Introduction
The current revised task force criteria for diagnosis of arrhythmogenic cardiomyopathy (ACM) includes only morphological criteria for right ventricle with no consideration for left ventricle criteria.1 However, accumulating evidence suggests increasing left ventricle involvement in ACM. One such phenotype is Carvajal syndrome, which is a primary left ventricle ACM that is inherited as a familial cardiocutaneous syndrome consisting of woolly hair, palmoplantar keratoderma, and cardiac involvement. High index of clinical suspicion and advanced imaging based on cardiac magnetic resonance imaging (MRI) are key to making the diagnosis. We describe here cardiac MRI findings and scar pattern that led to the diagnosis of Carvajal syndrome in our patient.
Key Teaching Points.
-
•
Arrhythmogenic left-sided ventricular cardiomyopathy (ALVC) is a rare type of arrhythmogenic cardiomyopathy with a phenotype that is distinct from the traditional arrhythmogenic right ventricular cardiomyopathy.
-
•
High index of clinical suspicion and cardiac magnetic resonance imaging are key to the diagnosis of ALVC.
-
•
A circumferential/ring-like pattern of epicardial or midmyocardial delayed enhancement pattern is increasingly recognized as a hallmark for clinically suspected ALVC with desmosomal mutation (Carvajal syndrome).
Case report
History of presentation
A 47-year-old man with newly diagnosed heart failure, physical findings of woolly hair, palmoplantar keratoderma, and family history of arrhythmogenic right ventricular cardiomyopathy (ARVC) was referred for further evaluation. He had 1 prior episode of presyncope that was preceded by palpitations and another episode of isolated palpitations. He was otherwise asymptomatic with good functional capacity and was very active, including daily jogging and bicycle riding. His cardiac exam was generally unremarkable, but skin exam was notable for woolly hair and callused hands and feet.
Past medical history
His medical conditions prior to diagnosis of heart failure include type 2 diabetes that was well controlled on an oral antidiabetic agent, hyperlipidemia, and obstructive sleep apnea that was managed with continuous positive airway pressure. He smoked 1 pack per day for 30 years and quit smoking recently.
Family history
Family history was significant for cardiac disease and Naxos syndrome (Figure 1). He has 5 male siblings and 4 out of his 5 brothers had cutaneous features of Naxos syndrome. Two of his brothers with Naxos syndrome died of sudden cardiac death (SCD) at ages 36 and 55, of which the younger one was confirmed to have ARVC on autopsy. Two other siblings had been diagnosed with Naxos syndrome owing to the characteristic woolly hair and callused hands and feet, similar to our patient. He had 1 brother, aged 57 years, who was reported to be unaffected. His father died suddenly at the age of 66, and did not have a Naxos phenotype. His mother died of breast cancer at 51.
Figure 1.
A 3-generation family tree showing our patient in green, live family members in white, and dead family members in pattern. The patient is shown in gray. Square shape indicates male sex and oval shape indicates female sex. The numbers indicate the age for each relative at the time of patient visit (if still alive) or death. ARVC = arrhythmogenic right ventricular cardiomyopathy; SCD = sudden cardiac death.
Differential diagnosis
The differential diagnosis is broad and includes nonischemic cardiomyopathy—including inflammatory, dilated, infiltrative, and genetic etiology—as well ischemic cardiomyopathy. However, his skin findings, significant family history of Naxos syndrome, cardiomyopathy, and SCD made us consider a genetic mechanism as the underlying etiology.
Investigations
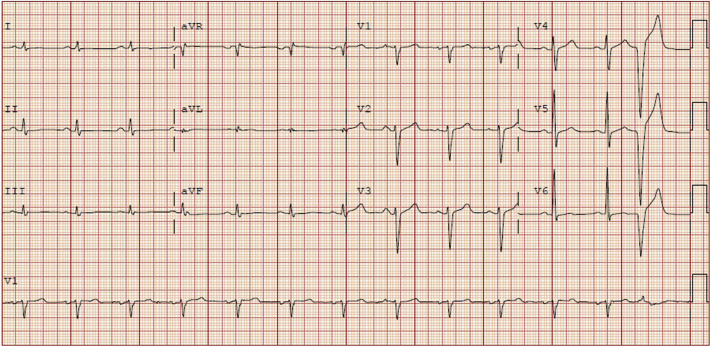
Prior to presenting to our clinic, the patient had extensive cardiac work-up by his primary physician. These included transthoracic echocardiogram, which showed mild-to-moderate global hypokinesis of the left ventricle with ejection fraction (EF) of 40%–45%. The right ventricle was well visualized and was adjudged to be normal. Subsequent work-up included nuclear myocardial perfusion imaging, which showed a large inferior wall fixed defect with associated regional inferior wall hypokinesis. A follow-up angiogram, however, revealed normal coronary arteries. He also had a 48-hour Holter monitor that revealed 1.9% ventricular ectopy, 0.5% supraventricular ectopy, and 1 atrial triplet. Twelve-lead electrocardiogram showed nonspecific T-wave abnormalities and 1 premature ventricular complex (Figure 2).
