Abstract
Background
FLAURA, the prospective trial of osimertinib as a first-line therapy compared with first-generation epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), did not show superior survival benefit for osimertinib in either the subgroup of Asians or the subgroup with the L858R mutation. In addition, the superiority of osimertinib compared with second-generation EGFR-TKI is thus far unclear.
Patients and methods
We reviewed the clinical data of all consecutive patients who were treated with osimertinib or afatinib as first-line therapy between May 2016 and October 2019 from 15 institutions in Japan. We defined the groups based on first-line EGFR-TKI as the afatinib group and the osimertinib group. Outcomes included time to discontinuation of any EGFR-TKI (TD-TKI), overall survival (OS), and time to treatment failure, with propensity score analysis carried out as an exploratory analysis in the survival and subgroup analyses.
Results
A total of 554 patients were enrolled. Data on 326 patients in the osimertinib group, and 224 patients in the afatinib group were analyzed. TD-TKI adjusted by propensity score in the afatinib and osimertinib groups was 18.6 months (95% confidence interval 15.8 to 22.0) and 20.5 months (95% confidence interval 13.8 to not reached), respectively, without significant difference (P = 0.204). OS adjusted by propensity score favored the afatinib group with a significant difference (P = 0.018). Subgroup analysis with propensity score showed that patients with L858R and without brain metastasis had superior survival benefit with afatinib compared with osimertinib (P < 0.001).
Conclusions
TD-TKI in the afatinib group was not significantly prolonged compared with the osimertinib group in the practical data. In the exploratory analysis of patients with L858R-mutated non-small-cell lung cancer without brain metastasis, afatinib showed more benefit in OS over osimertinib.
Key words: non-small-cell lung cancer, EGFR mutation, osimertinib, afatinib, real world data
Highlights
-
•
The large-scale practical data of 550 patients who were treated with osimertinib or afatinib as first-line therapy were analyzed.
-
•
The superiority of osimertinib compared with afatinib could not be demonstrated in all populations.
-
•
Osimertinib therapy showed effectiveness in patients with brain metastasis.
-
•
Afatinib therapy showed potential benefit in patients with L858R mutation and without brain metastasis.
Introduction
Epidermal growth factor receptor (EGFR) mutation is one of the driver oncogenes of non-small-cell lung cancer (NSCLC), leading tumorigenesis and tumor growth by activated EGFR pathway.1 EGFR tyrosine kinase inhibitors (EGFR-TKIs) have been established as standard therapy based on the evidence of prospective clinical trials. First-generation EGFR-TKIs, gefitinib and erlotinib, and the second-generation EGFR-TKI, afatinib, have been proven to significantly prolong progression-free survival (PFS) in EGFR-mutated NSCLC compared with chemotherapy in phase III trials.2, 3, 4, 5, 6, 7 Osimertinib, a third-generation EGFR-TKI, had survival benefit versus first-generation EGFR-TKIs, with a less toxic profile in the FLAURA trial. This resulted in its approval as a standard first-line treatment for EGFR-mutated NSCLC.8
Osimertinib was first approved for treatment of patients with the T790M mutation after progression during first- or second-generation EGFR-TKI. In the AURA3 trial, the improvement of PFS for osimertinib over chemotherapy was proven in patients with T790M-positive NSCLC who were pretreated with first-generation EGFR-TKIs; however, the patients who were pretreated with a second-generation EGFR-TKI were not included.9 As a result, the PFS of osimertinib after afatinib failure was not yet clear in this prospective trial. The combined analysis of the Lux-Lung 3, 6, and 7 trials indicated prolonged overall survival (OS) in patients who received afatinib therapy as first-line therapy follow up by osimertinib therapy as a sequential therapy compared with patients who received gefitinib therapy follow up by osimertinib therapy.10 The Gio-Tag trial, a retrospective study using the real-world data of 203 patients who were treated with afatinib follow up by osimertinib, demonstrated that the PFS of osimertinib after the failure of afatinib was 14.3 months.11,12 In addition, this study showed that the Asian population had prolonged PFS compared with the non-Asian population. These data indicated that second-generation EGFR-TKIs had the potential to prolong the total period of EGFR-TKI therapy, especially in the Asian population, resulting in an improvement of OS. The supportive data are, however, insufficient.
