Learning objectives.
By reading this article you should be able to:
-
•
Recall the functional anatomy of the nose and sinuses.
-
•
Recognise the indications for common elective rhinological procedures.
-
•
Detail how the conduct of anaesthesia can help reduce blood loss and optimise conditions for surgery.
-
•
Identify safety aspects of rhinological surgery such as throat packs, airway devices and use of topical vasoconstrictors.
Key points.
-
•
The head-up position, topical vasoconstrictors and hypotensive anaesthesia are used to aid haemostasis in the vascular sinonasal mucosa during rhinological surgery.
-
•
Hypotensive anaesthesia optimises the surgical field but can be associated with perioperative adverse events and patients must be selected with care.
-
•
Topical application of cocaine-based compounds increases the risk of cardiotoxic adverse events; alternatives such as phenylephrine are recommended
-
•
Routine insertion of throat packs by the anaesthetist is not recommended
More than 45,000 surgical procedures on the nose and sinuses took place in England in 2018–9, of which more than 70% were day cases.1 The most common operations are functional endoscopic sinus surgery (FESS), nasal polypectomy and septorhinoplasty. Patients for elective surgery are commonly young, with few comorbidities, but pulmonary disease or obstructive sleep apnoea may coexist with rhinological pathology and increase likelihood of perioperative difficulties.
Nose and sinus surgery is mostly minimally invasive, often involving endoscopic techniques. Bleeding from the vascular capillary beds of the sinonasal mucosa compromises the surgical field and increases operative time and risk of complications. Anaesthetists can assist haemostasis with a multifaceted approach, including use of topical vasoconstrictors and hypotensive anaesthesia. These techniques can be associated with rare but significant adverse effects and the patient should be evaluated carefully before they are used.
Clinical anatomy and physiology
Bones and cartilage
The nose is a bony and cartilaginous protuberance, the organ of olfaction and a key constituent of the innate immune system. Nasal hair and columnar ciliated epithelium with mucus-secreting goblet cells entrap foreign bodies and microbes, with a large vascular surface area enabling inspired air to reach body temperature and 100% relative humidity by the nasopharynx.
Smell is mediated by olfactory receptors in the upper nasal septum and lateral nasal walls termed the ‘olfactory zone’. Signals are transmitted along the olfactory nerve fibres through the cribriform plate of the ethmoid bone which synapse with the olfactory bulb. Damage to the cribriform plate during trauma or iatrogenically during surgery may cause anosmia and provides a route of infection into the central nervous system.
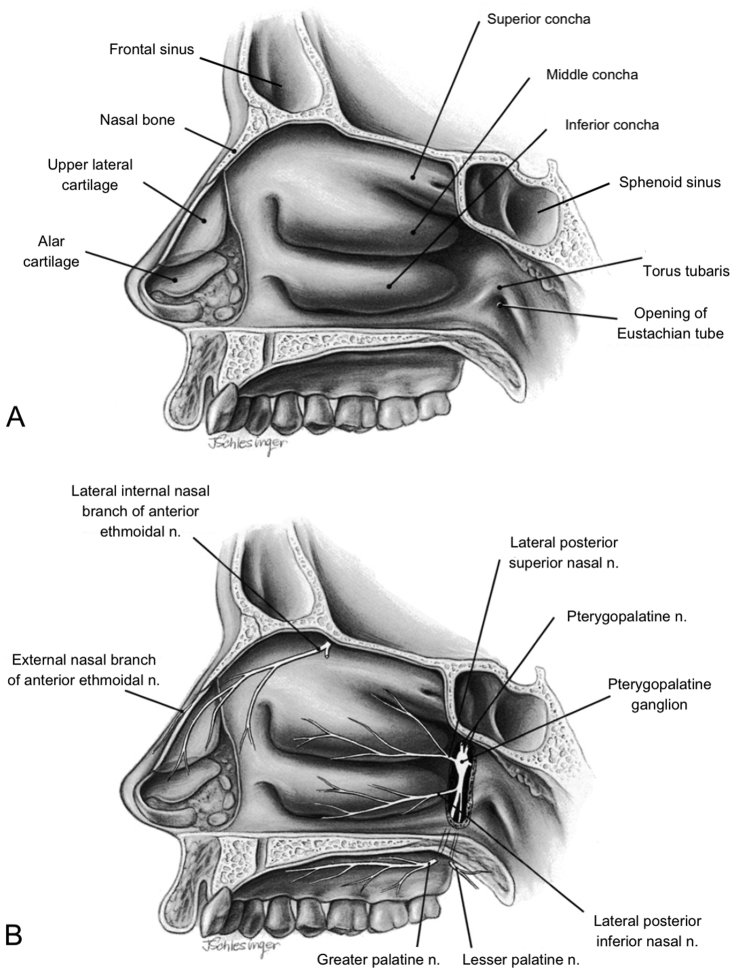
The roof of the nasal cavity is formed from the nasal and frontal bones. The nasal septum is composed superiorly of the vomer and ethmoid bones, and inferiorly by the large supporting septal cartilage. The lateral walls of the nose are formed from the ethmoid, maxilla and palatine bones, and consist of the three conchae or turbinate bones: superior, middle and inferior, with meatuses in between which form passageways for airflow (Fig. 1A). Located in the meatuses are various ostia which drain the frontal, maxillary and ethmoidal sinuses and nasolacrimal duct. The Eustachian tube, connecting the nose to the middle ear, is situated posteriorly below the inferior concha.
