Abstract
Background
Many ginsenosides have been shown to be efficacious for major depressive disorder (MDD), which is a highly recurrent disorder, through several preclinical studies. We aimed to review the literature assessing the antidepressant effects of ginsenosides on MDD animal models, to establish systematic scientific evidence in a rigorous manner.
Methods
We performed a systematic review on the antidepressant effects of ginsenoside evaluated in in vivo studies. We searched for preclinical trials from inception to July 2019 in electronic databases such as Pubmed and Embase. In vivo studies examining the effect of a single ginsenoside on animal models of primary depression were included. Items of each study were evaluated by two independent reviewers. A meta-analysis was conducted to assess behavioral changes induced by ginsenoside Rg1, which was the most studied ginsenoside. Data were pooled using the random-effects models.
Results
A total of 517 studies were identified, and 23 studies were included in the final analysis. They reported on many ginsenosides with different antidepressant effects and biological mechanisms of action. Of the 12 included articles assessing ginsenoside Rg1, pooled results of forced swimming test from 9 articles (mean difference (MD): 20.50, 95% CI: 16.13-24.87), and sucrose preference test from 11 articles (MD: 28.29, 95% CI: 22.90-33.69) showed significant differences compared with vehicle treatment. The risk of bias of each study was moderate, but there was significant heterogeneity across studies.
Conclusion
These estimates suggest that ginsenosides, including ginsenoside Rg1, reduces symptoms of depression, modulates underlying mechanisms, and can be a promising antidepressant.
Keywords: Depression, Ginsenosides, Ginsenoside Rg1, Antidepressive agents, Neuroinflammation
1. Introduction
Depression has a high mortality and prevalence rate, as well as a high probability of relapse and complications such as suicide or substance abuse, which can impose a social burden. According to a World Health Organization (WHO) report, over 264 million people of all ages are affected by depression, and it was the third leading cause of years lived with disability (YLDs) [1]. In 2008, the WHO reported major depressive disorder (MDD) as the third cause of burden of disease, and predicted that it will be the first in 2030 [2]. Depression has the disadvantage of not only being expensive to treat, but also poses a social burden such as burden of family care and reduced workplace productivity. Depression is also 2-3 times more likely to develop in people with multiple diseases compared with those without multiple diseases or chronic physical conditions; therefore, a large population is vulnerable to this disease [3].
The drug market for depressive disorder in 2015 was $3.18 billion, and is expected to grow to $5.79 billion by 2025 [4]. Antidepressants are also used for various psychiatric conditions such as panic disorder, post-traumatic stress disorder, and somatic diseases. This extensive use of antidepressants is required in diverse fields, thus the social demand for new drugs with less side effects is increasing. For this reason, there is a growing interest in natural therapeutics which were already recognized for their safety and effectiveness through previous preclinical and clinical studies.
Ginseng refers to the roots of some plants of the species Panax sp.. It consists of the major commercial ginsengs such as Panax ginseng Meyer (P. ginseng; Korean ginseng), Panax quinquefolius (American ginseng), and Panax notoginseng (Chinese ginseng), and also includes Panax japonicum (Japanese ginseng) and Panax vietnamensis (Vietnamese ginseng). These ginsengs have been used to treat a wide range of diseases for more than 2,000 years worldwide. P. ginseng was commonly used as an apoptogenic agent in East Asia, including Korea, China, and Japan, to improve physical performance, increase vitality, increase resistance against stress and aging, and activate the immune system. As its active ingredients, more than 40 types of ginsenosides have been identified and isolated [5]. Ginsenosides are classified into the protopanaxadiol type (Ginsenoside Ra1-3, Rb1-2, Rc, Rd, Rg3, Rh2-3), protopanaxatriol type (ginsenoside Re, Rf, Rg1-2, Rh1), oleanolic acid type (ginsenoside Ro), and ocotillol type (Makonoside-Rs from Vietnamese ginseng) [5,6]. P. ginseng has traditionally been used in various ailments in the nervous system including MDD. The antidepressant effect of P. ginseng itself was studied using a chronic mild stress model [7], chronic restraint stress model [8], menopause depression model [9], and an addiction-withdrawal model [10] so far. Various studies have been performed on each type of ginsenosides to demonstrate their antidepressant effects, and various mechanisms were suggested to contribute to this.
