoma | 3 |
| 4 | F | 58 | Submandibular gland | Low grade salivary gland neoplasm with basaloid features | 1 | Submandibular gland | Adenoid cystic carcinoma | 2 |
| 5 | M | 48 | Parotid | Basaloid salivary gland neoplasm | N/A | Partoid | Adenoid cystic carcinoma | 2 |
| 6 | M | 59 | Parotid | Consistent with adenoid cystic carcinoma | 2 | Bone | Metastatic adenoid cystic carcinoma | 2 |
| 7 | M | 84 | Lung | Consistent with adenoid cystic carcinoma | N/A | Lung | Adenoid cystic carcinoma | 3 |
| 8 | F | 50 | Breast | Suspicious for ductal carcinoma | 3 | Breast | Adenoid cystic carcinoma, solid variant | 3 |
| 9 | M | 86 | Parotid | Carcinoma with basaloid features | 1 | Partoid | Poorly differentiated carcinoma with basaloid features | 2 |
| 10 | M | 48 | Parotid | Basaloid salivary gland neoplasm | 1 | Partoid | Pleomorphic adenoma | 1 |
| 11 | F | 58 | Parotid | Basaloid salivary gland neoplasm | 0 | Partoid | Pleomorphic adenoma | 0 |
| 12 | F | 58 | Cervical lymph node | Metastatic salivary gland carcinoma | 0 | Partoid | Acinar cell carcinoma | 0 |
| 13 | F | 69 | Parotid | Basaloid salivary gland neoplasm | 0 | Partoid | Pleomorphic adenoma | 0 |
| 14 | F | 76 | Parotid | Ductal type salivary gland carcinoma | 0 | Partoid | Salivary gland adenocarcinoma, NOS | 0 |
| 15 | F | 55 | Parotid | Low grade salivary gland neoplasm with basaloid features | 0 | Partoid | Mammary analogue secretory carcinoma | 0 |
| 16 | M | 70 | Parotid | Basaloid salivary gland neoplasm | 0 | Partoid | Basal cell adenocarcinoma | 0 |
| 17 | F | 59 | Parotid | Low grade salivary gland neoplasm with basaloid features | 0 | Partoid | Pleomorphic adenoma | 0 |
| 18 | F | 81 | Parotid | Basaloid salivary gland neoplasm | N/A | Partoid | Basal cell adenocarcinoma | 0 |
*Three cytology cell blocks did not preserve sufficient tumor cells after processing
Three cytology cell blocks did not contain sufficient tumor cells after processing and were excluded from further analysis. All other cases demonstrated similar pattern and intensity of MYB labeling between FNA cell blocks and surgical specimens except two minor discrepancy. Staining concordance was 100% between cytology specimens and their corresponding surgical resection (Table 1). Minor discrepancy was observed in one case of submandibular gland AdCC and one case of poorly differentiated carcinoma with basaloid features. In both cases, FNA cell blocks showed relatively weak, score 1 MYB staining, while subsequent surgical resections demonstrated stronger and diffuser MYB staining and were scored as 2.
Strong diffuse nuclear staining (score 3, N = 5) was exclusively observed in AdCC cases both in cytology and surgical specimens. Only one pleomorphic adenoma and one poorly differentiated basaloid carcinoma are positive staining for MYB (score 1 to 2). Any degree of nuclear MYB labeling was seen in 100% AdCC cases (N = 8/8) compared with of 20% (N = 2/10) of all other non-AdCC cases (P = 0.0001) (Fig. 2). In addition, we did not see any correlation between MYB expression intensity and AdCC tumor grades, histological patterns, or anatomic locations (data not shown).
