Abstract
Background
Integration of specialist palliative care (PC) into standard oncology care is recommended. This study investigated how integration at the Cantonal Hospital St. Gallen (KSSG) was manifested 10 years after initial accreditation as a European Society for Medical Oncology (ESMO) Designated Center (ESMO-DC) of Integrated Oncology and Palliative Care.
Methods
A chart review covering the years 2006-2009 and 2016 was carried out in patients with an incurable malignancy receiving PC. Visual graphic analysis was utilized to identify patterns of integration of PC into oncology based on the number and nature of medical consultations recorded for both specialties. A follow-up cohort collected 10 years later was analyzed and changes in patterns of integrating specialist PC into oncology were compared.
Results
Three hundred and forty-five patients from 2006 to 2009 and 64 patients from 2016 were included into analyses. Four distinct patterns were identified using visual graphic analysis. The ‘specialist PC-led pattern’ (44.9%) and the ‘oncology-led pattern’ (20.3%) represent disciplines that took primary responsibility for managing patients, with occasional and limited involvement from other disciplines. Patients in the ‘concurrent integrated care pattern’ (18.3%) had medical consultations that frequently bounced between specialist PC and oncology. In the ‘segmented integrated care pattern’ (16.5%), patients had sequences of continuous consultations provided by one discipline before alternating to a stretch of consultations provided by the other specialty. In the 2016 follow-up, while the ‘oncology-led pattern’ occurred significantly less frequently relative to the ‘specialist PC-led pattern’ and the ‘segmented integrated care pattern’, the ‘concurrent integrated care pattern’ emerged more frequently when compared with the 2006-2009 follow-up.
Conclusion
The ‘specialist PC-led pattern’ was the most prominent pattern in this data. The 2016 follow-up showed that a growing number of patients received a collaborative pattern of care, indicating that integration of specialist PC into standard oncology can manifest as either segmented or concurrent care pathways. Our data suggest a closer, more dynamic and flexible collaboration between oncology and specialist PC early in the disease course of patients with advanced cancer and concurrent with active treatment.
Key words: oncology, palliative care, integration, designated center
Highlights
-
•
Specialist palliative care into oncology was evaluated in an ESMO Designated Center 10 years following accreditation.
-
•
In the 2016 follow-up, there was a significant increase of integrative procedures implemented in clinical practice.
-
•
Notwithstanding, 20% of patients with incurable cancer did not receive specialist palliative care with concurrent treatment of cancer.
-
•
There is a persisting need to overcome barriers to early palliative care in medical oncology.
Introduction
Early integration of specialist palliative care (PC) into standard oncology care is recommended.1,2 Evidence shows that advanced cancer patients whose oncologists provided early referrals to PC experienced improvement of their quality of life and greater satisfaction with their treatment.3, 4, 5, 6, 7 Over the past decades, there has been an increase towards offering specialist PC services on an outpatient basis alongside anticancer treatment.8
Several models of integration of PC into oncology have been proposed. Bruera and Hui9 suggested three models to describe how oncology and PC work together, emphasizing integrated care as the preferred model. Partridge et al.10 built upon this work by discussing necessary elements for successfully putting integration into practice. Through integration, the strengths of both specialties can be maximized, thereby improving patient care and reducing strain on health care systems.11,12 The European Society for Medical Oncology's (ESMO's) Designated Centers (ESMO-DCs) of Integrated Oncology and Palliative Care program has played a prominent role in the evolution of PC delivery models.13 In order to qualify for an ESMO-DC, self-nominating hospitals are evaluated anonymously but rigorously on the basis of a list of 13 qualitative criteria related to program infrastructure, clinical processes, education and research (Table 1).14 Cancer centers, which have earned Designated Center accreditation, demonstrate that patients and their caregivers have access to specialist PC services on an inpatient and outpatient basis following a patient-centered care approach. Close collaboration between oncologists and PC specialists is the cornerstone of the Designated Center program.15 Despite the growing emphasis on the integration of PC and oncology, little is known about how integration is manifested in clinical practice. The key randomized, controlled trials (RCTs) focusing on early integration provide extensive details on the PC interventions offered, but information about oncology care and joint visits is not provided.4, 5, 6
Table 1.