The OS secondary analysis in the FLAURA trial indicated that the Asian and the L858R-mutated populations had hazard ratios (HRs) of ∼1.0, which did not show a benefit of OS over first-generation EGFR-TKIs in these subgroups due to a small, underpowered sample size.13 Interestingly, race (Asian or non-Asian) and EGFR mutational subtype (exon 19 deletion or exon 21 L858R) are just two stratified factors in the randomization of this study. This fact indicated that these two factors independently had a negative influence on the clinical benefit of osimertinib. Asians account for the majority of EGFR-mutated NSCLC patients in terms of prevalence, with ∼40% of Asians having adenocarcinoma versus ∼20% in the non-Asian population. This confirms that reproducibility of clinical benefit in the Asian population with EGFR-mutated NSCLC is crucial in this tumor subtype.
Based on this background information, we hypothesize that first-line afatinib treatment, follow up by osimertinib, if T790M is detected at the point of afatinib failure (afatinib–osimertinib sequence strategy), has the potential for superior efficacy compared with first-line osimertinib treatment. The aim of this study is to explore the potential of an afatinib–osimertinib sequence strategy compared with a first-line osimertinib strategy, and to identify which subpopulations benefit from sequential afatinib–osimertinib treatment, with special interest in the subgroup analysis of patients with the L858R mutation for the aforementioned reasons.
Material and methods
Patients
In this observational cohort study, all consecutive patients who were treated with osimertinib (80 mg once daily as initial dose) or afatinib (40 mg once daily as initial dose) as a first-line therapy between May 2016 and October 2019 were enrolled from 15 institutions in Japan. The data cut-off was 31 December 2019. We defined the patients who were treated with afatinib or osimertinib in first-line settings as the afatinib and osimertinib groups, respectively.
Data extraction and management
Clinical data were extracted from the electronic medical records of each institution. Invitation to this study was sent to all institutions in the Central Japan Lung Study Group (CJLSG); case report forms were sent to the institutions after confirmation of intent to participate in this study, and the staff described the case details in the case report forms. Before data collection, a statistical plan was created by the designated statistician. The protocol was registered with the University Hospital Medical Information Network (UMIN; Clinical Trial Number: UMIN000041251). The data cleaning was carried out in accordance with protocols of data cleaning, and data were fixed after they were confirmed by the designated biostatistician. Data sharing is planned after publication of the main results.
Outcome
The primary endpoint of this study was defined as time to discontinuation of any EGFR-TKI (TD-TKI), which has been described in a previous report.14 TD-TKI was defined as the period from the induction of afatinib/osimertinib in first-line settings to the date of discontinuation of afatinib/osimertinib if absence of follow up osimertinib/afatinib therapy, or the period from the same point to the discontinuation of sequential osimertinib/afatinib therapy if the patient received follow up osimertinib/afatinib therapy. The secondary endpoint was OS, which was defined as the period from the introduction of afatinib or osimertinib as first-line treatment to the date of death due to any cause. Time to treatment failure (TTF) was defined as the period from the introduction of afatinib/osimertinib to the date of discontinuation of afatinib/osimertinib therapy, without progression being an event due to the evaluation of cases where continuing afatinib/osimertinib therapy was maintained beyond PD. PFS was calculated as the period from the start of afatinib/osimertinib therapy to the progression during afatinib/osimertinib therapy, with the discontinuation of afatinib/osimertinib before progression being censored at the discontinuation. The objective response rate was calculated in accordance with RECIST version 1.1.
Exploratory analysis
Based on the results of the subgroup analysis in the FLAURA trial, we had great interest in the efficacy of osimertinib in practice in patients with the L858R mutation. Previous reports indicated that osimertinib had the potential to be effective for brain metastasis; therefore we carried out a subgroup analysis of patients with EGFR mutational subtype and brain metastasis as an exploratory analysis. The full analysis set included patients with uncommon mutations, which might have biased the clinical outcome of each EGFR-TKI. Therefore, we carried out separate subgroup analyses for patients with L858R mutation, and for patients with exon 19 deletion. Consequently, subgroup analyses were planned in patients with or without brain metastasis, with exon 19 deletion, and with L858R point mutation. Survival analyses of subgroups were conducted by adjusting the propensity score excluding the factor, brain metastasis, or EGFR mutational subtype.