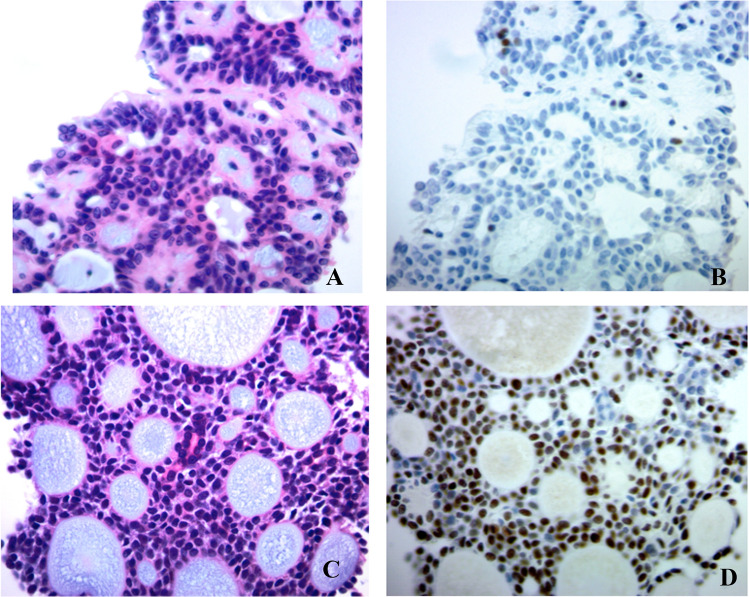
Fig. 2.
Immunohistochemical labeling for MYB in basaloid salivary neoplasm in FNA cell blocks. a, b A pleomorphic adenoma display scatter to no nuclear staining of MYB. c, d An adenoid cystic carcinoma display strong and diffuse nuclear MYB staining in tumor cells
Overall, the sensitivity and specificity of any degree MYB positivity for detecting AdCC in cytology specimen is 100% and 78%. The presence of diffuse, moderate to strong MYB labeling (score 2 or 3) has a sensitivity of 100% in surgical specimens and a sensitivity of 83% in FNA cytology specimens. The specificity of strong MYB labeling is is 90% in surgical specimens, and 100% in cytology specimens, respectively.
Discussion
A novel fusion transcript t(6:9) has been detected in AdCC occurring in the head and neck, breast and other anatomic locations [11–13, 19–21]. The studies have demonstrated that this genetic alteration is unique and specific to AdCC, irrespective of anatomic locations [18, 22]. The MYB (c-MYB) oncogene is the cellular mammalian homolog of v-MYB, a retroviral oncogene inked to avian leukemia, which is essential for cellular differentiation in various organ systems [21, 23]. Wild-type MYB protein has a half-life of about 30 min, and thus its expression is low in normal adult salivary glands [23]. The over-expression of 5′ portion of MYB due to t(6:9) MYB-NFIB gene fusion can be detected by IHC [13, 14]. Variable immunoreactivity for MYB by IHC has been reported in the literature, but may be due to variation of detection capability of different MYB antibody clones, or due to heterogeneous expression of the fusion protein in different tumor cells [24]. Though there are growing reports of MYB over-expression in AdCC at various anatomic locations, most reports are based on histologic specimens and only few based on the analysis on cytology materials [17]. In current study, we employed paired cytology—histologic resection specimens, to validate the utilization of MYB in diagnosis of AdCC.
Several previous reports state that the strong intensity MYB labeling was largely restricted to myoepithelial/basal cell component, whereas the inner epithelial cells lacked staining, especially in the tubular and cribriform types, suggesting preferential activation of MYB in myoepithelial cells [16, 18, 22, 24]. Other studies did not specify the preferential myoepithelial expression of MYB in AdCC [17, 25]. In our series, restricted strong expression of MYB in basaloid myoepithelial staining was noted in both cytology samples and surgical specimens.
In AdCC, the presence of MYB-NFIB fusion transcript ranges from 28 to 86%, while MYB protein expression has been noted in 55–97% of cases on IHC [13, 15–17, 19, 22, 24]. It has been also noted that MYB overexpression also occur in cases without fusion, indicating that expression of MYB may be mediated by other pathways as well [18]. In our study, the sensitivity of any degree MYB positivity for detecting AdCC in cytology specimen is nearly 100%. Intriguingly, one relatively low expression case (score 1) was found in 4 investigated pleomorphic adenomas. This may due to unspecific cross labeling of MYB antibody. A larger cohort study to investigate the incidence and grade of MYB expression in pleomorphic adenomas is warranted.
The cytology of AdCC is characteristic with extracellular matrix spheres. However, in the salivary glands, differentiating AdCC from pleomorphic adenomas and other basaloid neoplasms in FNA materials can be challenging, especially when aspirated lack extracellular hyaline globules or predominantly with solid morphology [7, 15]. In breast, AdCC also can be difficult to distinguish from other cribriform or basal-type lesions, including collagenous spherulosis and microglandular adenosis [2, 26]. In our study, specificity of any degree MYB positivity for detecting AdCC at all anatomic locations in cytology specimen was 78%. The presence of diffuse, moderate-strong MYB labeling has a specificity of 100% in FNA cytology specimens. Our results also suggested that overexpression of MYB may be utilized for AdCC diagnosis in difficult cases.
