Highlights
-
•
Recombinant production of soluble, active enterokinase (EK) is challenging.
-
•
Maltose binding protein-fusion improves EK solubility but reduces activity.
-
•
GroEL/ES and Erv2/PDI induces correct refolding and improves EK activity.
-
•
Replacing free cysteine with serine dramatically improves EK activity.
Abbreviations: D4K, (Aspartic Acid)4 Lysine; EK, enterokinase; bEKL, bovine enterokinase light chain; hEKL, human enterokinase light chain; IPTG, isopropyl β-D-1-thiogalactopyranoside; MBP, maltose-binding protein; TEV, tobacco etch virus
Keywords: Human enterokinase light chain, Escherichia coli, Recombinant protein, Fusion technology, Self-cleavage
Abstract
Human enterokinase light chain (hEKL) specifically cleaves the sequence (Asp)4-Lys↓X (D4K), making this a frequently used enzyme for site-specific cleavage of recombinant fusion proteins. However, hEKL production from Escherichia coli is limited due to intramolecular disulphide bonds. Here, we present strategies to obtain soluble and active hEKL from E. coli by expressing the hEKL variant C112S fused with maltose-binding protein (MBP) through D4K and molecular chaperons including GroEL/ES. The fusion protein self-cleaved in vivo, thereby removing the MBP in the E. coli cells. Thus, the self-cleaved hEKL variant was released into the culture medium. One-step purification using HisTrap™ chromatography purified the hEKL variant exhibiting an enzymatic activity of 3.1 × 103 U/mL (9.934 × 105 U/mg). The approaches presented here greatly simplify the purification of hEKL from E. coli without requiring refolding processes.
Recombinant fusion technology has been used to enhance the expression level and solubility of target proteins, and to facilitate their purification [1,2]. Proteases including Factor Xa, thrombin, tobacco etch virus (TEV) protease, and enterokinase (EK) are used for the site-specific cleavage of recombinant tags from fusion proteins [[3], [4], [5], [6]]. While Factor Xa, thrombin, and TEV protease cleave inside the recognition site, EK cleaves outside the site, thus it has a proteolytic activity regardless of the P1′ position sequence.
Human EK (hEK) (DDDDK↓, D4K↓) is produced by cells in the duodenum and intestinal brush-border [[7], [8], [9]]. EK activates trypsin by cleavage of trypsinogen [[10], [11], [12]]. hEK consists of an 86 kDa heavy chain and a 28 kDa light chain that are connected by a single disulphide bond. The heavy chain contains an intestinal brush-border membrane-binding motif. The light chain harbours the classical catalytic triad (chymotrypsin His57, Asp102, and Ser195) with four intramolecular disulphide bonds. The hEK light chain (hEKL) can cleave the fusion protein to obtain the authentic form of the protein [13]. In addition, hEKL is an attractive protease for use in protein purification due to its broad range of reaction conditions (pH 4.5–9.5 and temperature 4–45 °C), tolerance against various detergents, and reusability [10,12].
hEKL has a 10-fold higher catalytic efficiency (kcat/KM) than bEKL [14,15]. However, several reports show that hEKL is expressed in inclusion bodies in E. coli [10] that necessitates refolding using dialysis [[16], [17], [18], [19]], dilution [18,[20], [21], [22]], or on-column methods [18,[23], [24], [25]].