Statistical methods
In this study, the primary endpoint was TD-TKI, using propensity scoring analysis by the inverse probability of treatment weighting (IPTW) method, adjusting by the designated factors in the statistical plan if appropriate. Secondary endpoints included OS, PFS, and TTF. Subgroup analyses based on EGFR mutational subtype and brain metastasis were carried out as exploratory analyses, as these secondary analyses were conducted as sensitivity analyses. The analyses of qualitative variable characteristics were conducted using a two-sided Fisher's exact test or chi-square test where appropriate, and quantitative variables were analyzed using the Student's t-test. Survival curves were estimated by the Kaplan–Meier method, and a comparison between the two survival curves was conducted using the log-rank test, calculating HR and 95% confidential intervals (CIs) using the Cox hazard model. Adjusting by propensity score was planned in the survival analysis, with propensity score calculated using logistic regression by 10 factors as follows: institutions, age, sex, histologic subtype, smoking history, EGFR mutational status, clinical stage, Eastern Cooperative Oncology Group performance status, level of PD-L1 expression, and the presence of brain metastasis. The C statistic of the model was 0.663 (95% CI, 0.616-0.710). Adjusting by propensity score was conducted using stabilized IPTW, truncated (at the 99th percentile) IPTW, and matching method by three ratios: 1 : 1, 1 : 2, and 1 : 3 without replacement, respectively. P values <0.05 were defined as a significant difference in this study. All statistical analyses were conducted using SAS version 9.4 software (SAS Institute Inc, Cary, NC). The protocol was registered with the UMIN (Clinical Trial Number: UMIN000041251). Data analyses were conducted in accordance with the statistical analysis plan, and data sharing is planed after publication of the main results.
Ethical considerations
The protocol was approved by the institutional review board of each institution (approval number in Matsusaka Municipal Hospital: J-63-191216-3-1). The study was carried out in accordance with the Declaration of Helsinki, and individual data were anonymized before enrollment of the data to this study. The research did not receive funding from any company, and final support was provided only by the CJLSG.
Results
Patients
A total of 554 patients, 329 patients in the osimertinib group and 225 patients in the afatinib group, were enrolled from 15 institutions in Japan. Three patients in the osimertinib group, and one patient in the afatinib group were excluded from the analysis because one patient in the afatinib group received afatinib based on the protocol of another clinical trial, and three patients in the osimertinib group received osimertinib outside of the protocol period (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100115). For the whole population, the median age was 70 (range 31-93) years; females accounted for 56.7%, adenocarcinoma was present in 96.0% of the cases. Exon19 deletion, exon 21 L858R point mutation, and uncommon mutation rates were 50.4%, 45.8%, and 9.8%, respectively. The median follow-up period, which was calculated by the reverse Kaplan–Meier method, was 14.0 months, with 9.4 months in the osimertinib group, and 26.2 months in the afatinib group. The comparative analysis of the two groups' backgrounds showed a significant difference in the EGFR mutational subtype, and the trend of difference in the proportion of patients with brain metastasis, L858R mutation, and uncommon mutation (Table 1). In the afatinib group, 164 patients (73.5% in afatinib group) had already discontinued afatinib at the data cut-off, and 56 patients (34.1% of patients discontinued afatinib therapy) in the afatinib group received sequential osimertinib therapy after afatinib failure. In the osimertinib group, 96 patients (29.4% in the osimertinib group) experienced discontinuation of osimertinib therapy, and only 2 patients (2.1% of patients discontinued osimertinib therapy) received afatinib therapy after the discontinuation of osimertinib due to adverse event. The prevalence of brain metastasis based on EGFR mutational status was 29.6% (82/277) in those with Ex19del mutation, 23.6% (54/229) in those with L858R mutation, and 55.1% (27/49) among those with uncommon mutations including compound mutation, suggesting that patients with an uncommon mutation had significantly more brain metastasis compared with those with a major mutation.
Table 1.
Demographicsa
| Afatinib group (N = 224) | Osimertinib group (N = 326) | P value | |
|---|---|---|---|
| Age (years), mean | 68.82 | 70.14 | 0.152 |
| Sex | |||
| Male/Female | 105/119 | 133/193 | 0.162 |
| Smoking status | |||
| Never/former or current/unknown | 111/107/6 | 185/137/4 | 0.153 |
| Clinical stage (8th edition TNM stage classification) | |||
| 1-2/3/4/R | 5/25/152/42 | 10/24/228/64 | 0.469 |
| Histologic subtypeb | |||
| ADC | 215 | 313 | 1 |
| SCC | 5 | 7 | 1 |
| Other | 5 | 6 | 0.765 |
| EGFR mutationb | |||
| Ex19del | 114 | 163 | 0.862 |
| L858R | 74 | 155 | 0.001 |
| Uncommon | 39 | 9 | <0.001 |
| ECOG PS | |||
| 0/1/2 or more/Unknown | 67/124/30/3 | 114/159/47/6 | 0.328 |
| Pleural effusion | |||
| Yes | 65 | 114 | 0.165 |
| Brain metastasis | |||
| Yes | 75 | 85 | 0.07 |
| PD-L1 | |||
| <1/1-49/50≤/Unknown | 53/56/41/74 | 102/79/55/90 | 0.233 |
ADC, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma.