Definitions of the four patterns of integration between oncology and specialist palliative care
| A ‘medical consultation’ may reflect either outpatient or inpatient patient care. A ‘switch’ is defined as a change in service provision from specialist PC to oncology OR a change in service provision from oncology back to specialist PC. |
A patient whose case history meets the following criteria was classified in ONC pattern:
|
A patient whose case history meets the following criteria was classified in PALL pattern:
|
A patient whose case history meets the following criteria was classified in SEG pattern:
|
A patient whose case history meets the following criteria was classified in CONC pattern:
|
CONC pattern, concurrent integrated care pattern; ONC pattern, oncology-led pattern; PALL pattern, palliative care-led pattern; PC, palliative care; SEG pattern, segmented integrated care pattern.
This paper examines how integration of PC was exhibited at the Cantonal Hospital St. Gallen (KSSG), which has been accredited an ESMO-DC since October 2005.16 KSSG is an 800-bed tertiary care center serving northeast Switzerland. The oncology department comprises 37 beds and treats an annual patient load of approximately 1500 inpatients and 30 000 outpatient consultations. The specialist PC department has an 11-bed inpatient unit and three outpatient clinics managed by a PC consultation team of 12 physicians. The oncology and specialist PC departments are co-located and have a deeply intertwined relationship, with emphasis on reciprocal education and interdisciplinary patient care. Oncologists complete 40 h of PC skills training plus a 3-month rotation in the inpatient specialist PC unit. PC physicians complete a 6-month rotation in oncology.
This study sought to answer the following questions. Are there different patterns that characterize the integration of specialist PC with oncology? What characteristics are observed in patients who fall into different trajectories of care? Do established integration patterns change over time?
Patients and methods
Patients and design
A retrospective chart review was conducted for all patients with advanced incurable malignancy (i.e. metastatic or locally advanced) seen at specialist PC over a period of 3 years from 2006 to 2009. Eligible patients had at least one specialist PC consultation. Charts were reviewed from patient's first contact with specialist PC and followed until death. In the 2016 follow-up, data were collected over a year.
Measures
Data were collected on patient characteristics, as well as the dates and details of the medical services provided. Medical consultations were categorized as palliative, oncological, joint (oncologists and PC specialists were involved simultaneously in service provision), or neutral (emergencies or non-cancer-related treatment) during the course of patients' treatment trajectories. The core measure of interest was the number of times a patient switched between oncology and PC specialties. A switch was operationalized as a medical consultation that changed from PC to oncology or from oncology back to PC.
Statistical methods
Visual graphic analysis (VGA) is an innovative technique that can provide researchers with an alternative method of quantitative statistical analysis that is more sensitive to individual change and variation. In this study, VGA was utilized to analyze longitudinal care trajectories and to compare individual care trajectories over time.17 VGA provides a method that retains nuances of individual case details while simultaneously accounting for patterns across the sample.18 Individual patient data were visualized to reveal convergences and divergences in the sample and identify groupings.19 In order to visualize the tabular data, each service delivery date was color-coded so that PC contacts were immediately distinguishable from oncology contacts, and a visual pattern could be seen clearly. In order to define the care trajectory patterns, an interdisciplinary group of professionals (three PC specialists, two oncologists, two ward nurses and two study nurses) identified trajectories of care according to the different sequences of oncologic and PC consultations. A directed approach that was theory and research guided (concept-driven coding) was utilized to identify the coding categories (patterns). Data were analyzed using a stepwise process to assign predefined codes to 55 patients in order to develop the pattern schemes. Following individual analysis by each professional, the research team met to share findings. Pattern definitions were discussed and reworked until consensus was reached. Subsequently, the resulting pattern coding scheme was used for analysis among all patients in the sample. VGA was then carried out on the full sample. To ensure a rigorous process of validation and verification we used an interdisciplinary consensus process, blinded double-coding of each case by at least two professionals. These quality measures provide confidence that patterns were accurately identified and assigned.2
Analysis of patient characteristics and disease status was carried out using descriptive statistics: age at referral, gender, tumor type, number of days between referral and death and number of joint consultations were examined. All statistical analyses were carried out using Excel (Microsoft Corporation, Redmond, WA) and Stata IC 12.1 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.).