In summary, we validated MYB on cytologic FNA cell blocks and found MYB over-expression in FNA materials is highly concordant with corresponding subsequent surgical resection specimens. Strong and diffuse nuclear IHC labeling for MYB may serve as an important adjunct tool in AdCC of all grades and histological patterns in FNA cytology diagnosis in variety anatomic locations.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tong Sun, Email: tong.sun@mgh.harvard.edu, Email: tong.sun@yale.edu.
Tao Zuo, Email: tao.zuo@umassmemorial.org, Email: tao.zuo@bmc.org.
References
- 1.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: a review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38:620–627. doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agafonoff S, Sobolewski A, Braverman TS. Adenoid cystic carcinoma of the breast—discordant size on imaging and pathology: a case report and review of literature. Ann Med Surg (Lond) 2019;43:1–4. doi: 10.1016/j.amsu.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchiano E, Chin OY, Fang CH, Park RC, Baredes S, Eloy JA. Laryngeal adenoid cystic carcinoma: a systematic review. Otolaryngol Head Neck Surg. 2016;154:433–439. doi: 10.1177/0194599815621884. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya T, Bahl A, Kapoor R, Bal A, Das A, Sharma SC. Primary adenoid cystic carcinoma of lung: a case report and review of the literature. J Cancer Res Ther. 2013;9:302–304. doi: 10.4103/0973-1482.113399. [DOI] [PubMed] [Google Scholar]
- 5.Layfield LJ. Fine-needle aspiration in the diagnosis of head and neck lesions: a review and discussion of problems in differential diagnosis. Diagn Cytopathol. 2007;35:798–805. doi: 10.1002/dc.20769. [DOI] [PubMed] [Google Scholar]
- 6.Kundu R, Handa U, Punia RS, Dass A, Saini V. Adenoid cystic carcinoma: a study of 19 cases of salivary and extra-salivary tumours diagnosed by fine needle aspiration cytology. Diagn Cytopathol. 2018;46:1004–1009. doi: 10.1002/dc.24075. [DOI] [PubMed] [Google Scholar]
- 7.Miller JA, An D, Shafique K, Song S, Rao RA, Viswanathan K, Eykman E, Wiles A, Ali SZ, Field A, Fadda G, Barkan GA, Layfield LJ, Rossi ED, Powers CN, Siddiqui MT, Kholova I, Baloch Z, Maleki Z. Mucoepidermoid carcinoma, acinic cell carcinoma, and adenoid cystic carcinoma on fine-needle aspiration biopsy and the milan system: an international multi-institutional study. J Am Soc Cytopathol. 2019 doi: 10.1016/j.jasc.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Al-Abbadi MA, Aziz L. Performance characteristics of adenoid cystic carcinoma of the salivary glands in fine-needle aspirates. Arch Pathol Lab Med. 2016;140:1183. doi: 10.5858/arpa.2016-0138-LE. [DOI] [PubMed] [Google Scholar]
- 9.Aisagbonhi OA, Tulecke MA, Wilbur DC, Goldar-Najafi A, Iqbal S, Sadow PM, Faquin WC. Fine-needle aspiration of epithelial-myoepithelial carcinoma of the parotid gland with prominent adenoid cystic carcinoma-like cribriform features: avoiding a diagnostic pitfall. Am J Clin Pathol. 2016;146:741–746. doi: 10.1093/ajcp/aqw128. [DOI] [PubMed] [Google Scholar]
- 10.Pal S, Mondal PK, Sharma A, Sikder M. Fine needle aspiration cytology of basal cell adenoma of parotid simulating adenoid cystic carcinoma. J Cytol. 2018;35:55–57. doi: 10.4103/JOC.JOC_46_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca FP, Sena Filho M, Altemani A, Speight PM, Vargas PA. Molecular signature of salivary gland tumors: potential use as diagnostic and prognostic marker. J Oral Pathol Med. 2016;45:101–110. doi: 10.1111/jop.12329. [DOI] [PubMed] [Google Scholar]