Early-stage patients received EGFR-TKI therapy in first-line settings due to intolerability for operation or radiation therapy resulting from factors such as advanced age or poor PS.
The cases with multiple factors were counted in each group of the categories of Histologic subtype and EGFR mutation.
Treatment duration of TKI (TD-TKI)
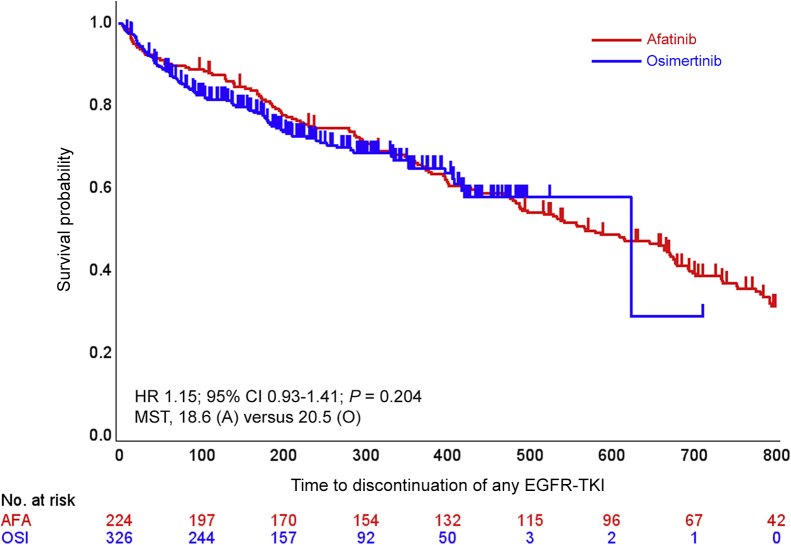
There was no significant difference in TD-TKI between groups with a P value of 0.204 after propensity score adjustment (Figure 1A). Median TD-TKI was 18.6 months (95% CI 15.8-22.0) in the afatinib group and 20.5 months (95% CI 13.8-not estimated) in the osimertinib group (adjusted HR 1.146, 95% CI 0.929-1.414). HRs adjusted by other propensity scoring methods are shown in Table 2 [see data related to TD-TKI (primary endpoint)]. The HRs are estimated using a statistical model, and the estimates (HRs) are appropriate under some mathematical assumptions. Showing that similar trends can be obtained by employing several statistical models with different assumptions will support the robustness of the main analysis results.
Figure 1.
Survival curves of TD-TKI.
Kaplan–Meier curves of TD-TKI with hazard ratio (HR) calculated by propensity score analysis using the inverse probability of treatment weighting (IPTW) method.
A/AFA, afatinib; CI, confidence interval; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; MST, median survival time; O/OSI, osimertinib; TD-TKI, time to discontinuation of any EGFR-TKI.
Table 2.