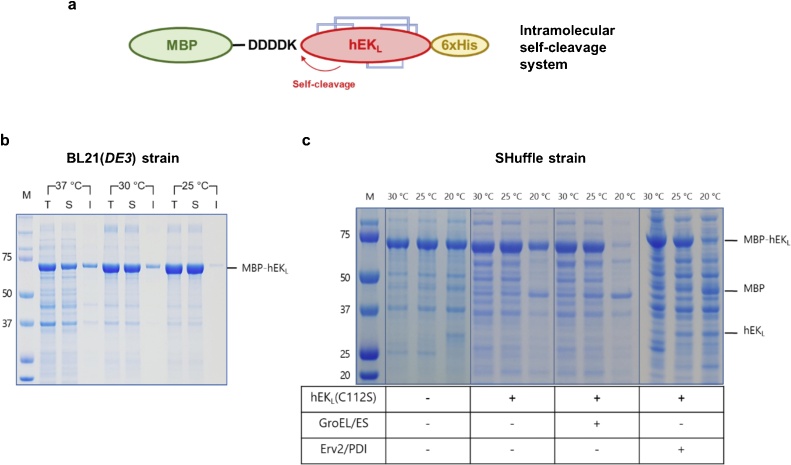
In this study, we present strategies to produce active hEKL in E. coli cytoplasm. We report production of soluble, active hEKL with improved folding efficiency that can be used in-house. To produce active, cytoplasmic hEKL with the correct disulphide bonds, we constructed hEKL fused with MBP through the D4K cleavage site and expressed this in E. coli cells expressing chaperone proteins (Fig. 1a). A previous report demonstrated expression of soluble and active MBP-tagged hEKL [26]. However, we found that MBP-hEKL was unable to self-cleave, indicating an absence of the enzymatic activity (Figs. S1 and 1b). To test whether removal of MBP could restore the hEKL activity, an hEKL variant was constructed by replacing the D4K with the TEV protease recognition site (ENLYFQ). However, hEKL obtained by TEV cleavage of MBP-hEKLwas still inactive (data not shown). To investigate whether the loss of activity resulted from a limited reduction of disulphide bonds or misfolding, we conducted a refolding process to rearrange disulphide bonds. Detection of self-cleaved forms of refolded hEKL indicated that the refolded enzyme was partially active (Fig. S2). These results demonstrated that MBP fusion enhances the solubility of hEKL but does not allow its correct folding. We speculated that hEKL misfolding might result from incorrect disulphide bonds formed during expression in E. coli.
Fig. 1.
The expression and activity analysis of hEKL in flask culture. (a) Construction of MBP-hEKL fusion connected through the EK cleavage sequence. (b) Expression of MBP-D4K-hEKL in E. coli BL21 (DE3) at different temperatures. (c) The expression of hEKL C112S in E. coli SHuffle strain. The blue lane in 1a indicates disulphide bonds. M, Protein marker; I, Insoluble protein; S, soluble protein; T, Total protein.
Therefore, to promote the formation of the correct disulphide bonds in E.coli-expressed hEKL, we employed three strategies: (i) use of a trxB−, gor−, ahpC*+mutant expressing cytoplasmic DsbC (SHuffle strain) for oxidative folding, (ii) replacement of the free cysteine with serine (C112S), which bound to heavy chain, to reduce misfolding, and (iii) co-expression of molecular chaperones that isomerize disulphide bonds. First, when the SHuffle strain was used, self-cleaved hEKL was successfully detected, although at a low level (7.9 % of total MBP-D4K-hEKL), in cells grown at 20 °C (Fig. 1c). Use of the C112S mutated hEKL dramatically improved the ratio of self-cleaved hEKL to up to ∼49.5 % in cells grown at 20 °C, which may be caused by the reduced mispairing of multiple disulphide bonds [12,27]. Remarkably, fully self-cleaved hEKL was detected from cell co-expressing GroEL/ES and Erv2/PDI grown at 20 °C. In particular, the activity was slightly higher upon GroEL/ES co-expression. Notably, hEKL was not visible in the SDS-PAGE gel even when hEKL activity was observed. However, as shown in Fig. S3, when inactivated hEKL was produced by TEVp, hEKL was visible in the SDS-PAGE gel. Therefore, we assumed that the visibility of hEKL in the SDS-PAGE gel was influenced by its folding.