Fig 1.
Right lateral wall of the nasal cavity viewed from the left side with left nasal wall and septum removed. Image reproduced and adapted with permission. (A) Bones, cartilage and three conchae. (B) Nerve supply to the nasal cavity and paranasal sinuses.
The floor of the nasal cavity consists of the maxilla and palatine bone. Successful insertion of medical devices, including nasogastric tubes, nasopharyngeal airways, temperature probes, endoscopes or nasotracheal tubes, is backwards along the floor of the nose, beneath the inferior concha.
Blood supply
The anterior and posterior ethmoidal arteries, which branch from the internal carotid artery, supply the upper part of the nose, and branches of the maxillary (sphenopalatine and greater palatine arteries) and facial artery (superior labial) supply the lower part of the nose.
The most vascular area of the nose is the anterior inferior part of the septum, termed Little's area, or Kiesselbach's plexus, where these arteries anastomose. Ruptured blood vessels in Little's area are the most common origin of epistaxis, with 10% of cases originating posteriorly from branches of the sphenopalatine artery.
Submucosal venous plexuses drain into the sphenopalatine, facial and ophthalmic veins. They link to the superior ophthalmic vein, which drains partially via the cavernous sinus, and small venous branches which drain across the cribriform plate. Central nervous system infection or cavernous venous sinus thrombosis can be a complication of infections involving the nose or sinuses.
Nerve supply
Olfaction is mediated by the first cranial (olfactory) nerve.
Sensation to the nose, septum, nasal cavity and sinuses is supplied by ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.
The ophthalmic nerve (V1) branches to the nasociliary nerve, which gives off the anterior ethmoidal nerve, innervating the uppermost part of the septum, nasal tip and anterior lateral walls. V1 branches also innervate the ethmoidal, sphenoid and frontal sinuses.
The maxillary nerve (V2) traverses the pterygopalatine fossa, where it forms the pterygopalatine ganglion (Fig. 1B). Branches including the greater and lesser palatine nerves emerge, which innervate the septum and lateral walls of the nose. The terminal branch of the maxillary nerve is the infraorbital nerve, which supplies the maxillary sinus and the skin immediately lateral to and beneath the nose. Postganglionic parasympathetic fibres (from the facial nerve) travel with the maxillary nerve branches to facilitate secretomotor functions of the nasal and sinus mucosa.
Types of surgery
Rhinologists will often combine procedures, aiming to reduce symptoms of nasal obstruction and chronic rhinosinusitis. Functional endoscopic sinus surgery is offered when chronic sinus disease is not responsive to medical therapy. Diseased tissue is removed in key areas in order to restore adequate aeration and drainage of the sinuses, and enable topical therapies. If nasal polyps are present, nasal polypectomy can be performed to optimise normal nasal functioning.