Hazard ratio based on method of propensity score analysis
| Method | TD-TKI (primary endpoint) |
Overall survival (secondary endpoint) |
||||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | P value | N | HR (95% CI) | P value | |
| IPTW | 540 | 1.146 (0.929-1.414) | 0.204 | 540 | 1.470 (1.070-2.021) | 0.018 |
| Sensitivity analysis | ||||||
| Unadjusted | 550 | 1.090 (0.814-1.459) | 0.565 | 550 | 1.217 (0.783-1.893) | 0.383 |
| Matching (1 to 1) | 376 | 1.010 (0.701-1.455) | 0.959 | 376 | 1.141 (0.639-2.037) | 0.657 |
| Stabilized IPTW | 540 | 1.134 (0.842-1.528) | 0.408 | 540 | 1.450 (0.924-2.275) | 0.106 |
| Truncated IPTW | 529 | 1.169 (0.943-1.450) | 0.155 | 529 | 1.385 (0.998-1.922) | 0.052 |
| Method | TTF (secondary endpoint) |
PFS (secondary endpoint) |
||||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | P value | N | HR (95% CI) | P value | |
| IPTW | 540 | 0.924 (0.755-1.131) | 0.443 | 540 | 1.020 (0.812-1.281) | 0.864 |
| Sensitivity analysis | ||||||
| Unadjusted | 550 | 0.882 (0.667-1.167) | 0.379 | 550 | 0.980 (0.716-1.340) | 0.898 |
| Matching (1 to 1) | 376 | 0.817 (0.575-1.163) | 0.262 | 376 | 1.003 (0.686-1.468) | 0.987 |
| Stabilized IPTW | 540 | 0.914 (0.687-1.214) | 0.534 | 540 | 1.000 (0.726-1.377) | >0.99 |
| Truncated IPTW | 529 | 0.932 (0.758-1.145) | 0.501 | 529 | 1.038 (0.825-1.305) | 0.751 |
CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; PFS., progression-free survival.
Overall survival, time treatment of failure, and progression-free survival
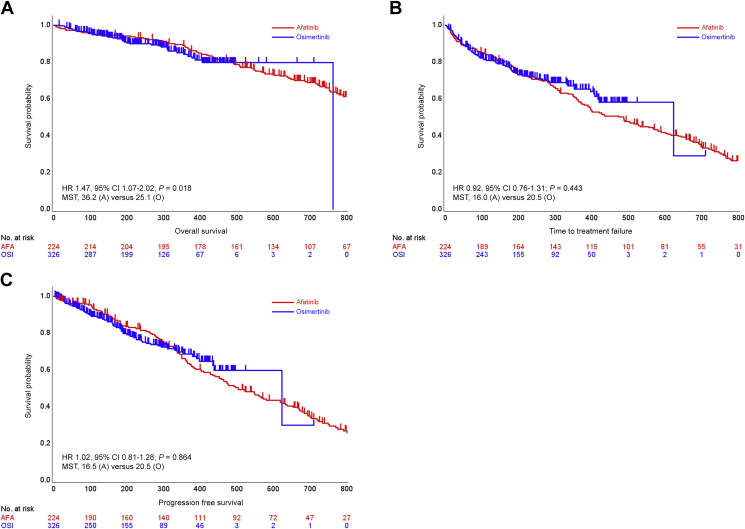
OS analysis showed a significant difference between the afatinib and osimertinib groups with a P value of 0.018 after adjusting by propensity scoring [Figure 2A and Table 2, see data related to OS (secondary endpoint)]. Median OS was 36.2 months (95% CI 30.6-55.3) in the afatinib group and 25.1 months (95% CI not estimated) in the osimertinib group (adjusted HR 1.47, 95% CI 1.07-2.02). In TTF analysis and PFS analysis, no significant difference was shown between groups (Figure 2B and C). Median TTF and median PFS in the afatinib and osimertinib groups are shown with HRs in Table 2 [see data related to TTF (secondary endpoint) and PFS (secondary endpoint)]. Median TTF of osimertinib after afatinib failure was 9.9 months (95% CI 3.59-16.2) for all 56 patients, 14.5 months (95% CI 11.65-17.32) in the 34 patients with Ex19del, and 5.55 months (4.74-6.37) in the 16 patients with L858R.
Figure 2.
Survival curves of secondary endpoints.
Kaplan–Meier curves of (A) overall survival (OS), (B) TTF, and (C) progression-free survival (PFS) with hazard ratio (HR) calculated by propensity score analysis using the inverse probability of treatment weighting (IPTW) method.
A/AFA, Afatinib; CI, confidence interval; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; O/OSI, osimertinib.