Results
In total, 345 patients from 2006 to 2009 and 64 patients from 2016 were included for the analysis.
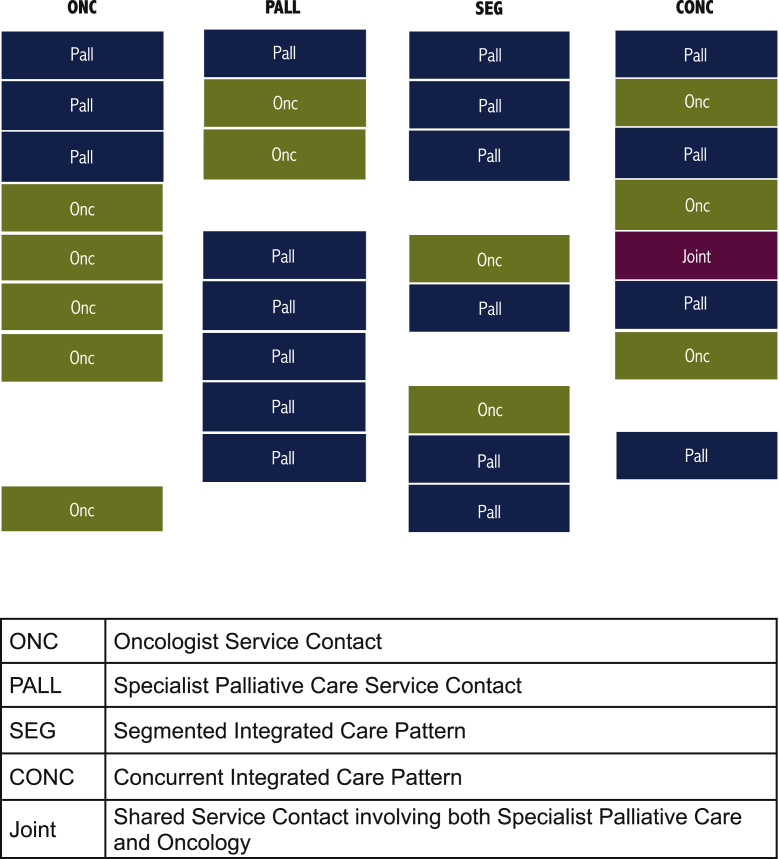
Four patterns of integration of specialist PC and oncology were characterized (Figure 1) by means of VGA.
-
(i)
In the oncology-led pattern (ONC pattern), a short series of one to three consultations with PC specialists was followed up with service provided exclusively by oncologists until the patient's death.
-
(ii)
In the palliative care-led pattern (PALL pattern), the patient had a maximum of three serial oncology visits, subsequently, the remaining medical consultations were provided by PC specialists until life's end.
-
(iii)
The segmented integrated care pattern (SEG pattern) showed a trajectory in which a patient had sequences of continuous consultations within a discipline before alternating to a stretch of service provided by the other specialty, and the alternation between blocks of oncology and specialist PC occurred a maximum of four times before death.
-
(iv)
Patients in the concurrent integrated care pattern (CONC pattern) had medical consultations that frequently moved between specialist PC and oncology with five or more switches between the specialties, shorter time spans between switches and more joint visits.
-
(v)
Table 1 provides the full definition for each trajectory of care pattern.
Figure 1.
Patterns of integration.
Patient characteristics in each pattern (Table 2)
Table 2.