Patients with a deviated septum may experience nasal obstruction, which may require correction, termed septoplasty. A bent septum may occur with a twist in the outside shape of the nose. In these cases, septal surgery may be combined with nose re-shaping surgery, termed septorhinoplasty, to straighten the nose.
Functional endoscopic sinus surgery was originally performed under topical anaesthesia with sedation, and local anaesthesia is still considered suitable for some minor procedures. However, general anaesthesia allows for adequate airway protection, control of cardiovascular function, and an immobile surgical field with the patient unconscious so that the surgeon can perform a more extensive resection.
Preoperative assessment
The majority of rhinological surgery is ideal for day-case surgery, because it is minimally invasive, with low complication rates, minimal blood loss and postoperative pain is minimal. Patients are typically young and of ASA grade 1 or 2, but those with obesity or obstructive sleep apnoea with otherwise good functional status should not usually be excluded from day surgery pathways. Avoidance of longer-acting opioid analgesia and other sedatives, and extended prophylaxis against deep vein thrombosis should be considered in these patients.2
Patients undergoing surgery with a higher risk of serious bleeding or postoperative complications, or children with obstructive sleep apnoea are amongst those for whom an inpatient stay should be planned.
The key considerations when assessing patients for sinus and nasal surgery are highlighted in Table 1.
Table 1.
Key aspects of preoperative assessment in rhinological surgery
| Airway assessment |
|
| Comorbidities |
|
| Drug history |
|
COPD, chronic obstructive pulmonary disease.
Intraoperative care and haemostasis
The paranasal sinuses are situated somewhat precariously between the orbits and skull base, with close proximity to optic nerves. It is recommended to leave the eyes uncovered during sinus surgery, allowing the surgeon access to the globe to inspect for complications such as a breach in the orbital wall. Eyes should be carefully lubricated to prevent corneal damage.
Blood in the narrow nasal cavity will compromise endoscopic visualisation whilst operating and risk damage to local structures. Limiting bleeding from the vascular mucosa of the nose and sinuses is therefore a key priority. Reduced blood loss has obvious benefits in terms of haemodynamic stability – reducing postoperative anaemia, postoperative nausea and vomiting, and risk of postoperative aspiration. An improved surgical field of view reduces operating time and increases the likelihood of successful surgery. Effective haemostasis reduces the need for postoperative nasal packing and prevents haematoma formation, which may cause adhesions, scarring and need for repeat surgery.
Non-pharmacological and pharmacological methods to limit blood loss during rhinological surgery should be considered.
Non-pharmacological means to limit blood loss
The reverse Trendelenburg head-up position limits venous congestion, reduces preload by causing venous pooling in the lower limbs, and decreases blood pressure in the arteries supplying the surgical field.3 Elevation of the head by 10–20° achieves a balance between improved surgical field and reduced cerebral perfusion pressure.4 The risk of venous air embolism is rare.
Limiting high PEEP during controlled ventilation may limit venous pressure increase and thus bleeding, but the application of PEEP up to 5 cmH2O does not appear to affect surgical field quality.5
Avoidance of hypercapnoea appears to be logical in limiting vasodilatation, but in a randomised trial, there was no difference in the surgical field or blood loss between patients assigned to normocapnoea, hypocapnoea or hypercapnoea.6
Regional nerve blockade
Regional nerve blocks targeting the innervation of the nose and sinuses (see Fig. 1B) can provide useful intraoperative and postoperative analgesia, in addition to vasoconstriction of the surgical field. The anterior ethmoidal nerve can be blocked by endonasal infiltration of the middle turbinate with a local anaesthetic, and the infraorbital nerve can be blocked intraorally or transnasally as it emerges through the infraorbital foramen. Pterygopalatine fossa injection via the greater palatine canal with local anaesthetic and adrenaline (epinephrine) effectively induces vasoconstriction of the sphenopalatine artery, optimising the surgical field and producing analgesia in the distribution of V2.3,7
Topical vasoconstrictors
A variety of topical vasoconstrictors are used in rhinological surgery including cocaine, adrenaline, phenylephrine and oxymetazoline. Agents are used individually or in various combinations with or without local anaesthetic agents such as lidocaine, levobupivacaine or tetracaine. Case reports of serious adverse effects attributable to systemic absorption necessitate caution, particularly in those with cardiovascular comorbidities.