Subgroup analysis
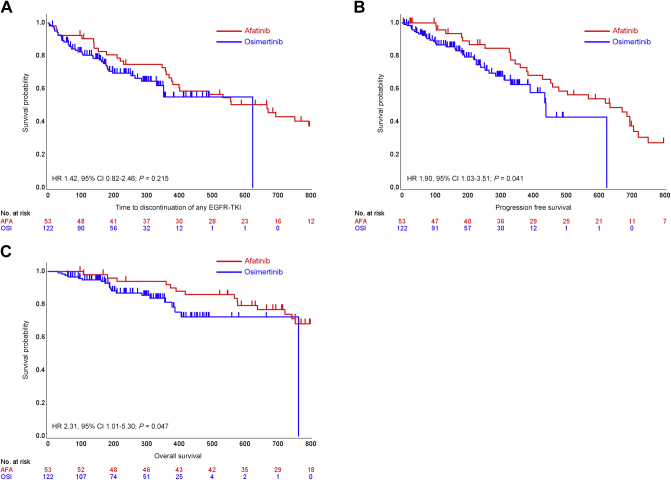
The survival curves of the subgroup analysis based on EGFR mutational subtype showed the afatinib had a trend of better survival benefit in patients with the L858R mutation compared with osimertinib (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100115). By contrast, osimertinib indicated a trend of superior clinical benefit compared with afatinib in patients with brain metastasis (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100115). We carried out a subgroup analysis of patients with L858R, and without brain metastasis. There was a significant difference in OS between groups with a P value of 0.047 (Figure 3). Propensity score analysis in the subgroups showed significant differences in PFS and OS between the two groups (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100115), and the HRs favored afatinib across propensity scoring methods, with a P value of <0.001 by the IPTW method (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100115).
Figure 3.
Survival curves of patients with L858R and without brain metastasis.
Kaplan–Meier curves of (A) TD-TKI, (B) progression-free survival (PFS), and (C) overall survival (OS) of patients with non-small-cell lung cancer (NSCLC) harboring L858R without brain metastasis (red, the afatinib group; blue, the osimertinib group).
CI, confidence interval; HR, hazard ratio.
Discussion
The FLAURA trial showed improved survival benefit for osimertinib over first-generation EGFR-TKIs in first-line settings, while the superiority of osimertinib to second-generation EGFR-TKIs has not yet been investigated. To our best knowledge, this is the first report evaluating the efficacy of osimertinib compared with a second-generation EGFR-TKI in clinical practice, using the largest-scale practical data of patients treated with osimertinib in first-line settings. In addition, almost all of the patients included in our data were Asians, therefore our data provided some insights into possible solutions to several clinical issues highlighted by the data in the FLAURA trial.
The FLAURA trial indicated OS superiority for osimertinib over first-line EGFR-TKIs; however, the subgroup analysis failed to show a superior survival benefit of osimertinib versus first-generation EGFR-TKIs in the subgroups with the L858R mutation, and Asians, with HRs of ∼1.0. Most important is that EGFR mutational status (Ex19Del or L858R) and race (Asian or non-Asian) were just two factors stratified due to randomization in the FLAURA study, suggesting that these two subgroup analyses should be discussed separately from other subgroup analyses. Asian data already have been reported, demonstrating that the OS benefit of osimertinib was superior to that of first-generation EGFR-TKIs.15 These data, however, did not include a subgroup analysis of OS in patients with the L858R mutation. The OS analysis in our study, in which almost all patients were Asians, showed that the OS curve of the afatinib group was better than that of the osimertinib group among patients with L858R.
We hypothesized that the enhanced efficacy of osimertinib after afatinib therapy improved the OS benefit of afatinib therapy. The Gio-Tag trial, which was a retrospective study to evaluate the sequential therapy of afatinib follow up by osimertinib in practice, indicated that osimertinib was prolonged to a greater degree after afatinib therapy than previous reported.11 The proportion of patients treated with osimertinib after afatinib therapy among those who discontinued afatinib therapy was more frequent than historical data indicated, which is consistent with the better results in our study. In our study, however, the median TTF of osimertinib after progression on afatinib therapy was 9.9 months, where patients with L858R exhibited a much shorter TTF than those with the Ex19Del (median 5.55 months versus 14.5 months). The AURA3 trial also indicated that the HR of PFS against chemotherapy in patients with the Ex19Del showed a better trend compared with that of the L858R group.9 These analyses continued to raise the question of what induced the superiority of afatinib over osimertinib among the patients with L858R.