Patient characteristics, by integration pattern
| Sample | PALL pattern | ONC pattern | CONC pattern | SEG pattern | |
|---|---|---|---|---|---|
| n (% of sample) | 345 | 155 (44.9) | 70 (20.3) | 63 (18.3) | 57 (16.5) |
| Age (years) | |||||
| Mean ± SD | 65 ± 13 | 66 ± 13 | 64 ± 11 | 60 ± 12 | 62 ± 13 |
| Median | 65 | 67 | 63 | 62 | 63 |
| Range | 20-91 | 20-91 | 38-82 | 23-85 | 27-84 |
| Male (%) | 64.4 | 64.5 | 64.3 | 57.1 | 71.9 |
| Tumor type n (% of sample) | |||||
| Gastrointestinal | 115 | 53 (46.1) | 23 (20.0) | 19 (16.5) | 20 (17.4) |
| Thoracic | 63 | 28 (44.4) | 15 (23.8) | 7 (11.1) | 13 (20.6) |
| Genitourinary | 49 | 16 (32.7) | 10 (20.4) | 12 (24.5) | 11 (22.5) |
| Ear, nose and throat | 41 | 24 (58.5) | 3 (7.3) | 11 (26.8) | 3 (7.3) |
| Gynecological | 30 | 11 (36.7) | 10 (33.3) | 5 (16.7) | 4 (13.3) |
| Hematological | 11 | 3 (27.3) | 3 (27.3) | 2 (18.2) | 3 (27.3) |
| Other | 36 | 20 (55.6) | 6 (16.7) | 7 (19.4) | 3 (8.3) |
| Joint palliative-oncology visits | |||||
| n (% of sample/pattern group) of patients with ≥1 joint visits | 83 (24.1) | 14 (9.0) | 17 (24.3) | 28 (44.4) | 24 (42.1) |
| Mean number of joint visits for patients with ≥1 joint visits | 1.27 | 1.0 | 1.0 | 1.6 | 1.3 |
| Months from first palliative outpatient consultation until death | |||||
| Mean | 7.63 | 4.44 | 9.04 | 12.85 | 8.84 |
| Median | 4.5 | 2.17 | 4.92 | 12.3 | 6 |
| Range | 0.06-39.67 | 0.06-37.63 | 0.06-39.67 | 1.6-36.23 | 0.27-35.1 |
CONC pattern, concurrent integrated care pattern; ONC pattern, oncology-led pattern; PALL pattern, palliative care-led pattern; SEG pattern, segmented integrated care pattern.
Compared with the other groups, patients in the PALL pattern were older on average, while younger patients were seen in the CONC pattern. Gastrointestinal, thoracic, genitourinary, ear, nose and throat tumors as well as gynecological tumors were seen predominantly in the PALL pattern relative to the other groups. Patients in the SEG and CONC patterns had more joint oncology/PC visits (42% and 44%) in comparison with patients in other patterns of integration. Patients treated within the CONC pattern lived approximately 6 months longer than patients in other patterns of integration [t (343) = 6.04, P < 0.001].
Follow-up
Changes in the distribution of pattern types were observed in the 10-year follow-up data. In the 2016 data, while the PALL pattern and the SEG pattern became marginally less frequent, although not statistically significant compared with the 2006-2009 data (Table 3), the ONC pattern occurred significantly less frequently in the 2016 data [χ2 (1, N = 409) = 5.61, P = 0.018]. In contrast, the CONC pattern increased significantly from 18% of cases in 2006-2009 to 45% of cases in 2016 [χ2 (1, N = 409) = 22.66, P < 0.001].
Table 3.
Results—pattern characteristics and comparison 2006-2009/2016
| Samples |
ONC pattern |
PALL pattern |
SEG pattern |
CONC pattern |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2016 | 2006 | 2016 | 2006 | 2016 | 2006 | 2016 | 2006 | 2016 | |
| n (% of sample) | 345 | 64 | 70 (20) | 5 (8) | 155 (45) | 21 (33) | 57 (17) | 9 (14) | 63 (18) | 29 (45) |
| Age (years) | ||||||||||
| Mean ± SD | 65 ± 13 | 64 ± 14 | 64 ± 11 | 58 ± 17 | 66 ± 13 | 65 ± 16 | 62 ± 13 | 68 ± 12 | 60 ± 12 | 66 ± 14 |
| Median | 65 | 64 | 63 | 55 | 67 | 69 | 63 | 71 | 62 | 63 |
| Range | 20-91 | 36-92 | 38-82 | 37-79 | 20-91 | 40-90 | 27-84 | 52-88 | 23-85 | 35-92 |
| Male (%) | 64 | 45 | 64 | 20 | 65 | 52 | 72 | 33 | 57 | 48 |
| Tumor type, n (% of sample) | ||||||||||
| Gastrointestinal | 115 (33) | 9 (14) | 23 (20) | 0 | 53 (46) | 2 (10) | 20 (17) | 2 (22) | 19 (17) | 5 (17) |
| Thoracic | 63 (18) | 20 (30) | 15 (23) | 2 (40) | 28 (44) | 5 (24) | 13 (21) | 5 (56) | 7 (11) | 8 (28) |
| Genitourinary | 49 (14) | 6 (9) | 10 (20) | 0 | 16 (33) | 2 (10) | 11 (23) | 1 (11) | 12 (25) | 3 (10) |