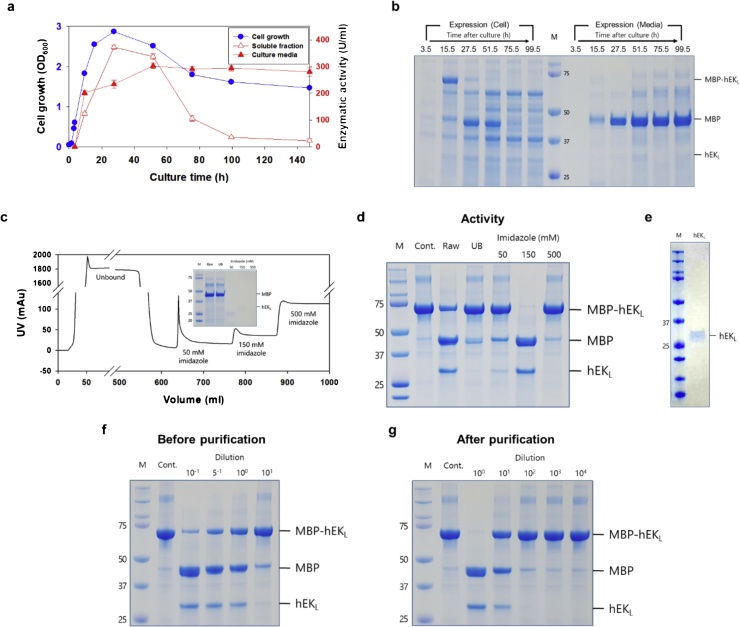
We further monitored the time profiles for cell growth and enzymatic activity of hEKL C112S (Fig. 2a and b). After 27.5 h of culture, the cell growth reached the maximum (2.87 OD600) and then sharply decreased. At that time, the hEKL activity in the soluble fraction reached the maximum value (372 U/mL) and then decreased to ∼22 U/mL. In contrast, hEKL in culture supernatants reached the maximum value (303 U/mL) after 75.5 h of culture. These results indicated that hEKL may be released into the extracellular fraction by autolysis of cell.
Fig. 2.
Expression and purification of hEKL C112S. (a) Time-profiles of cell growth and activity of hEKL C112S in flask culture. (b) SDS-PAGE analysis of flask culture samples. (c) Chromatogram of hEKL C112S purification. The inlet indicates SDS-PAGE of each fraction (raw: load fraction, UB: unbounded fraction). (d) Indirect conformation of enzymatic activity of each eluted fraction. MBP-D4K-hEKL (25 μg) was treated with 1 μl of purified hEKL, and incubated at 37 °C for 1 h. (e) SDS-PAGE and western blot of purified hEKL C112S. Enzymatic activity of hEKL C112S (f) before purification and (g) after purification. MBP-D4K-hEKL (25 μg) was treated with 1 μl of diluted culture supernatant or purified hEKL, and incubated at 37 °C for 1 h. M, Protein marker; Cont., MBP-D4K-hEKL.
We attempted to obtain highly pure hEKL C112S from culture supernatants. The culture supernatant of E. coli SHuffle expressing pET-30a-MBP-D4K-hEKL C112S and pACYC-GroEL/ES was loaded on the affinity chromatography (HisTrap™) along with 1 mM DTT to improve the binding efficacy (Fig. 2c). The enzymatic activity was 306 ± 0 U/mL and 3085 ± 43 U/mL before and after purification, respectively (Fig. 2d–g). A previous report [11] showed that a low-yield hEKL (10 %) can be purified from the culture media of P. pastoris using a two-step purification with several pre-treatment steps [11]. However, we could purify hEKL at high purity (>99 %) and yield (>99 %) using a simplified one-step method. Purified hEKL C112S had affinity to GD4K-na with KM = 0.287 ± 0.079 mM, turnover number Kcat = 6.725 × 104 ± 1.230 × 104 s−1, and catalytic efficiency KM/Kcat = 2.385 × 105 mM−1 s−1.
In conclusion, we could purify soluble and active hEKL at a high yield using an MBP tag, replacing the free cysteine with serine, using E. coli strain promoting oxidative folding, co-expressing molecular chaperone that isomerise disulphide bonds, and culturing at low temperature. These findings provide strategies for purification of the complex, multiple disulphide-bonded hEKL from E. coli.
Author contributions
Y.S.K, H. Lee and S.H. Park designed experiments and collected data. Y.K and J.A. supervised the research project and guided the design of experiments. Y.S.K and H.L drafted the manuscript. All authors read the manuscript and agree to submission to Journal of Biotechnology
Data statement
All data reported in the paper are available from the corresponding author upon reasonable request. Materials and Methods in this study are described in the Supplementary information.