Cocaine-containing preparations were used by 68% of UK-based ear, nose and throat (ENT) surgeons surveyed in 2010.8 Cocaine is an ester local anaesthetic that exerts a vasoconstrictor effect by blocking the reuptake of noradrenaline (norepinephrine) at peripheral nerve endings; its dual function explains its popularity. Despite this, cocaine appears to cause more cardiotoxic effects such as tachycardias and arrhythmias than other vasoconstrictors; sustained hypertension, myocardial infarction and acute angle closure glaucoma have also been reported, even in younger patients and with lower drug concentrations.9,10
Cocaine is commonly used as Moffett's solution: a mixture of 1–2 ml cocaine 5%, 1 ml adrenaline 1:1000 solution, 2 ml sodium bicarbonate 8.4%, made up to 10 ml with 0.9% sodium chloride. Variations in this recipe are common. Adrenaline is a non-selective α1-, α2-and β-adrenoceptor agonist that theoretically reduces the systemic absorption of cocaine and enhances its local vasoconstrictive action; however, its combination with cocaine has also been linked to an increased incidence of adverse effects.
Adrenaline can be used as a sole agent in various concentrations together with local anaesthetics. Applied topically, adrenaline has shown to be as effective as cocaine, with fewer adverse effects.11 Adrenaline is commonly infiltrated into the nasal submucosa, but this appears to confer no additional benefit and is associated with more systemic absorption compared with topical application.12 Systemic absorption of low doses of adrenaline can lead to hypotension, attributable to activation of β2 adrenoceptors in skeletal muscle, causing vasodilation. High doses of adrenaline are more likely to cause hypertension and tachycardia because of preferential stimulation of α and β1 adrenoceptors.10
Drugs with a predominant α1 agonist action such as phenylephrine and oxymetazoline are effective in achieving an optimal surgical field, and have fewer reported adverse effects than cocaine and adrenaline. Phenylephrine is as effective as cocaine in the quality of vasoconstriction and reduction in blood loss in nasal surgery, and can be given with lidocaine as co-phenylcaine (lidocaine 5% with phenylephrine 0.5%).13 Oxymetazoline has a strong safety profile and can be considered for children or those at higher risk of cardiovascular complications; it is commonly combined with lidocaine or tetracaine.14,15 Topical or infiltrated levobupivacaine has vasoconstrictor actions at concentrations <0.25%, with benefits for postoperative analgesia.
Topical agents can be given using pledgets (patties) gauze or paste placed in the nasal cavity, or applied to the nose using a spray, i.v. cannula, or mucosal atomisation device. When they are used the patient should be in the 30° head down position with their eyes protected, and the head turned to each side sequentially to facilitate adequate spread across the mucosa. Topical vasoconstrictors should be given shortly after induction of anaesthesia, as 15–30 min is required for full efficacy.
With all topical agents, there is a risk of accidental i.v. injection, especially as most are presented as clear liquids, which are easily confused with other injectable medicines. Clear labelling, separate storage and other precautions should be taken.
Corticosteroids
Preoperative oral or intranasal steroids reduce bleeding, operative time and postoperative pain, and improve surgical field quality in FESS.16 The ideal dosing and duration of treatment and the potential impact of adverse effects has not been clearly established. Dexamethasone, given intraoperatively, has benefits as an antiemetic in addition to reducing oedema and bleeding.