| Ear, nose and throat | 41 (12) | 10 (16) | 3 (7) | 0 | 24 (59) | 3 (14) | 3 (7) | 1 (11) | 11 (27) | 6 (21) |
| Gynecological | 30 (9) | 6 (9) | 10 (33) | 2 (40) | 11 (37) | 4 (19) | 4 (13) | 0 | 5 (17) | 0 |
| Hematological | 11 (3) | 6 (9) | 3 (27) | 0 | 3 (27) | 1 (5) | 3 (27) | 0 | 2 (18) | 5 (17) |
| Other | 36 (10) | 7 (11) | 6 (17) | 1 (20) | 20 (56) | 4 (19) | 3 (8) | 0 | 7 (19) | 2 (7) |
| Joint palliative-oncology visits | ||||||||||
| Patients with ≥1 joint visits, n (%) | 83 (24) | 31 (48) | 17 (24) | 3 (60) | 14 (9) | 3 (14) | 24 (42) | 3 (33) | 28 (44) | 22 (76) |
| Mean number of joint visits | 1.27 | 1.84 | 1.0 | 1.3 | 1.0 | 1.3 | 1.3 | 1.3 | 1.6 | 2.0 |
| Months from first palliative outpatient consultation until death occurred | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n = 345 | n = 14 | n = 70 | n = 0 | n = 155 | n = 7 | n = 57 | n = 2 | n = 63 | n = 5 |
| Mean | 7.63 | 2.83 | 9.04 | N/A | 4.44 | 2.52 | 8.84 | 1.02 | 12.85 | 3.98 |
| Median | 4.5 | 1.95 | 4.92 | N/A | 2.17 | 1.23 | 6.00 | 1.02 | 12.30 | 3.97 |
| Range | 0.06-39.67 | 0.53-8.00 | 0.06-39.67 | N/A | 0.06-37.63 | 0.53-5.27 | 0.27-35.1 | 0.73-1.30 | 1.60-36.23 | 0.70-8.00 |
CONC pattern, concurrent integrated care pattern; ONC pattern, oncology-led pattern; PALL pattern, palliative care-led pattern; SEG pattern, segmented integrated care pattern.
Discussion
Within an ESMO-DC, two types of close collaboration between oncology and specialist PC were present (SEG and CONC). There was also evidence of oncology handing-off care to specialist PC (PALL), as well as specialist PC providing limited service to oncology patients (ONC pattern).
Overall, the traditional model of PC (PALL pattern) was the most prominent. Probably specialist PC was the appropriate care provider for these patients because of complex needs, symptom severity and/or suffering in end of life preparation. Patients in this group often do not have anticancer treatment options that would justify continuing oncology consultations. As for the 20% of patients who received care in the ONC pattern, we hypothesize that these patients might have been referred to specialist PC for a specific symptom, such as a specialized nutrition/fatigue clinic or advanced pain management.
In the 2006 cohort, patient survival in the CONC group (median 12.3 months) is comparable with the 14-month survival reported for the intervention group in Bakitas et al.3 and the 11.6-month survival in Temel's4 intervention group. This is rather due to a correlation than a cause, because patients with a longer life span qualify more for integrated patterns.
In contrast, a follow-up 10 years later showed a development towards more concurrent and collaborative patterns as the percentage of patients treated in the CONC group increased from 18% to 45% during this time span. This might reflect a change of culture and a more established collaborative approach with double-boarded physicians, joint consultations, and a subjective sense of trust and rapport between individual providers. We assume that the desired integrative procedure at the KSSG was implemented into clinical practice through several factors. First, oncologists were supported to engage in specialized education. Second, the acknowledgement of seminal work by Bakitas et al.7 and Temel et al.4 and the ESMO position papers, and physicians' own research contribution to evidence-based practice and policy making in cancer care,8,15,20,21 promoted the integration of oncology and palliative care at the KSSG. Third, the outpatient clinic became more widely used as time passed. Finally, the notion that early integration of specialist PC into standard oncology was experienced beneficial also by the patients further strengthened the collaborative approach.