Declaration of Competing Interest
The authors have no competing interests to declare
Acknowledgements
The authors are grateful for the support of the Ministry of Trade, Industry and Energy of the Republic of Korea (20009121), and Korea Research Institute of Bioscience and Biotechnology Research Initiative Program (1711134081) of the Republic of Korea.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2021.e00626.
Contributor Information
Yeu-chun Kim, Email: dohnanyi@kaist.ac.kr.
Jungoh Ahn, Email: ahnjo@kribb.re.kr.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Abdelhamid M.A., Motomura K., Ikeda T., Ishida T., Hirota R., Kuroda A. Affinity purification of recombinant proteins using a novel silica-binding peptide as a fusion tag. Appl. Microbiol. Biotechnol. 2014;98:5677–5684. doi: 10.1007/s00253-014-5754-z. [DOI] [PubMed] [Google Scholar]
- 2.Karikari T.K., Turner A., Stass R., Lee L.C., Wilson B., Nagel D.A., Hill E.J., Moffat K.G. Expression and purification of Tau protein and its frontotemporal dementia variants using a cleavable histidine tag. Protein Expr. Purif. 2017;130:44–54. doi: 10.1016/j.pep.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington J.C., Dougherty W.G. A viral cleavage site cassette: identification of amino acid sequences required for tobacco etch virus polyprotein processing. Proc. Natl. Acad. Sci. U. S. A. 1988;85:3391–3395. doi: 10.1073/pnas.85.10.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale A.J., Yegneswaran S., Xu X., Pellequer J.L., Griffin J.H. Characterization of a factor Xa binding site on factor va near the Arg-506 activated protein C cleavage site. J. Biol. Chem. 2007;282:21848–21855. doi: 10.1074/jbc.M702192200. [DOI] [PubMed] [Google Scholar]
- 5.Waugh D.S. An overview of enzymatic reagents for the removal of affinity tags. Protein Expr. Purif. 2011;80:283–293. doi: 10.1016/j.pep.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Zhang W., Yi Z., Wang S., Li Z. Identification of a thrombin cleavage site and a short form of ADAMTS-18. Biochem. Biophys. Res. Commun. 2012;419:692–697. doi: 10.1016/j.bbrc.2012.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunitz M. Formation of trypsin from crystalline trypsinogen by means of enterokinase. J. Gen. Physiol. 1939;22:429–446. doi: 10.1085/jgp.22.4.429. Available online: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maroux S., Baratti J., Desnuelle P. Purification and specificity of porcine enterokinase. J. Biol. Chem. 1971;246:5031–5039. [PubMed] [Google Scholar]
- 9.Zheng X.L., Kitamoto Y., Sadler J.E. Enteropeptidase, a type II transmembrane serine protease. Front. Biosci. 2009;1:242–249. doi: 10.2741/E23. [DOI] [PubMed] [Google Scholar]
- 10.Gasparian M.E., Ostapchenko V.G., Dolgikh D.A., Kirpichnikov M.P. Biochemical characterization of human enteropeptidase light chain. Biochemistry. 2006;71:113–119. doi: 10.1134/S0006297906020015. [DOI] [PubMed] [Google Scholar]
- 11.Pepeliaev S., Krahulec J., Černý Z., Jílková J., Tlustá M., Dostálová J. High level expression of human enteropeptidase light chain in Pichia pastoris. J. Biotechnol. 2011;156:67–75. doi: 10.1016/j.jbiotec.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Simeonov P., Berger-Hoffmann R., Hoffmann R., Sträter N., Zuchner T. Surface supercharged human enteropeptidase light chain shows improved solubility and refolding yield. Protein Eng. Des. Sel. 2011;24:261–268. doi: 10.1093/protein/gzq104. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Ren L., Ge L., Cui Q., Cao X., Hou Y., Bai F., Bai G. A strategy for fusion expression and preparation of functional glucagon-like peptide-1 (GLP-1) analogue by introducing an enterokinase cleavage site. Biotechnol. Lett. 2014;36:1675–1680. doi: 10.1007/s10529-014-1526-1. [DOI] [PubMed] [Google Scholar]
