Introduction
In patients with functional bradycardia or neurally mediated syncope (NMS), autonomic modification through catheter ablation of atrial ganglionated plexuses (GP) has been proposed as an alternative and promising therapeutic approach.1, 2, 3, 4, 5, 6, 7, 8, 9 However, to date, there is no consensus on the best strategy for identifying atrial GP sites, either physiologically guided (by means of spectral mapping or high-frequency stimulation) or anatomically guided, and also on the extension of GP ablation necessary to attenuate the vagal activity in these patients. In addition, there is no consensus about how to establish the vagal denervation endpoints.10
We report the case of a 38-year-old male patient, who was referred to our institution for implantation of permanent pacemaker owing to nocturnal episodes of advanced atrioventricular (AV) block and ventricular pauses longer than 2 seconds on 24-hour Holter monitoring, and right bundle branch block (RBBB) on resting electrocardiogram (ECG). After a comprehensive evaluation, ruling out dysfunction of the His-Purkinje system, the patient underwent anatomically guided ablation of interatrial septum GP, with vagal denervation endpoints evaluated by extracardiac vagal stimulation (ECVS), resulting in normalization of AV conduction and suppression of ventricular pauses in short- and medium-term follow-up.
Key Teaching Points.
-
•
In patients with functional bradycardia or neurally mediated syncope, autonomic modification through catheter ablation of atrial ganglionated plexuses (GP) has been proposed as a new therapeutic approach.
-
•
The optimal technique for identifying atrial GP sites, either physiologically guided (by means of spectral mapping or high-frequency stimulation) or anatomically guided, has not been established yet. Further, there is no consensus on the extension of GP ablation for attenuating the vagal activity in these patients. In addition, there is no consensus about how to establish the vagal denervation endpoints.
-
•
Our case highlights that anatomically guided ablation of atrial GP, addressing both sides of the interatrial septum, with vagal denervation endpoints evaluated by extracardiac vagal stimulation, may be an alternative therapy for functional atrioventricular (AV) block, resulting in normalization of AV conduction in short- and medium-term follow-up.
Case report
A 38-year-old male patient was referred to our institution after recommendation of permanent pacemaker implantation in another service, owing to several nocturnal episodes of advanced AV block associated with reduced sinus rate and 4879 ventricular pauses longer than 2 seconds, the longest of 3.5 seconds, on 24-hour Holter monitoring (Figure 1B), consistent with vagally mediated bradycardia. He also presented a first-degree AV block (PR interval of 360 ms) associated with RBBB (QRS duration of 155 ms) on resting ECG (Figure 1A). Serological tests for Chagas disease were negative. The echocardiogram showed no evidence of structural heart disease. Exercise stress testing revealed a normal AV nodal conduction response to exercise. Thyroid function tests were normal. Polysomnography was normal and excluded obstructive sleep apnea. The patient was mainly sedentary, his family history was unremarkable, and no drug or condition could explain the AV block.
Figure 1.
A: Baseline 12-lead electrocardiogram (ECG) showing sinus rhythm, prolonged PR interval (360 ms), and intraventricular conduction delay with right bundle branch block morphology and QRS duration of 155 ms. B: The 24-hour Holter recordings displaying episodes of nocturnal advanced atrioventricular block (3:1) associated with sinus rate slowing, resulting in ventricular pauses longer than 3 seconds. C, D: Electrophysiologic recordings before and after ganglionated plexus (GP) ablation. After GP ablation, there was a marked decrease in sinus cycle length (P-P interval, 1281 vs 810 ms), PR interval (290 vs 180 ms), and AH (atrial-His) interval (177 vs 108 ms). From top to bottom, ECG leads and electrograms from His bundle (His p; His d) are shown.
Based on these findings, we initially considered the nocturnal episodes of advanced AV block to be functional. However, pending the results of the electrophysiological study to assess the integrity of the His-Purkinje system, it was discussed with the patient that a permanent pacemaker might not be recommended for his clinical condition. In this sense, if the dysfunction of the His-Purkinje system was excluded, other treatment options could be used, including, preferably, clinical follow-up or autonomic atrial denervation. The patient was very concerned about his clinical findings and chose to perform an electrophysiological study and, in case of exclusion of dysfunction from the His-Purkinje system, to proceed with an atrial autonomic denervation, with full knowledge of its experimental nature, and gave informed consent for both procedures.