Demographic and clinical characteristics of patients provided no clear explanations of why patients fell into a particular pattern. Our data showed a tendency for older patients to be cared for in the PALL pattern. This result corresponds with the higher incidence of multimorbidity in older patients.22
Comparison to the literature
The significance of early PC has been assessed in two prominent studies. Bakitas et al.3 demonstrated that the integration of a PC intervention, concurrent with anticancer treatments early in the treatment trajectory, improved end of life quality of life and lowered depressed mood. Similarly, a more recent study by Temel et al.4 found that early PC improved not only quality of life and mood, but resulted also in a better prognostic understanding with less aggressive treatment decisions at the end of life and longer survival. Although PC in Europe is provided in different settings, manifestation of early integration of oncology and PC in hospitals remains imprecise and evidence stems from single-center studies.4,5,23,24 Results from international studies indicated that a majority of patients still receive PC in their terminal phase of life25 with a median of 20 days in cancer patients, 122 days in heart failure and 10 days in patients with chronic obstructive pulmonary disease (COPD) before death.26 The integration of PC into oncology was evaluated in a large survey sent to members of the Multinational Association of Supportive Care in Cancer (MASCC), ESMO and the European Association of Palliative Care (EAPC) across different European institutions. Findings revealed that PC services were limited to inpatient consultation service and out-patient PC clinics. Only a minority of the respondents, however, reported to have a PC unit with acute hospital beds and institution-operated hospice services. According to this survey, the most important barriers to integration of PC were financial limitations, a lack of adequately trained PC physicians and poor reimbursement for services.20 Of note, when integration was compared between non-designated cancer centers, urban hospitals and ESMO-DCs, only ESMO-DCs had constantly endeavored to integrate PC into oncology during the past 10 years with employment of well-educated specialist PC physicians and implementation of highly developed infrastructure, including ambulatory, consultative and acute inpatient services. This discrepancy may be a result of the ESMO accreditation.20,27
Clinical implications
In the ESMO-DC at KSSG, a growing number of patients receiving a collaborative pattern of care showed that interaction between oncology and specialist PC can manifest as either segmented or concurrent care pathways, demonstrating that early integration of PC into medical oncology is feasible. Nevertheless, early referral to PC is still underrepresented even in ESMO-DCs.14 Likewise, in our study, 20% of patients with incurable cancer did not receive specialist PC with concurrent treatment of cancer. These results highlight the persisting need to overcome barriers to early introduction of PC in medical oncology. Delivering patient-centered care is an integral component of state-of-the-art treatment in oncology independent of diagnosis, prognosis and treatment intention.28 The requirements of patient-centered care, however, can only be met if oncology and PC is fully integrated. In order to promote integration of oncology and PC, the Lancet Oncology Commission29 recommend standardized care pathways and multidisciplinary teams. Consensus-based components of integration were formulated by Hui et al.,14 addressing clinical structures (inpatient and outpatient PC services) and clinical processes in oncological care (e.g. presence of interdisciplinary team, routine screen monitoring, documentation of advance care planning and pain assessment, continuous education and training for oncologist and PC specialists, rotation in PC for oncology fellows, etc.). First and foremost, the integration of oncology and PC can only be successfully implemented through national and international actions, support by national health care authorities and the follow-up of the Commission's recommendations at national and regional levels.29
Future directions
An integrated approach requires oncologists to understand and value PC specialists' expertise in managing physical and psychosocial symptoms in patients with advanced and incurable diseases.21 At KSSG, many oncologists have substantial PC education, and many PC specialists are also trained as medical oncologists. This overlapping education interferes with this study's ability to predict oncologists' role in collaboration with specialist PC. Additionally, the strength of working relationships between physicians certainly influences referral and collaboration. Future research is warranted to better understand why integration patterns change over time and what factors contribute to the change of culture. Employing social network analysis in future research could provide an insight into patterns of integration.18 Transition from a condition in which interventions aim to treat the cancer to a status where the overall goal of the treatment is symptom control and improving quality of life is continuous. The transition requires the need for integration of PC into standard oncological treatment and incorporation of standard principles of PC into training and education of oncologists.30
Strengths and limitations
Several limitations to this research must be mentioned. First, data collection was limited to a single center, which restricts the extrapolation of results to the general population. Second, the number of patients included in the 2016 comparison group is much lower than the initial group studied. The statistical techniques used, however, allow for unequal sample sizes and can control for the differences. Third, our results are subject to researcher bias as the pattern schemes were concept-driven. There were, however, clear efforts made to operationalize the concepts by quantifying the number of consecutive visits and the number of switches. This helped to provide objective structure to the coding process. Fourth, additional patient data would prove useful in understanding why a patient was treated in a particular pattern—however, incomplete data and limited availability of variables is inherent to the retrospective design of the analysis. Not collected but useful patient variables would include: specialist PC needs, symptom assessments, socioeconomic background and degree of social support.31 Fifth, factors related to physicians, such as oncologists' reason for referral or palliative education, were also not evaluated although they would provide additional information. Finally, the analysis did not account for the content of services.