However, to date, no systematic review has been performed with regards to preclinical studies of ginsenosides on depression. Studies conducted on the specific ginsenosides explore various behavioral changes and underlying mechanisms using many kinds of models, doses, and administration routes. They sometimes report contradictory results despite the same research methods. Due to their small sample sizes, the results of some previous studies had low statistical power. Therefore, in this study, we conducted a rigorous and overall systematic review and meta-analysis on the antidepressant effects of ginsenosides, to provide evidence and a potential reference for its clinical uses.
2. Materials and methods
2.1. Search strategy
Two trained researchers independently performed searches on ginsenosides on depressive disorder using electronic databases including Pubmed and Embase for original studies published up to 23 July 2019. We used the search strategy in Pubmed as follows: (Ginsenosides [Mesh] OR Panax [Mesh] OR Ginseng [Tiab] OR panax [tiab] OR ginsan [tiab] OR "jen shen" [tiab] OR shinseng [tiab] OR "ren shen" [tiab] OR renshen [tiab] OR schinseng [tiab] OR ninjin [tiab] OR ginsenoside [tiab] OR gingilone [tiab] OR panaxoside [tiab] OR protopanaxa [tiab] OR protopanaxadiol [tiab] OR protopanaxatriol [tiab] OR panaxagin [tiab] OR ginsenol [tiab] OR ginsenine [tiab] OR "ginseng saponin"[tiab]) AND (Depression [Mesh] OR "Depressive disorder" [Mesh] OR Depress∗[tiab] OR “emotional disorder”[tiab] OR “psychological disorder”[tiab] OR “psychological distress”[tiab] OR “emotional distress”[tiab] OR "emotional stress"[tiab]). We also performed a hand search. The reference lists from relevant publications were used to identify further relevant research articles and reviews.
2.2. Inclusion criteria and exclusion criteria
The inclusion criteria were (i) studies which used each ginsenoside as a pure chemical compound alone; (ii) in vivo studies on animal subjects; and (iii) studies that primarily focused on depressive disorder. The exclusion criteria were (i) studies that used the whole ginseng extract or mixture which contained many different ginsenosides and other components as the intervention; (ii) studies that used metabolites of ginsenosides produced after digestion; (iii) case reports, clinical trials, abstracts, comments, reviews, or editorials; (iv) studies that did not test the efficacy of ginsenoside on depression. To determine their eligibility for inclusion, two authors (YK and SC) assessed the titles and abstracts of the identified articles, and obtained copies of the articles to review the study design and methodology that administered ginsenoside and measured behavioral changes in MDD model rodents. We did not have any restrictions on the language, publication year, and study design.
2.3. Data extraction
All the data were extracted from the included studies independently by two participants (YK and SC). The following details of the study design were extracted from each study: (1) first author's name and the publication year; (2) individual data were obtained for each study, including sample size, species, sex, weight, and type of MDD model; (3) main experimental groups; substances used as experimental and control treatments; the dose, method, and timing of ginsenoside administration; (4) outcome measures were included.
Ginsenoside Rg1 (Rg1) was most abundantly investigated among the included studies and we extracted the results of the forced swimming test (FST) and sucrose preference test (SPT) to perform meta-analysis. The primary outcome of interest were behavioral tests to assess depressive-like behavior, including the FST and SPT.
When data were only reported graphically, we used a digital screen ruler (Adobe ruler) to detect values. When data for each group were measured at different timelines, we adopted data from the final time point. When a multiple doses were assessed, we extracted the data of respective results. If we could not find whether the reported measure of variance was SD or SEM, we assumed that it was SEM, because the study might otherwise cause inadequate weight in the meta-analysis. All data were extracted by two independent reviewers.
2.4. Quality assessment
We assessed the methodological quality regarding risk of bias based on a modified scale from the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) [11]. The scale was modified from CAMARADES according to the features preclinical studies on depression and includes the following criteria: (1) peer reviewed publication; (2) control of temperature; (3) random allocation to groups; (4) blinded induction of depression; (5) blinded assessment of behavioral outcome; (6) use of anesthetic without significant intrinsic neuroprotective activity; (7) calculation of the sample size necessary to achieve sufficient power; (8) appropriate animal model which uses animals without relevant comorbidities (aged, diabetic, or hypertensive); (9) compliance with animal welfare regulations; (10) statement of potential conflict of interests. Two authors (YK and SC) independently assessed study quality and discussed any difference in opinions. Each study was given a quality score out of a possible total of 10 points, and the average score of was calculated.