Under conscious sedation, electrode catheters (Biosense Webster, Inc, Irvine, CA) were percutaneously introduced through femoral veins and positioned in the right atrium (RA), coronary sinus, right ventricle, and His bundle region. The baseline electrophysiological evaluation showed prolonged PR interval (290 ms) and AH (atrial-His) interval (177 ms), normal HV (His-ventricular) interval (45 ms), and QRS with an RBBB pattern and duration of 159 ms (Figure 1C). The programmed atrial stimulation showed an impaired AV nodal conduction (Wenckebach cycle length of 840 ms with suprahisian block) and ruled out sustained tachycardias, as well as primary dysfunction of the sinus node and the His-Purkinje system.
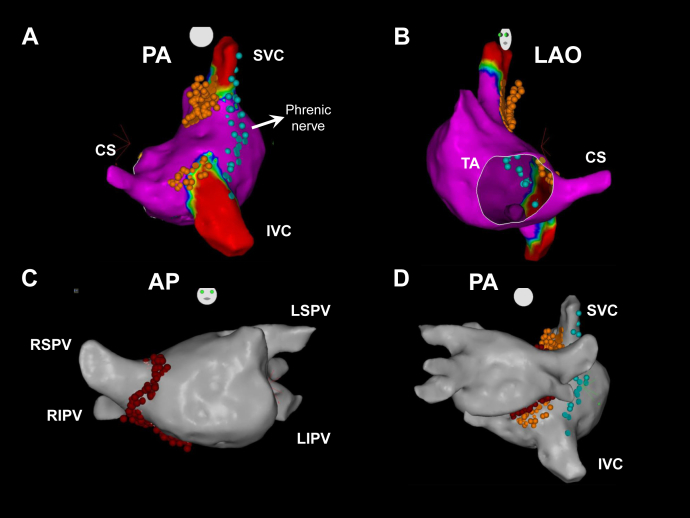
A steerable quadripolar catheter was fluoroscopically advanced in both the right and left jugular veins up to the level of the upper wisdom tooth, and ECVS (50 Hz, pulse width 50 μs, pulse amplitude 1 V/kg up to 70 V, duration 5–10 seconds) was performed before ablation, and at the end of GP ablation to confirm the denervation endpoints10 (Figure 2). We did not evaluate the vagal response induced by ECVS after ablation of each specific GP site. After accessing of the left atrium (LA) by transseptal puncture, an activated clotting time was maintained over 300 seconds with intravenous heparin. The 3-dimensional electroanatomical mapping (CARTO 3 System; Biosense Webster, Inc, Irvine, CA) of both atria and pulmonary veins was carried out and excluded the presence of low-voltage areas that could suggest atrial scarring adjacent to the conduction system (Figure 3). In order to avoid the right phrenic nerve injury during ablation of GP, high-amplitude stimulation was delivered to the lateral border of the superior vena cava, RA, and inferior vena cava, and sites where phrenic nerve was captured were annotated on the 3-dimensional mapping geometry (Figure 3).
Figure 2.
A: Anteroposterior fluoroscopic view showing the position of the steerable quadripolar catheter within the right internal jugular vein for extracardiac vagal stimulation (ECVS). Any repeated ECVS during the procedure was performed with the steerable catheter in the same radiological position. B, C: Examples of ECVS before and after septal ganglionated plexus ablation. Before ablation (B), ECVS induced sinus pauses and transitory atrioventricular block. After ablation (C), similar ECVS showed abolishment of the vagal response. From top to bottom, electrocardiogram leads and right atrium electrograms (RA p; RA d) are shown.
Figure 3.
A, B: Three-dimensional mapping of the right atrium (RA); A: posteroanterior (PA) view; B: left anterior oblique (LAO) view. C: Three-dimensional mapping of left atrium (LA), anteroposterior (AP) view. D: Three-dimensional mapping showing both atria, PA view. Blue tags indicate sites where phrenic nerve was captured before radiofrequency (RF) delivery to ganglionated plexus sites. Orange tags indicate RF application sites through the RA. Red tags indicate RF application sites through the LA. CS = coronary sinus ostium; IVC = inferior vena cava; LIPV = left inferior pulmonary vein; LSPV = left superior PV; RIPV = right inferior PV; RSPV = right superior PV; SVC = superior vena cava; TA = tricuspid annulus.
Radiofrequency (RF) energy was delivered to the right and left sides of the interatrial septum at sites consistent with GP locations6,9: in the RA: (1) the superoposterior area, at the junction of the superior vena cava; (2) the inferoposterior septal area, at the coronary sinus ostium; in the LA: (3) at the right anterior GP (RAGP), extending inferiorly to the right inferior pulmonary vein; (4) at the right inferior GP (RIGP) (Figure 3). Considering that the exact anatomic edges of the GP are variable, we delivered an expanded number of RF applications to the presumed GP areas, using a standard irrigated catheter (ThermoCool SmartTouch; Biosense Webster Inc, Diamond Bar, CA), with power limit of 35 W, contact force range of 10–20 g, and RF application duration of 20–30 seconds.