This research has two implications for clinical practice. Firstly, it supports the use of a dynamic and flexible system of interaction between oncology and specialist PC in order to provide patients with service tailored to their needs. Secondly, our results reinforce efforts to support oncologists in providing intermediate palliative interventions.
Future research is needed to validate our findings through reproducing the results in other centers, with particular interest in how patterns may be different based on the health care model. More research is warranted to study potential mechanisms or triggers that lead to the assessment of the need for involvement of PC (e.g. once a specific event occurs or a specific threshold of patient consultations is reached etc.). The clinical and cost-effectiveness of different patterns is also an area of future research interest.
Conclusion
The traditional model of PC (PALL pattern) was the most prominent pattern of care for patients in both time periods evaluated. Our data revealed a change toward more collaborative patterns. Nevertheless, early referral to PC is still underrepresented even in ESMO-DCs. The data suggest a closer, more dynamic and flexible collaboration between oncology and specialist PC early in the disease course of patients with advanced cancer and concurrent with active treatment.
Acknowledgements
The authors would like to acknowledge Aurelius Omlin (KSSG) and Tobias Silzle (KSSG) for their help in conducting this study.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Dalgaard K.M., Bergenholtz H., Nielsen M.E., Timm H. Early integration of palliative care in hospitals: a systematic review on methods, barriers, and outcome. Palliat Support Care. 2014;12(6):495–513. doi: 10.1017/S1478951513001338. [DOI] [PubMed] [Google Scholar]
- 2.Mazanec P., Daly B.J., Pitorak E.F., Kane D., Wile S., Wolen J. A new model of palliative care for oncology patients with advanced disease. J Hosp Palliat Nurs. 2009;11(6):324–331. [Google Scholar]
- 3.Bakitas M., Lyons K.D., Hegel Mark T. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer. J Am Med Assoc. 2009;302(7):741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temel J.S., Greer J.A., Muzikansky A. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann C., Swami N., Krzyzanowska M. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 6.Bakitas M., Bishop M.F., Caron P., Stephens L. Developing successful models of cancer palliative care services. Semin Oncol Nurs. 2010;26(4):266–284. doi: 10.1016/j.soncn.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakitas M.A., Tosteson T.D., Li Z. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strasser F., Blum D. Integration von palliativen interventionen in der behandlungspfade von unheilbaren Krebspatienten durch onkologieteams und spezialisierte palliative care. Schweiz Krebsbull. 2013;1:21–24. [Google Scholar]
- 9.Bruera E., Hui D. Integrating supportive and palliative care in the trajectory of cancer: establishing goals and models of care. J Clin Oncol. 2010;28(25):4013–4017. doi: 10.1200/JCO.2010.29.5618. [DOI] [PubMed] [Google Scholar]
- 10.Partridge A.H., Seah D.S.E., King T. Developing a service model that integrates palliative care throughout cancer care: the time is now. J Clin Oncol. 2014;32(29):3330–3336. doi: 10.1200/JCO.2013.54.8149. [DOI] [PubMed] [Google Scholar]
- 11.Quill T.E., Abernethy A.P. Generalist plus specialist palliative care – creating a more sustainable model. N Engl J Med. 2013;368(13):1173–1175. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 12.Weissman D.E., Meier D.E. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the center to advance palliative care. J Palliat Med. 2011;14(1):17–23. doi: 10.1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 13.Jordan K., Aapro M., Kaasa S. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018;29(1):36–43. doi: 10.1093/annonc/mdx757. [DOI] [PubMed] [Google Scholar]