2.5. Statistical analysis
The statistical analyses and forest plots were conducted using the RevMan 5.3.5 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). For the meta-analysis, in terms of the pooled results, continuous data were expressed as mean differences (MD) with associated 95% confidence intervals (CI) using the random-effects model. Mean difference for the value at post-treatment was used. Random-effects models were used because the number of animals of the included studies was small and heterogeneity among studies need to be considered. I2 statistic was used to assess statistical heterogeneity. Subgroup meta-analysis was performed according to dose (mg/kg). Publication bias was looked for using funnel plotting. P-values < 0.05 were considered statistically significant.
3. Results
3.1. Study inclusion
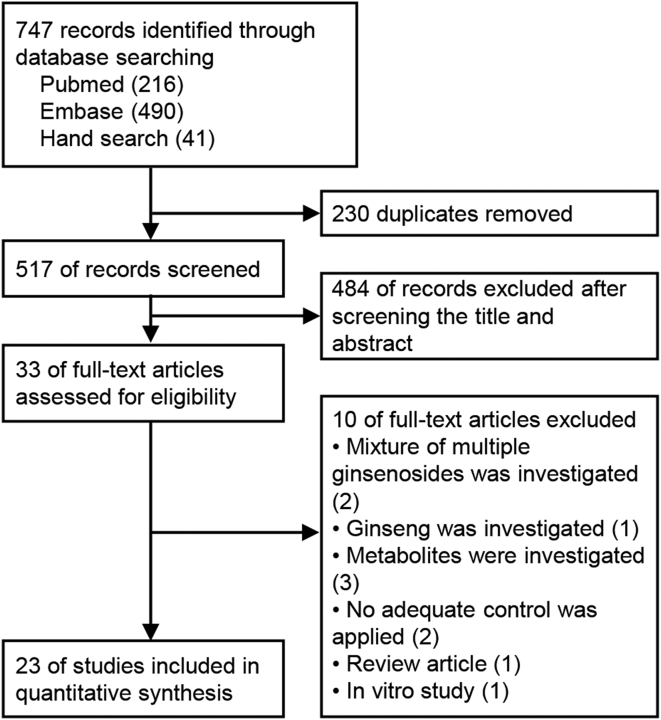
After searching in electronic databases and removing duplicates, we identified 517 records. Of these, 484 records were excluded as they did not meet our inclusion criteria during screening of the titles and abstracts. Then, 33 full-text articles were assessed for eligibility, and 10 records were excluded after reading the full article text for the following reasons: mixture of multiple ginsenosides was investigated [12,13], ginseng was investigated [14], metabolites were investigated [[15], [16], [17]], no adequate control was applied [18,19], a review article [20], cell culture study [21]. Finally, 23 studies were finally included (Fig. 1).
Fig. 1.
Flow chart of the included studies. 747 records identified, 517 records screened, 33 reviewed in detail, and 23 included for analysis.
3.2. Characteristics of included studies
A total of 606 animals were included, of which 332 were under ginsenosides treatment and 274 were under vehicle treatment. Of the 23 included studies, one article was written in Chinese and the rest were in English (Table 1).
Table 1.