Tranexamic acid
Tranexamic acid is an antifibrinolytic agent that when given i.v. reduces intraoperative and postoperative blood loss, improves the surgical field in nasal and sinus surgery and has an excellent safety profile.17 Topical application may also reduce bleeding and improve the surgical field during FESS.11
Hypotensive anaesthesia
Intraoperative bleeding from the capillaries of the sinonasal mucosa is a function of the MAP and central venous pressure.
Hypotensive anaesthesia, also known as controlled, induced or deliberate hypotension, is defined as a decrease in MAP to between 50 and 65 mmHg, or a 30% reduction in baseline MAP.4 Hypotensive anaesthesia is effective at reducing blood loss and improving the quality of the surgical field.
The benefits of reduced blood flow to the nasal mucosa must be balanced against the risks of organ hypoperfusion. For those with impaired autoregulation of blood flow or high risk of organ dysfunction such as those with ischaemic heart disease, carotid atherosclerosis, cerebrovascular disease, chronic hypertension, autonomic neuropathy, or chronic kidney disease, hypotensive anaesthesia to the levels described above is unsafe. Stronger evidence is emerging of the harmful effects of intraoperative hypotension, even in younger patients and for short durations.18 Individualised targets for arterial pressure according to the patient's age, comorbidities and perceived benefit relevant to the operation in question, should be stated during the preoperative team brief. In the typical patient without vascular comorbidity, a modest time-limited reduction in blood pressure aiming for a systolic of 90 mmHg, MAP of >65 mmHg and a HR of 60 beats min−1 appears reasonable. Close communication between surgeon and anaesthetist, and continuous physiological monitoring are vital.
When approaching methods to achieve hypotension, it should be noted that MAP depends on both the systemic vascular resistance (SVR) and cardiac output (CO), and that CO is a product of HR and stroke volume.
SVR can be reduced using vasodilators, such as calcium channel blockers, sodium nitroprusside and inhalational anaesthetic agents. However, in the context of nasal surgery, vasodilatation can paradoxically increase blood flow to the nasal mucosa; it can also cause reflex tachycardia, which may restore CO, precluding any benefit. Stroke volume can be reduced by using drugs that decrease contractility or preload, such as β blockers (propranolol or esmolol), combined α- and β-adrenergic receptor blocking drugs (labetalol), or remifentanil. Bradycardia increases end-diastolic filling time, possibly increasing venous return and reducing venous bleeding, and a HR of 60 beats min−1 appears optimal.11
Remifentanil, combined with propofol or inhalational agents, is often considered first choice in anaesthesia for rhinological surgery. It reduces HR and MAP independently, dampening haemodynamic responses to surgical stimuli, can be titrated rapidly, and reduces the need for additional antihypertensive medication. It also has a short context-sensitive half-time and produces a fast postoperative recovery.
Selective α2 adrenergic agonists, such as clonidine and dexmedetomidine, cause hypotension without substantial tissue vasodilation, and reduce bleeding and improve surgical visualisation in FESS. They have additional analgesic and anaesthesia-sparing benefits, but increase postoperative sedation. This may limit their usefulness, and further research is required.19
Total intravenous anaesthesia with propofol, with or without remifentanil or an alternative opioid, is a popular option for facilitating hypotensive anaesthesia. Propofol causes vasodilation by depressing central sympathetic tone, in contrast to inhalational agents, which cause non-selective vasodilation of peripheral and cerebral blood vessels.11 Enthusiasts favour the haemodynamic stability, titratability and reliability in achieving hypotension that TIVA can provide. A potential drawback may be difficulty positioning electrodes reliably for EEG-based depth of anaesthesia monitoring during rhinological surgery. Furthermore, the patient must be deeply anaesthetised until endoscopic surgery is completed, and emergence after propofol infusion may be delayed, which may be counterproductive in the day-surgery setting.