- 14.Gasparian M.E., Ostapchenko V.G., Schulga A.A., Dolgikh D.A., Kirpichnikov M.P. Expression, purification, and characterization of human enteropeptidase catalytic subunit in Escherichia coli. Protein Expr. Purif. 2003;31:133–139. doi: 10.1016/S1046-5928(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 15.Smith E.T., Johnson D.A. Human enteropeptidase light chain: bioengineering of recombinants and kinetic investigations of structure and function. Protein Sci. 2013;22:577–585. doi: 10.1002/pro.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins-Racie L.A., McColgan J.M., Grant K.L., DiBlasio-Smith E.A., McCoy J.M., LaVallie E.R. Production of recombinant bovine enterokinase catalytic subunit in Escherichia coli using the novel secretory fusion partner DsbA. Biotechnology (N Y) 1995;13:982–987. doi: 10.1038/nbt0995-982. [DOI] [PubMed] [Google Scholar]
- 17.Higaki J.N., Light A. Independent refolding of domains in the pancreatic serine proteinases. J. Biol. Chem. 1986;261:10606–10609. [PubMed] [Google Scholar]
- 18.Pepeliaev S., Krahulec J., Tlustá M., Černý Z., Jílková J. Expression and purification of the light chain of human enteropeptidase in E. coli. Minerva Biotechnol. 2012;24:42–52. [Google Scholar]
- 19.Tengattini S., Rinaldi F., Piubelli L., Kupfer T., Peters B., Bavaro T., Calleri E., Massolini G., Temporini C. Enterokinase monolithic bioreactor as an efficient tool for biopharmaceuticals preparation: on-line cleavage of fusion proteins and analytical characterization of released products. J. Pharm. Biomed. Anal. 2018;157:10–19. doi: 10.1016/j.jpba.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Yi J., Zhang Y.X. Refolding of the fusion protein of recombinant enterokinase light chain rEKL. Chin. J. Biotechnol. 2006;22:811–816. doi: 10.1016/S1872-2075(06)60058-7. [DOI] [PubMed] [Google Scholar]
- 21.Skala W., Goettig P., Brandstetter H. Do-it-yourself histidine-tagged bovine enterokinase: a handy member of the protein engineer’s toolbox. J. Biotechnol. 2013;168:421–425. doi: 10.1016/j.jbiotec.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan H., Wang J., Zhao Z.K. Purification and refolding optimization of recombinant bovine enterokinase light chain overexpressed in Escherichia coli. Protein Expr. Purif. 2007;56:40–47. doi: 10.1016/j.pep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Lemercier G., Bakalara N., Santarelli X. On-column refolding of an insoluble histidine tag recombinant exopolyphosphatase from Trypanosoma brucei overexpressed in Escherichia coli. J. Chromatogr. B. 2003;786:305–309. doi: 10.1016/S1570-0232(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu H., Zhou X., Zhang Y. A comparative investigation on different refolding strategies of recombinant human tissue-type plasminogen activator derivative. Biotechnol. Lett. 2006;28:457–463. doi: 10.1007/s10529-006-0001-z. [DOI] [PubMed] [Google Scholar]
- 25.Suh C.W., Park S.H., Park S.G., Lee E.K. Covalent immobilization and solid-phase refolding of enterokinase for fusion protein cleavage. Process Biochem. 2005;40:1755–1762. doi: 10.1016/j.procbio.2004.06.050. [DOI] [Google Scholar]
- 26.Niu L.X., Li J.Y., Ji X.X., Yang B.S. Efficient expression and purification of recombinant human enteropeptidase light chain in Esherichia coli. Braz. Arch. Biol. Technol. 2015;58:154–165. doi: 10.1590/S1516-8913201400094. [DOI] [Google Scholar]
- 27.Ivanenkov V.V., Murphy-Piedmonte D.M., Kirley T.L. Bacterial expression, characterization, and disulfide bond determination of soluble human NTPDase6 (CD39L2) nucleotidase: implications for structure and function. Biochemistry. 2003;42:11726–11735. doi: 10.1021/bi035137r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.