Figure 2.
Electrocardiogram showing normal sinus rhythm, nonspecific T abnormalities in inferolateral leads, and a premature ventricular complex.
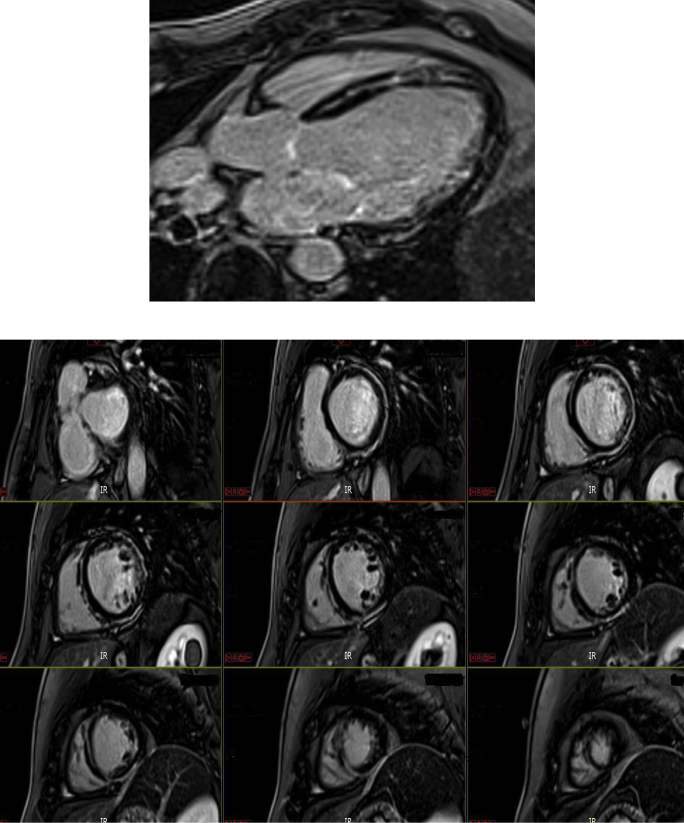
Given his significant history and the suspicion for underlying nonischemic cardiomyopathy, we sent the patient for cardiac MRI, which showed a dilated left ventricle with moderately reduced global systolic function (EF 38%). Right ventricle was normal in size and global systolic function, and there was no evidence of major or minor MRI criteria for ARVC (Supplemental Video). Late gadolinium enhancement imaging, however, was abnormal with extensive, circumferential/ring-like epicardial to midmyocardial enhancement of the left ventricle, extending from the base to the apex, with some skip segments in-between (Figure 3). In addition, genetic testing was positive for pathogenic mutation in desmoplakin gene DSP c.6510_6511insCT (p.Asn2171Leufs∗17) and a variant of uncertain significance in the DSP gene c.273+5G>A (Intronic). Given the MRI findings with predominant left ventricular involvement and the results of his genetic testing, he was diagnosed with left-sided ACM. The predominant left ventricular involvement is in favor of Carvajal syndrome, a variant of Naxos disease.
Figure 3.
Delayed-enhancement imaging with extensive, circumferential epicardial to midmyocardial enhancement of the left ventricle, extending from the base to the apex, with some skip segments in between.
Management
He was commenced on guideline-directed medical therapy for heart failure, including beta-blocker and angiotensin II receptor blocker. For his left-sided predominant ACM (Carvajal syndrome), he was recommended genetic testing in first-degree relatives and counseled extensively to refrain from high-intensity and competitive sports, as high-intensity exercise has been shown to accelerate progression of ARVC and increase ventricular arrhythmia. In addition, he received an implantable cardioverter-defibrillator (ICD) for prevention of SCD, based on his risk factors for SCD, including family history of SCD, personal history of presyncope that was presumed to be due to ventricular arrhythmia, and confirmed ACM. In addition, he has 1 major criterion (left ventricle EF <50%) and 3 minor criteria for ICD (male sex, >1000 premature ventricular contractions per 24 hours, and 2 desmosomal variants) to warrant at least a class IIb ICD indication.