A recent RCT comparing TIVA with inhalational anaesthesia demonstrated improved intraoperative visualisation and decreased blood loss in patients undergoing FESS.20 A meta-analysis of RCTs suggested that TIVA is beneficial in optimising surgical field and reducing blood loss compared with inhalational anaesthesia.21 Despite study heterogeneity necessitating further higher quality confirmatory evidence, the European Position Statement on Rhinosinusitis and Nasal Polyps 2020 advises using TIVA in rhinological surgery.14
Airway management
Nasal surgery involves a shared airway, frequently obscured under drapes at the opposite end of the operating theatre table to the anaesthetic machine. It is essential that the airway is adequate and secured for the duration of surgery. The supraglottic airway has become standard for most day-case rhinology procedures. A reinforced, flexible laryngeal mask airway (LMA) has several advantages: a large cuff protects the airway from blood and secretions; neuromuscular block is not required; and it can be used with a spontaneous breathing technique, pressure support mode or positive pressure ventilation. Minimal sympathetic stimulation on insertion and removal further lessens bleeding from the surgical field.11 Caution is advised in the patient who is obese, for whom the default use of supraglottic airways has been associated with a disproportionate number of adverse events, as highlighted in the 4th National Audit Project (NAP4) study.22
A need for a tracheal tube may be anticipated in advance, because of factors related to the patient (obesity, significant acid reflux, predicted difficult airway, pulmonary disease) and to the surgery (degree of bleeding, length of surgery, inaccessibility of airway). A supraglottic airway may need to be replaced with a tracheal tube if the former provides a poor seal or poor stability.
If a tracheal tube is used, the first choice may be a preformed cuffed tube such as south-facing RAE (Ring–Adair–Elwyn) tube, secured in the midline. Endobronchial intubation may occur in the patient with a short neck as these tubes are of a fixed length. Other options include a standard reinforced tracheal tube, which can be taped to one side in unilateral surgery.
Throat packs
A throat pack is a surgical swab or sponge placed in the pharynx, which provides an absorptive barrier to airway soiling by blood, secretions or surgical fluids. The most serious complication of a throat pack is failure to remove it before tracheal extubation, resulting in airway obstruction. At least one death and several cases of significant morbidity have resulted from retained throat packs, and it constitutes an ‘NHS Never Event’ – defined as an incident that is judged wholly preventable by following existing guidance.22,23 The risk of throat pack retention is increased by physical and human factors such as a change of anaesthetist, additional packs placed during the procedure and an unexpectedly rapid recovery.
Throat packs are also associated with an increased incidence of sore throat postoperatively or dental or soft tissue damage as a result of insertion. Throat packs do not appear to reduce postoperative nausea and vomiting from intraoperative blood drainage into the stomach.23 The lack of beneficial evidence, and risk of rare but potentially serious complications, resulted in a consensus statement in 2018 by the Difficult Airway Society (DAS), the British Association of Oral and Maxillofacial Surgery (BAOMS) and the British Association of Otorhinolaryngology (ENT-UK).23 The routine insertion of throat packs by anaesthetists can no longer be recommended, and if a throat pack is required in selected patients, it should be inserted by the surgeon, using gauze with a radiopaque thread that is part of the swab count.
Despite this recommendation, throat packs are still placed by anaesthetists. To prevent accidental retention, the National Patient Safety Agency (NPSA) guidelines recommend at least one visual check, such as part of the throat pack protruding, attached to the airway device, or a mark on the patient, and one written check, such as recording insertion and removal on the swab count board.24 The WHO Safer Surgery checklist should reference the throat pack, and a ‘step back before you pack’ pause considered before throat pack insertion.25
Postoperative care
Ensuring a dry airway
At the end of surgery, a Valsalva manoeuvre may be requested by the surgeon to identify bleeding points. The anaesthetist should inspect and suction the pharynx before reversal of any neuromuscular block and under sufficiently deep anaesthesia, to ensure that the airway is clear of blood and secretions.23 A sizeable blood clot, developing because of pooled blood in the nasopharynx, and hidden behind the soft palate, may become dislodged and cause airway obstruction in the larynx or trachea. This so-called ‘Coroner's clot’ resulted in two cases in the NAP4 report of postoperative airway obstruction with one death and one case of serious harm.22
Postoperative pain and bleeding
Pain after elective rhinological surgery is mild to moderate and results from surgical trauma and nasal packing with tampons or gauze. Paracetamol and oral NSAIDs, if not contraindicated, are usually sufficient for day-case surgery, with supplementary opioid analgesia rarely required. Soaking nasal packs with analgesics such as fentanyl or local anaesthetics may improve postoperative pain scores. Nasal packs may also be soaked in tranexamic acid, steroids or other vasoconstrictors to ensure haemostasis. A head-up position during recovery from anaesthesia reduces bleeding.