Compared with baseline, sinus cycle length (P-P interval, 1281 vs 810 ms), sinus node recovery time (1494 vs 1044 ms), PR interval (290 vs 180 ms), AH interval (177 vs 108 ms) (Figure 1C, 1D), and AV node Wenckebach cycle length (840 vs 510 ms) were decreased after interatrial GP ablation; however, there were no changes in PA interval, HV interval, and QRS duration. Preablation ECVS induced sinus pauses and transitory AV block (Figure 2B), whereas postablation ECVS showed abolishment of the cardiac vagal response (Figure 2C), demonstrating the acute efficacy of the vagal denervation. The procedure was uneventful, and the patient was discharged on the next day. A 24-hour Holter monitoring was repeated at 1, 6, and 11 months after ablation (Table 1). Nocturnal ventricular pauses longer than 2 seconds were reduced from 4879 (preablation) to 2 pauses / 24 hours at 1 month; and a slight increase in the frequency of ventricular pauses, to 9 and 26 pauses / 24 hours, was observed at 6 and 11 months, respectively.
Table 1.
Follow-up 24-hour Holter recording
| Minimum/average/maximum HR (bpm) | Pauses >2 s | Maximum pause length (s) | Atrial ectopy (n) | Ventricular ectopy (n) | |
|---|---|---|---|---|---|
| Pre-abl | 27/58/108 | 4879 | 3.5 | 2 | 4 |
| 7 days | 47/67/105 | 2 | 2.2 | 7 | 2 |
| 6 months | 38/66/113 | 9 | 2.4 | 9 | 5 |
| 11 months | 35/61/107 | 27 | 2.6 | 1 | 4 |
abl = ablation; bpm = beats per minute; HR = heart rate.
Discussion
We reported the case of 38-year-old male patient who was referred to our institution for implantation of a permanent pacemaker owing to episodes of advanced AV block and ventricular pauses on 24-hour Holter monitoring, and RBBB on resting ECG. Although a specific therapy is rarely required for asymptomatic/oligosymptomatic vagally mediated bradycardias, the patient was referred to our institution for implantation of a permanent pacemaker, which was avoided in the present case after ruling out dysfunction of the His-Purkinje system, and by performing anatomically guided ablation of interatrial septal GP, resulting in acute normalization of AV conduction and suppression of ventricular pauses.
Pachón and colleagues1 were the first to report the successful vagal denervation by RF ablation targeting GP in 21 patients with symptomatic functional bradycardias and NMS, including 7 with high-degree AV block, with procedural endpoint of normalization of AV conduction. In these patients, the denervation was performed only through the RA in 3 patients, but 1 patient still experienced nocturnal second-degree Mobitz I AV block during follow-up. Ever since, several strategies for atrial autonomic denervation have been reported. However, the optimal technique for identifying atrial GP sites, either physiologically guided (spectral mapping, high-frequency stimulation) or anatomically guided, has not been established yet.1,2,3,7,9,11 Further, there is no consensus on the extension of GP ablation for attenuating the vagal activity for treatment of functional bradycardias and NMS, whether ablating all atrial GP, selectively ablating GP on both sides of the interatrial septum, or targeting exclusively specific areas of the RA or LA GP.1, 2, 3, 4, 5, 6, 7, 8, 9
The intrinsic cardiac autonomic nervous system is composed of an extensive neural network of axons and parasympathetic neurons, interconnecting within and between clusters of autonomic ganglia (GP), embedded within epicardial fat pads. Experimental and clinical studies11,12 suggested that despite multiple interconnections and significant regional overlap among the atrial GP, the RAGP serves as the main integration center for the sinus node and the RAGP and RIGP seem to serve as integration centers for the AV node. Thus, as a hypothesis, to obtain an effective autonomic denervation of the AV node, it is necessary to perform the selective ablation of both RAGP and RIGP.
Since 2009, the Arrhythmia Unit of the Heart Institute of Sao Paulo started to perform physiologically (guided by high-frequency stimulation or spectral mapping) or anatomically guided biatrial ablation of GP, either targeting all atrial GP or selectively ablating GP on both sides of the interatrial septum for the management of refractory cardioinhibitory NMS or symptomatic functional bradycardias.3,6,9 In 2016, Rivarola and colleagues6 reported a favorable outcome for a 38-year-old man presenting with asymptomatic nocturnal episodes of advanced AV block, who was treated with vagal denervation targeting the interatrial septal GP guided by spectral mapping.