Characteristics of studies included in systematic review of antidepressant effects of ginsenosides
| Ginsenoside | Depression model | Animal species (sex, n) | Weight | Experimental group | Control group | Outcome | Intergroup difference | Method of administration | Time of ginsenoside administration | First author, year |
|---|---|---|---|---|---|---|---|---|---|---|
| Rb1 | CUMS | ICR mice (male, 10/10) | 20-25g | Rb1 (10 mg/kg, po) for 5w + CUMS for 7w | vehicle for 5w + CUMS for 7w |
|
|
po | 30 min before stress | Yao, 2012 [28] |
| Rb1 | CUMS | Wistar rats (male, 6/6) | 200-220g | Rb1 (4, 8, 16 mg/kg, po) for 3w+CUMS for 4w | Vehicle for 3w+CUMS for 4w |
|
|
po | 60 min prior to the experiment | Wang, 2017 [33] |
| Rb3 |
|
NIH mice (male, (A) 19/21, (B) 11/11) |
|
|
|
|
Cui, 2012 [31] | |||
| Rd | CUMS | ICR mice (male, 10/10) | 20-25g | Rg1 (10 mg/kg, po) for 5w + CUMS for 7w | vehicle for 5w + CUMS for 7w |
|
|
po | 30 min before stress | Yao, 2012 [28] |
| Re | CUMS | ICR mice (male, 10/10) | 20-25g | Rg1 (10 mg/kg, po) for 5w + CUMS for 7w | vehicle for 5w + CUMS for 7w |
|
|
po | 30 min before stress | Yao, 2012 [28] |
| Re | CIS | Sprague-Dawley rats (male, 7/7) | 240-280 g | Re (10, 20, 50 mg/kg, ip) for 10d + CIS (2h/day) for 10d | Saline + CIS (2h/day) for 10d |
|
|
ip | 30 min before daily exposures to immobilization stress | Lee, 2012 [40] |
| Rf | Astrocyte ablation model | C57BL/6 mice (male, 7/7) | Rf (20 mg/kg, po) for 6d + L-AAA (ic,) for 2d | Vehicle (po) for 6d + L-AAA (ic,) for 2d |
|
po | 1w after injection | Kim, 2019 [22] | ||
| Rg1 | CUMS | C57BL/6 mice (male, 20/20) | NR | Rg1 (2.5, 5, 10, 20 mg/kg, ip) for 14d + CUMS for 8w | Saline for 14d + CUMS for 8w |
|
|
ip | Before CUMS | Jiang, 2012 [23] |
| Rg1 | CUMS | ICR mice (male, 10/10) | 20-25g | Rg1 (10 mg/kg, po) for 5w + CUMS for 7w | vehicle for 5w + CUMS for 7w |
|
|
po | 30 min before stress | Yao, 2012 [28] |
| Rg1 | CUMS | Sprague-Dawley rats (male, 10/10) | NR | Rg1 (5,10,20mg/kg, po) for 28d + CUMS for 28d | vehicle (po) for 28d + CUMS for 28d |
|
|
po | NR | Mou, 2017 [41] |
| Rg1 | CUMS | Sprague-Dawley rats (male, 10/10) | 250-300g | Rg1 (5,10,20mg/kg, po) for 28d + CUMS for 28d | CUMS for 28d |
|
|
po | NR | Huang, 2013 [42] |
| Rg1 | CUMS | Wistar rats (male, 12/12) | 180-200 g | Rg1 (40 mg/kg, ip) for 5w + CUMS for 5w | Vehicle (ip) for 5w |
|
|
ip | 30 min prior to the stress exposure | Zhu, 2016 [34] |
| Rg1 | CUMS | Sprague-Dawley rats (male, 10/10) | 250–300g | Rg1 (5,10,20mg/kg, po) for 29d + CUMS for 35d | water (po) for 29d + CUMS for 35d |
|
|
po | Before CUMS | Jin, 2017 [43] |
| Rg1 | CUMS | Wistar rats (male, 20/20) | 180–200g | Rg1 (40 mg/kg, ip) for 5w + CUMS for 5w | CUMS for 5w |
|
|
ip | 30 min prior to CUMS | Liu, 2016 [36] |
| Rg1 | CUMS | Wistar rats (male, 18/18) | 220–240 g | Rg1 (40 mg/kg, ip) for 5w+ CUMS for 5w | Saline + CUMS for 5w |
|
|
ip | 30 min prior to CUMS exposure | Fan, 2018a [37] |
| Rg1 | CUMS | Wistar rats (male, 12/12) | 160-180 g | Rg1 (40 mg/kg, ip) for 5w+ CUMS for 5w | CUMS for 5w |
|
|
ip | 60 min prior to CUMS exposure | Fan, 2018b [38] |
| Rg1 | CUMS | Wistar rats (male, 12/12) | 160-180 g | Rg1 (40 mg/kg, ip) for 5w + CUMS for 5w | CUMS for 5w |
|
|
ip | 30 min prior to stress exposure | Yu, 2018 [39] |
| Rg1 | CUMS | Sprague-Dawley rats (male, 12/12) | NR | Rg1 (20,40 mg/kg, po) for 21d+CUMS for 21d | Saline(po) for 21d+CUMS for 21d |