Summary
Anaesthetists use a multifaceted approach to reduce blood loss and optimise the surgical field in rhinological surgery. The use of hypotensive anaesthesia and topical vasoconstrictors must account for the age and comorbidities of the individual patient. Alternatives to topical cocaine are recommended. Current evidence favours TIVA over inhalational anaesthesia for reduced blood loss and improved surgical field; remifentanil and α2 adrenoceptor agonists also independently reduce bleeding. Throat packs are not recommended, and utmost care should be taken to ensure a dry airway before emergence from anaesthesia.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Declarations of interest
The authors declare that they have no conflicts of interest.
Biographies
Iona Murdoch BMedSci (Hons) MRCP(UK) MRCEM FRCA is a specialty registrar in anaesthesia at Guy's and St Thomas' NHS Foundation Trust, London.
Nhathien Nguyen-Lu BMedSci (Hons) FRCA is a consultant anaesthetist at Guy's and St Thomas’ NHS Foundation Trust with clinical interests in ENT, high-risk obstetrics and medical education.
Pavol Surda FRCS is a consultant ENT surgeon at Guy's and St Thomas' NHS Foundation Trust, with expertise in advance sinus surgery, nasal deformities and skull base surgery. His research focus is chronic rhinosinusitis and he has published widely on this topic. He sits on the executive board of the European Rhinologic Society.
Matrix codes: 1C01, 1C02, 2A03, 3A02, 3A06
References
- 1.National Statistics England . 2019. Hospital admitted patient care activity 2018–2019.https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19 Available from. [Google Scholar]
- 2.Bailey C.R., Ahuja M., Bartholomew K. Guidelines for day-case surgery 2019. Anaesthesia. 2019;74:778–792. doi: 10.1111/anae.14639. [DOI] [PubMed] [Google Scholar]
- 3.Carlton D.A., Govindaraj S. Anesthesia for functional endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2017;25:24–29. doi: 10.1097/MOO.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 4.Pant H. Hemostasis in endoscopic sinus surgery. Otolaryngol Clin North Am. 2016;49:655–676. doi: 10.1016/j.otc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Demaria S., Govindaraj S., Huang A. The influence of positive end-expiratory pressure on surgical field conditions during functional endoscopic sinus surgery. Anesth Analg. 2015;120:305–310. doi: 10.1213/ANE.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 6.Nekhendzy V., Lemmens H.J.M., Vaughan W.C. The effect of deliberate hypercapnia and hypocapnia on intraoperative blood loss and quality of surgical field during functional endoscopic sinus surgery. Anesth Analg. 2007;105:1404–1409. doi: 10.1213/01.ane.0000282781.56025.52. [DOI] [PubMed] [Google Scholar]
- 7.Shamil E., Rouhani M.J., Basetti S. Role of local anaesthetic nerve block in endoscopic sinus surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2018;43:1201–1208. doi: 10.1111/coa.13128. [DOI] [PubMed] [Google Scholar]
- 8.Fu B., Sharp H. Moffett’s solution — is it safe? The UK experience. Clin Otolaryngol. 2011;36:184–185. doi: 10.1111/j.1749-4486.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- 9.Lenders G.D., Jorens P.G., De Meyer T. Coronary spasm after the topical use of cocaine in nasal surgery. Am J Case Rep. 2013;14:76–79. doi: 10.12659/AJCR.883837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saif A.M., Farboud A., Delfosse E. Assessing the safety and efficacy of drugs used in preparing the nose for diagnostic and therapeutic procedures: a systematic review. Clin Otolaryngol. 2016;41:546–563. doi: 10.1111/coa.12563. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y.C., Psaltis A.J. Hemostasis in sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2016;24:26–30. doi: 10.1097/MOO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 12.Khosla A.J., Pernas F.G., Maeso P.A. Meta-analysis and literature review of techniques to achieve hemostasis in endoscopic sinus surgery. Int Forum Allergy Rhinol. 2013;3:482–487. doi: 10.1002/alr.21126. [DOI] [PubMed] [Google Scholar]