As mentioned earlier, the FLAURA study also failed to show the superiority of osimertinib in the subgroup of patients with the L858R mutation. By contrast, osimertinib had better penetration of the central nervous system, and clinical benefits have also been reported.16 Colclough et al.17 investigated the brain penetration of EGFR-TKIs using a preclinical model, with the results indicating that osimertinib displayed the best blood–brain-barrier penetration of the 16 irreversible and reversible EGFR-TKIs tested. Our study showed the superiority of osimertinib in patients with brain metastasis in clinical practice, which suggests that patients with brain metastasis are candidates for osimertinib as a first-line therapy. Therefore we focused on the patients with L858R, and without brain metastasis, and the propensity score analysis was carried out in this population as an exploratory analysis. As a result, the OS benefit showed a favorable trend for the afatinib group compared with the osimertinib group, as shown in Figure 3, and Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100115. The subgroup analysis of the Asian and non-Asian populations in the Gio-Tag trial indicated that afatinib resulted in a better outcome among the Asian population. Japanese data showed that 87% of Japanese patients received rebiopsy after first- or second-generation EGFR-TKI treatment failure in the prospective trial.18 Nadler et al.19 reported that only 19% of patients treated with EGFR-TKI in first-line settings were tested for T790M mutation in the United States.
This potentially indicates that the L858R mutation weakens the efficacy of osimertinib, while afatinib has a survival benefit, with an advantage among Asians, especially in Japan where sequential osimertinib therapy is available due to the approval of osimertinib and frequent rebiopsy in the Gio-Tag trial, and a disadvantage in patients with brain metastasis. Consequently, Japanese patients with the L858R mutation, and without brain metastasis showed an OS benefit in the afatinib group compared with the osimertinib group.
This study had some limitations. There was a bias due to the nature of retrospective study, although we carried out a subgroup analysis or propensity score analysis to address the bias as far as possible. The OS analysis is immature due to the short follow-up period, and osimertinib had more censored cases, which induced the trend of a better survival curve in the osimertinib group due to the bias of informative censoring. The difference in follow-up periods between groups is also a bias because the relatively shorter follow-up period in the osimertinib group might introduce some unrecognized bias. The results of the subgroup analyses should be applied with caution because of the multiplicity issue of testing. These subgroup analyses are included as exploratory analyses; therefore the results are not conclusive.
In conclusion, TD-TKI and OS in the afatinib group have not been proven to be superior to those in the osimertinib group. However, osimertinib also could not sufficiently show superior clinical benefit over afatinib. Osimertinib showed strong clinical benefit in patients with brain metastasis in our data. Meanwhile afatinib had the trend of having clinical benefit in patients with the L858R mutation compared with osimertinib. Consequently, the subgroup analysis with the propensity score method revealed that afatinib had a strong trend of prolonged TD-TKI and OS over osimertinib in patients with the L858R mutation and without brain metastasis. Our results are certainly not conclusive because of the retrospective design; however, this represents that the use of osimertinib is not yet conclusive for this particular population. This study supports further investigation, such as a prospective clinical trial of an Asian population, to confirm the results.
Acknowledgements
This study was supported by the Central Japan Lung Study Group. We thank the staff who contributed to this research at all investigational sites.
Funding
This study was supported through research funding, and managed by Central Japan Lung Study Group, a nonprofit organization (no grant number).
Disclosure