- 12.Thierauf J, Ramamurthy N, Jo VY, Robinson H, Frazier RP, Gonzalez J, Pacula M, Dominguez Meneses E, Nose V, Nardi V, Dias-Santagata D, Le LP, Lin DT, Faquin WC, Wirth LJ, Hess J, Iafrate AJ, Lennerz JK. Clinically integrated molecular diagnostics in adenoid cystic carcinoma. Oncologist. 2019 doi: 10.1634/theoncologist.2018-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Almeida-Pinto YD, Costa SFDS, de Andrade BAB, Altemani A, Vargas PA, Abreu LG, Fonseca FP. t(6;9)(MYB-NFIB) in head and neck adenoid cystic carcinoma: a systematic review with meta-analysis. Oral Dis. 2019;25:1277–1282. doi: 10.1111/odi.12984. [DOI] [PubMed] [Google Scholar]
- 14.Andersson MK, Afshari MK, Andrén Y, Wick MJ, Stenman G. Targeting the oncogenic transcriptional regulator MYB in adenoid cystic carcinoma by inhibition of IGF1R/AKT signaling. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx017. [DOI] [PubMed] [Google Scholar]
- 15.Rooney SL, Robinson RA. Immunohistochemical expression of MYB in salivary gland basal cell adenocarcinoma and basal cell adenoma. J Oral Pathol Med. 2017;46:798–802. doi: 10.1111/jop.12617. [DOI] [PubMed] [Google Scholar]
- 16.Poling JS, Yonescu R, Subhawong AP, Sharma R, Argani P, Ning Y, Cimino-Mathews A. MYB labeling by immunohistochemistry is more sensitive and specific for breast adenoid cystic carcinoma than MYB labeling by FISH. Am J Surg Pathol. 2017;41:973–979. doi: 10.1097/PAS.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 17.Vallonthaiel AG, Jain D, Singh V, Kaur K, Madan K, Kumar V, Iyer VK, Sharma MC. c-Myb overexpression in cytology smears of tracheobronchial and pulmonary adenoid cystic carcinomas. Acta Cytol. 2017;61:77–83. doi: 10.1159/000453103. [DOI] [PubMed] [Google Scholar]
- 18.Bell D, Roberts D, Karpowicz M, Hanna EY, Weber RS, El-Naggar AK. Clinical significance of Myb protein and downstream target genes in salivary adenoid cystic carcinoma. Cancer Biol Ther. 2011;12:569–573. doi: 10.4161/cbt.12.7.17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysocki PT, Izumchenko E, Meir J, Ha PK, Sidransky D, Brait M. Adenoid cystic carcinoma: emerging role of translocations and gene fusions. Oncotarget. 2016;7:66239–66254. doi: 10.18632/oncotarget.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016;6:176–187. doi: 10.1158/2159-8290.CD-15-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, Carey CD, Rodig SJ, Sholl LM, Afrogheh AH, Faquin WC, Queimado L, Qi J, Wick MJ, El-Naggar AK, Bradner JE, Moskaluk CA, Aster JC, Knoechel B, Bernstein BE. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48:265–272. doi: 10.1038/ng.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brill LB, 2nd, Kanner WA, Fehr A, Andrén Y, Moskaluk CA, Löning T, Stenman G, Frierson HF., Jr Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24:1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Kurimoto T, Taketo MM, Fujii S, Kikuchi A. The WNT/MYB pathway suppresses KIT expression to control the timing of salivary proacinar differentiation and duct formation. Development. 2016;143:2311–2324. doi: 10.1242/dev.134486. [DOI] [PubMed] [Google Scholar]
- 24.Mitani Y, Li J, Rao PH, Zhao YJ, Bell D, Lippman SM, Weber RS, Caulin C, El-Naggar AK. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–4731. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen TY, Keeney MG, Chintakuntlawar AV, Knutson DL, Kloft-Nelson S, Greipp PT, Garrity JA, Salomao DR, Garcia JJ. Adenoid cystic carcinoma of the lacrimal gland is frequently characterized by MYB rearrangement. Eye (Lond) 2017;31:720–725. doi: 10.1038/eye.2016.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas DN, Asarian A, Xiao P. Adenoid cystic carcinoma of the breast. J Surg Case Rep. 2019;2019:rjy355. doi: 10.1093/jscr/rjy355. [DOI] [PMC free article] [PubMed] [Google Scholar]