- 14.Hui D., Cherny N.I., Wu J., Liu D., Latino N.J., Strasser F. Indicators of integration at ESMO designated centres of integrated oncology and palliative care. ESMO Open. 2018;3(5):e000372. doi: 10.1136/esmoopen-2018-000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherny N., Catane R., Schrijvers D., Kloke M., Strasser F. European Society for Medical Oncology (ESMO) program for the integration of oncology and palliative care: a 5-year review of the designated centers' incentive program. Ann Oncol. 2010;21(2):362–369. doi: 10.1093/annonc/mdp318. [DOI] [PubMed] [Google Scholar]
- 16.ESMO . ESMO Accredited Designated Centres - Kantonsspital St. Gallen - Palliativzentrum, Switzerland. European Society for Medical Oncology (ESMO); Lugano, Switzerland: 2005. [Google Scholar]
- 17.Brown C.G., McGuire D.B., Beck S.L., Peterson D.E., Mooney K.H. Visual graphical analysis. Nurs Res. 2007;56(3):195–201. doi: 10.1097/01.NNR.0000270029.82736.5a. [DOI] [PubMed] [Google Scholar]
- 18.Chambers D., Wilson P., Thompson C., Harden M. Social network analysis in healthcare settings: a systematic scoping review. PLoS One. 2012;7(8):e41911. doi: 10.1371/journal.pone.0041911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson J.M., Beck S.L., Christian B. Patterns of fatigue in adolescents receiving chemotherapy. Oncol Nurs Forum. 2010;37(4):444–455. doi: 10.1188/10.ONF.444-455. [DOI] [PubMed] [Google Scholar]
- 20.Davis M.P., Strasser F., Cherny N. How well is palliative care integrated into cancer care? A MASCC, ESMO, and EAPC Project. Support Care Cancer. 2015;23(9):2677–2685. doi: 10.1007/s00520-015-2630-z. [DOI] [PubMed] [Google Scholar]
- 21.Hui D., Kim Y.J., Park J.C. Integration of oncology and palliative care: a systematic review. Oncologist. 2014;20(1):77–83. doi: 10.1634/theoncologist.2014-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie C.S., Kvale E., Fisch M.J. Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract. 2011;7(6):371–374. doi: 10.1200/JOP.2011.000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temel J.S., Greer J.A., El-Jawahri A. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834–841. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanbutsele G., Pardon K., Van Belle S. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol. 2018;19(3):394–404. doi: 10.1016/S1470-2045(18)30060-3. [DOI] [PubMed] [Google Scholar]
- 25.Pivodic L., Pardon K., Van den Block L. Palliative care service use in four European countries: a cross-national retrospective study via representative networks of general practitioners. PLoS One. 2013;8(12):e84440. doi: 10.1371/journal.pone.0084440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beernaert K., Cohen J., Deliens L. Referral to palliative care in COPD and other chronic diseases: a population-based study. Respir Med. 2013;107(11):1731–1739. doi: 10.1016/j.rmed.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Hui D., Cherny N., Latino N., Strasser F. The ‘critical mass’ survey of palliative care programme at ESMO designated centres of integrated oncology and palliative care. Ann Oncol. 2017;28(9):2057–2066. doi: 10.1093/annonc/mdx280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramello M., Audisio R.A. The value of patient centred care in oncology. Eur J Surg Oncol. 2021;47(3, Part A):492–494. doi: 10.1016/j.ejso.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Kaasa S., Loge J.H., Aapro M. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol. 2018;19(11):e588–e653. doi: 10.1016/S1470-2045(18)30415-7. [DOI] [PubMed] [Google Scholar]
- 30.Ferrell B.R., Temel J.S., Temin S. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2016;35(1):96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J.M., DiGiacomo M., Currow D.C., Davidson P.M. Social capital in a lower socioeconomic palliative care population: a qualitative investigation of individual, community and civic networks and relations. BMC Palliat Care. 2014;13(1):30. doi: 10.1186/1472-684X-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]