KI reports personal fees from Boehringer Ingelheim, AstraZeneca, Pfizer, Eli Lilly, Chugai Pharmaceutical, Merk Sharp & Dohme (MSD), Ono Pharmaceutical, and Taiho Pharmaceutical. MM reports grants from Boehringer Ingelheim, Eli Lilly; personal fees from Eli Lilly, Chugai, Astra Zeneca, Ono, Pfizer, MSD; nonfinancial support from F. Hoffmann-La Roche; others from Chugai, Astra Zeneca, Pfizer, Merk Serono, Kissei, Taiho, and Novartis. KW reports grants from Chugai Pharmaceutical Co., Ltd, AstraZeneca, Novartis, and Abbie; receiving personal fees from Chugai Pharmaceutical Co., Ltd, Taiho Pharmaceutical, Boehringer Ingelheim, Eli Lilly K.K., Ono Pharmaceutical, MSD, and AstraZeneca. OH reports grants from AstraZeneca, Novartis, Boehringer Ingelheim, Byer, Daiichi Sankyo, GlaxoSmithKline; personal fees from AstraZeneca, Novartis, and Boehringer Ingelheim. TS has patents pending with AstraZeneca, Bristol-Myers Squibb, Ono Pharmaceutical, and Takeda. KT reports personal fees from AstraZeneca, Boehringer Ingelheim, Chugai, Eli Lilly, MSD, Taiho, and Novartis. NF reports personal fees from Eli Lilly Japan, Chugai, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim Japan, Taiho, Ono, and Pfizer Japan. TK reports personal fees from Boehringer Ingelheim and AstraZeneca. SI reports grants from AstraZeneca and Chugai; personal fees from AstraZeneca, Boehringer Ingelheim, Ono, Taiho, Bristol Myers Squibb, Eli Lilly Japan, Pfizer, and Chugai. YK reports grants from MSD; personal fees from AstraZeneca, Chugai Pharmaceutical, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, and Eli Lilly. HY reports personal fees from Boehringer Ingelheim, AstraZeneca, Chugai pharmaceutical, Eli Lilly, Pfizer, Bristol-Myers Squibb, Ono Pharmaceutical, MSD KK, Novartis, Kyowa Hakko Kirin, Daiichi Sankyo, Delta-Fly Pharma, Nippon Kayaku, and Taiho Pharmaceutical. MK reports grants from Eli Lilly; receiving personal fees from Eli Lilly, CHUGAI PHARMACEUTICAL CO, AstraZeneca, MSD, ONO PHARMACEUTICAL CO, Bristol Myers Squibb, Takeda Pharmaceutical Company, and Boehringer Ingelheim. HS reports grants from AstraZeneca, MSD (Merk), Ono Pharmaceutical, Eli Lilly Japan, Chugai Pharmaceutical, Bristol Myers Squibb, Quintiles Transnational Japan, Chugai Pharmaceutical, Beyer Pharmaceuticals, Olympus, Boehringer Ingelheim Japan, West Japan Oncology Group, AC Medical, Novartis Pharma, Japan Blood Products Organization, CMIC HOLDINGS, Takeda, A2 Healthcare, Otsuka, Pfizer, Parexel International, Boston Scientific, Harada, Nobelpharma, Taisho Toyama Pham; personal fees from AstraZeneca, MSD (Merk), Ono Pharmaceutical, Eli Lilly Japan, Covidien Japan, Taiho Pharmaceutical, Becton, Dickinson and Company, AMCO Inc., Kyowa Hakko Kirin, KYORIN Pharmaceutical, Chugai Pharmaceutical, Olympus, Boehringer Ingelheim Japan, Novartis Pharma, Pfizer, Parexel International, and Boston Scientific. All other authors have declared no conflicts of interest.
Role of the funder/sponsor
The funding sources from CJLSG had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplementary data
References
- 1.Jorissen R.N., Walker F., Pouliot N. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M., Inoue A., Kobayashi K. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Sequist L.V., Yang J.C., Yamamoto N. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y.L., Zhou C., Hu C.P. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y.L., Zhou C., Liam C.K. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 8.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 9.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park K., Bennouna J., Boyer M. Sequencing of therapy following first-line afatinib in patients with EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2019;132:126–131. doi: 10.1016/j.lungcan.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Hochmair M.J., Morabito A., Hao D. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 2018;14:2861–2874. doi: 10.2217/fon-2018-0711. [DOI] [PubMed] [Google Scholar]
- 12.Hochmair M.J., Morabito A., Hao D. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019;15:2905–2914. doi: 10.2217/fon-2019-0346. [DOI] [PubMed] [Google Scholar]
- 13.Ramalingam S.S., Vansteenkiste J., Planchard D. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 14.Planchard D., Boyer M.J., Lee J.S. Postprogression outcomes for osimertinib versus standard-of-care EGFR-TKI in patients with previously untreated EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res. 2019;25:2058–2063. doi: 10.1158/1078-0432.CCR-18-3325. [DOI] [PubMed] [Google Scholar]
- 15.Cho B.C., Chewaskulyong B., Lee K.H. Osimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol. 2019;14:99–106. doi: 10.1016/j.jtho.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Ballard P., Yates J.W., Yang Z. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 17.Colclough N., Chen K., Johnström P. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27:189–201. doi: 10.1158/1078-0432.CCR-19-1871. [DOI] [PubMed] [Google Scholar]
- 18.Seto T., Nogami N., Yamamoto N. Real-world EGFR T790M testing in advanced non-small-cell lung cancer: a prospective observational study in Japan. Oncol Ther. 2018;6:203–215. doi: 10.1007/s40487-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadler E., Pavilack M., Espirito J.L., Clark J., Fernandes A. Observational study of treatment patterns in patients with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer after first-line EGFR-tyrosine kinase inhibitors. Adv Ther. 2020;37:946–954. doi: 10.1007/s12325-020-01221-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.