- 13.Alhaddad S.T., Khanna A.K., Mascha E.J. Phenylephrine as an alternative to cocaine for nasal vasoconstriction before nasal surgery: a randomised trial. Indian J Anaesth. 2013;57:163–169. doi: 10.4103/0019-5049.111844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fokkens W.J., Lund V.J., Hopkins C. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 15.Higgins T.S., Hwang P.H., Kingdom T.T. Systematic review of topical vasoconstrictors in endoscopic sinus surgery. Laryngoscope. 2011;121:422–432. doi: 10.1002/lary.21286. [DOI] [PubMed] [Google Scholar]
- 16.Pundir V., Pundir J., Lancaster G. Role of corticosteroids in functional endoscopic sinus surgery — a systematic review and meta-analysis. Rhinology. 2016;54:3–19. doi: 10.4193/Rhino15.079. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.H., Kim S., Kang H. Efficacy of tranexamic acid on operative bleeding in endoscopic sinus surgery: a meta-analysis and systematic review. Laryngoscope. 2019;129:800–807. doi: 10.1002/lary.27766. [DOI] [PubMed] [Google Scholar]
- 18.Gregory A., Stapelfeldt W.H., Khanna A.K. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg. 2020 doi: 10.1213/ANE.0000000000005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quijada-Manuitt M.A., Escamilla Y., Vallano A. Use of alpha2-adrenergic agonists to improve surgical field visibility in endoscopy sinus surgery: a systematic review of randomised controlled trials. Clin Ther. 2018;40:136–49 e19. doi: 10.1016/j.clinthera.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Brunner J.P., Levy J.M., Ada M.L. Total intravenous anesthesia improves intraoperative visualization during surgery for high-grade chronic rhinosinusitis: a double-blind randomized controlled trial. Int Forum Allergy Rhinol. 2018;8:1114–1122. doi: 10.1002/alr.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu V.M., Phan K., Oh L.J. Total intravenous versus inhalational anesthesia in endoscopic sinus surgery: a meta-analysis. Laryngoscope. 2020;130:575–583. doi: 10.1002/lary.28046. [DOI] [PubMed] [Google Scholar]
- 22.Cook T.M., Woodall N., Frerk C. Major complications of airway management in the UK: results of the 4th national Audit Project of the royal college of anaesthetists and the difficult airway society. Br J Anaesth. 2011;106:617–631. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 23.Athanassoglou V., Patel A., McGuire B. Systematic review of benefits or harms of routine anaesthetist-inserted throat packs in adults: practice recommendations for inserting and counting throat packs: an evidence-based consensus statement by the Difficult Airway Society (DAS), the British Association of Oral and Maxillofacial Surgery (BAOMS) and the British Association of Otorhinolaryngology, Head and Neck Surgery (ENT-UK) Anaesthesia. 2018;73:612–618. doi: 10.1111/anae.14197. [DOI] [PubMed] [Google Scholar]
- 24.NHS Improvement Safer practice notice — reducing the risk of retained throat packs after surgery (2009) in Recommendations from National Patient Safety Agency alerts that remain relevant to the Never Events list 2018. NHS Improvement 2018. 2018 https://improvement.nhs.uk/documents/2267/Recommendations_from_NPSA_alerts_that_remain_relevant_to_NEs_FINAL.pdf Available from. [Google Scholar]
- 25.Bailey C.R., Nouraie R., Huitink J.M. Have we reached the end for throat packs inserted by anaesthetists? Anaesthesia. 2018;73:535–538. doi: 10.1111/anae.14168. [DOI] [PubMed] [Google Scholar]