In the present report, we performed an anatomically guided ablation of RAGP and RIGP, addressing both sides of the interatrial septum, as shown in Figure 3, for attenuation of the vagal effects on the AV node. Additionally, an important issue highlighted in the present case is the use of the technique of ECVS to establish the denervation endpoints. In agreement with a previous study,10 the technique of ECVS, which can be performed repeatedly during the vagal denervation procedure (in contrast to atropine infusion, which action lasts for several hours), proved to be useful for monitoring the extension of the denervation and ultimately demonstrating the suppression of the cardioinhibitory response. Nevertheless, ECVS, as any other method to establish the vagal denervation endpoint, has its own limitations. Most cardiac sympathetic nerves originating from the right and left intrathoracic chains also carry parasympathetic output,13 and these extravagal sources are neglected as we use extracardiac vagal stimulation exclusively to determine procedure endpoints.
Finally, the 24-hour Holter monitoring showed that ventricular pauses longer than 2 seconds were reduced from 4879 (preablation) to 2 pauses per 24 hours 1 week after ablation, but there was an increase in the frequency of ventricular pauses, to 9 and 26 per 24 hours, after 6 and 11 months, respectively (Table 1). These data are consistent with a late reinnervation after atrial GP ablation, which has already been reported by other authors.2,3,6
Conclusion
In conclusion, this report highlights that anatomically guided ablation of atrial GP, addressing both sides of the interatrial septum, with vagal denervation endpoints evaluated by ECVS, may be an alternative therapy for functional AV block, resulting in normalization of AV conduction in short- and medium-term follow-up. Further large-scale studies are needed to assess the impact of this novel therapeutic approach in the setting of functional AV block.
Footnotes
Conflict of Interest Disclosures: None.
Funding Sources: None.
References
- 1.Pachón J.C., Pachón E.I., Pachón J.C. “Cardioneuroablation” - New treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Pachón J.C., Pachón E.I., Cunha Pachón M.Z. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace. 2011;13:1231–1242. doi: 10.1093/europace/eur163. [DOI] [PubMed] [Google Scholar]
- 3.Scanavacca M., Hachul D., Pisani C., Sosa E. Selective vagal denervation of the sinus and atrioventricular nodes, guided by vagal reflexes induced by high frequency stimulation, to treat refractory neurally mediated syncope. J Cardiovasc Electrophysiol. 2009;20:558–563. doi: 10.1111/j.1540-8167.2008.01385.x. [DOI] [PubMed] [Google Scholar]
- 4.Aksu T., Golcuk S.E., Guler T.E., Yalin K., Erden J. Functional permanent 2:1 atrioventricular block treated with cardioneuroablation: case report. HeartRhythm Case Rep. 2015;1:58–61. doi: 10.1016/j.hrcr.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukunaga M., Wichterle D., Peichl P. Differential effect of ganglionic plexi ablation in a patient with neurally mediated syncope and intermittent atrioventricular block. Europace. 2017;19:119–126. doi: 10.1093/europace/euw100. [DOI] [PubMed] [Google Scholar]
- 6.Rivarola E., Hardy C., Sosa E. Selective atrial vagal denervation guided by spectral mapping to treat advanced atrioventricular block. Europace. 2016;18:445–449. doi: 10.1093/europace/euv142. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y., Shi R., Wong T. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465. [DOI] [PubMed] [Google Scholar]
- 8.Sun W., Zheng L., Qiao Y. Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivarola E., Hachul D., Wu T. Targets and endpoints in cardiac autonomic denervation procedures. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004638. [DOI] [PubMed] [Google Scholar]
- 10.Pachón M.J.C., Pachón M.E.I., Santillana P.T.G. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. JACC Clin Electrophysiol. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Hou Y., Scherlag B.J., Lin J. Interactive atrial neural network: determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Malcolme-Lawes L.C., Lim P.B., Wright I. Characterization of the left atrial neural network and its impact on autonomic modification procedures. Circ Arrhythm Electrophysiol. 2013;6:632–640. doi: 10.1161/CIRCEP.113.000193. [DOI] [PubMed] [Google Scholar]
- 13.Van Stee E.W. Autonomic innervation of the heart. Environ Health Perspect. 1978;26:151–158. doi: 10.1289/ehp.7826151. [DOI] [PMC free article] [PubMed] [Google Scholar]