Discussion
Our patient represents a case of left-sided ACM with a cardiac phenotype that is distinct from the traditional ARVC based on the MRI findings. In accordance with the revised task force criteria for ARVC,1 our patient has 2 major criteria—family history of confirmed ARVC and positive pathogenic gene mutation—and at least 1 minor criterion of ventricular ectopy. Hence, he fulfills the criteria for definite ACM, which requires at least 2 major criteria. His cutaneous manifestations and left-sided involvement are consistent with the form of ACM (Carvajal syndrome) that tends to have cardiocutaneous manifestations. This rare ACM phenotype was first reported in the 1990s from families in India and Ecuador, and a causal mutation in the desmoplakin gene has subsequently been described.2, 3, 4
Cardiac MRI played a key role in the diagnosis of ACM in our patient. Pathology studies in Carvajal patients have identified epicardial and midmyocardial patterns of fibrosis, similar to the MRI findings in our patient.5 The epicardial involvement and pattern of abnormal enhancement similar to our patient has been described with desmosomal disease–causing variants and left-sided ACM from the United Kingdom.6 Cardiac MRI findings with Carvajal syndrome have reported noncompaction changes in the past.7 Although our patient did not have noncompaction, his pattern of enhancement and scarring can be seen with other desmosomal diseases.6 A similar ring-like pattern of enhancement has been reported in arrhythmogenic left ventricular cardiomyopathy (ALVC) cases with DSP mutation and correlates with histological evidence on fibro-fatty replacement.8,9 Our case also highlights that MRI can play an important role in the evaluation of ALVC and the differentiation between Naxos and Carvajal syndromes. While noncompaction has been recognized as a presentation of Naxos and Carvajal syndromes, we would like to note that the abnormal scarring of the left ventricle could be an important and under-recognized manifestation of this disease, and could also play a role in the arrhythmogenic potential of the disease.
A notable dilemma in the management of this patient was whether an ICD should be implanted, as there is currently a paucity of data on risk stratification in patients with predominant left-sided ACM. The final decision was guided by the presence of risk factors that had been shown to be prognostic in population with ARVC and potential risk-benefit discussion with the patient.
Conclusion
In conclusion, we present a case of ALVC, Carvajal syndrome, diagnosed with circumferential epicardial and midmyocardial enhancement pattern on cardiac MRI and abnormal genetic testing. Our case provides more evidence to support predominant left-sided cardiomyopathy as an entity that is part of the ACM spectrum but with a cardiac phenotype that is distinct from the traditional ARVC. This adds credence to the call for the inclusion of morphological left ventricle criteria, including late gadolinium enhancement, in the task force criteria for ACM in order to improve the identification of this currently underdiagnosed left ventricle–dominant disease.10
Footnotes
Disclosures: The authors do not have any conflicts of interest.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2021.01.024.
Appendix. Supplementary data
Four-chamber cine showing a dilated left ventricle with moderately reduced global systolic function and normal right ventricular systolic function.
References
- 1.Towbin J.A., McKenna W.J., Abrams D.J. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Alcalai R., Metzger S., Rosenheck S., Meiner V., Chajek-Shaul T. A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol. 2003;42:319–327. doi: 10.1016/s0735-1097(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 3.Carvajal-Huerta L. Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39:418–421. doi: 10.1016/s0190-9622(98)70317-2. [DOI] [PubMed] [Google Scholar]
- 4.Rao B.H., Reddy I.S., Chandra K.S. Familial occurrence of a rare combination of dilated cardiomyopathy with palmoplantar keratoderma and curly hair. Indian Heart J. 1996;48:161–162. [PubMed] [Google Scholar]
- 5.Protonotarios N., Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol. 2004;13:185–194. doi: 10.1016/j.carpath.2004.03.609. [DOI] [PubMed] [Google Scholar]
- 6.Norman M., Simpson M., Mogensen J. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation. 2005;112:636–642. doi: 10.1161/CIRCULATIONAHA.104.532234. [DOI] [PubMed] [Google Scholar]
- 7.Prompona M., Kozlik-Feldmann R., Mueller-Hoecker J., Reiser M., Huber A. Images in cardiovascular medicine. Magnetic resonance imaging characteristics in Carvajal syndrome (variant of Naxos disease) Circulation. 2007;116:e524–e530. doi: 10.1161/CIRCULATIONAHA.107.704742. [DOI] [PubMed] [Google Scholar]
- 8.Augusto J.B., Eiros R., Nakou E. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study. Eur Heart J Cardiovasc Imaging. 2020;21:326–336. doi: 10.1093/ehjci/jez188. [DOI] [PubMed] [Google Scholar]
- 9.Chen L., Song J., Chen X. A novel genotype-based clinicopathology classification of arrhythmogenic cardiomyopathy provides novel insights into disease progression. Eur Heart J. 2019;40:1690–1703. doi: 10.1093/eurheartj/ehz172. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D., Perazzolo Marra M., Zorzi A. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four-chamber cine showing a dilated left ventricle with moderately reduced global systolic function and normal right ventricular systolic function.