|
|
po | NR | Wu, 2012 [44] |
| Rg1 | LPS-challenged mice | Wistar rats (male, 6/6) | 250-270g | Rg1 (10, 30 mg/kg. ip) for 4d + LPS (5 μg, icv) | vehicle (ip) for 4d + LPS (5 μg, icv) |
|
|
ip | 3 consecutive days (once daily) and rightly after the central injection of LPS | Zheng, 2014 [35] |
| Rg2 | CUMS | C57BL/6 mice (male, 10/10) | NR | Rg2 (10,20 mg/kg, ip) for 2w+CUMS for 6w | Vehicle (ip) + CUMS for 6w |
|
|
ip | NR | Ren, 2017 [24] |
| Rg3 | CUMS | C57BL/6 mice (female, 10/10) | NR | Rg3 (50, 100, 150 mg/kr, po) for 4w + CUMS for 4w | vehicle (po) + CUMS for 4w |
|
|
po | Before stress | Zhang, 2017 [26] |
| Rg3 | CSDS | C57BL/6 mice (male, 10/10) | NR | Rg3 (10, 20 mg/kg, ip) for 2w+CSDS for 14d | vehicle (ip) +CSDS for 14d |
|
|
ip | A day after the stress period | You, 2017 [25] |
| Rg3 | LPS-challenged mice | ICR mice (male, 10/10) | NR | Rg3 (20, 40 mg/kg, po) twice daily for 4d+ LPS (0.83 mg/kg, ip) | LPS (0.83 mg/kg, ip) |
|
|
po | Before and the same day on LPS injection | Kang, 2017 [29] |
| Rg5 | CSDS | C57BL/6 mice (male, 10/10) | NR | Rg5 (5, 10, 20, 40 mg/kg, ip) for 14d+CSDS(10min) for 14d | Vehicle (ip)+CSDS(10min) for 14d |
|
|
ip | A day after the last stress | Xu, 2017 [27] |
| Rh2 | LPS-challenged mice | ICR mice (male, 10/10) | 18–22 g | Rh2 (7.5, 15, 30 mg/kg, po) for 7d + LPS (0.83 mg/kg, ip) | LPS (0.83 mg/kg, ip) |
|
|
po | Before LPS injection | Chen, 2019 [30] |
| Notoginsenoside R1 | CUMS | ICR mice (male, 10/10) | 20-25g | R1 (10 mg/kg, po) for 5w + CUMS for 7w | vehicle for 5w + CUMS for 7w |
|
(1) n.s. | po | 30 min before stress | Yao, 2012 [28] |
| Majonoside-R1 | socially isolated depression mouse model | Swiss albino mice (male, 10/10) | 18-22g | MR1 (5,10 mg/kg, ip) for 10d + isolation stress for 5w | vehicle (ip) + isolation stress for 5w |
|
|
ip | NR | Duong, 2016 [32] |
| Majonoside-R2 | socially isolated depression mouse model | Swiss albino mice (male, 10/10) | 18-22g | MR2 (5,10 mg/kg, ip) for 10d + isolation stress for 5w | vehicle (ip) + isolation stress for 5w |
|
|
ip | NR | Duong, 2016 [32] |
| vina-ginsenoside-R2 | socially isolated depression mouse model | Swiss albino mice (male, 10/10) | 18-22g | VR2 (5,10 mg/kg, ip) for 10d + isolation stress for 5w | vehicle (ip) + isolation stress for 5w |
|
|
ip | NR | Duong, 2016 [32] |
BNDF, brain-derived neutrophic factor; TrkB, tropomyosin-related kinase B; Sirt1, sirtuin type 1; Nrf2, nuclear-related factor 2; p-NF-κB, phosphorylated nuclear factor-κB; p-IκB-α, phosphorylated inhibitor of κB-α; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin 6; SOD, superoxide dismutase; NSFT, Novelty-suppressed feeding test; CSDS, chronic social defeat stress model; TST, tail suspension test; FST, forced swimming test; NR, not reported; n.s., not significant; MDA, malondialdehyde.
3.2.1. Animals
Six studies used C57BL/6 mice [[22], [23], [24], [25], [26], [27]], 3 studies used ICR mice [[28], [29], [30]], NIH mice [31], and Swiss albino mice [32] in each experiment. Seven studies used Wistar rats [[33], [34], [35], [36], [37], [38], [39]] and 5 used Sprague Dawley rats [[40], [41], [42], [43], [44]]. Except for one study [26], all other studies used male animals.
The chronic unpredicted mild stress (CUMS) model was the most frequently used model of MDD [23,24,26,28,31,33,34,[36], [37], [38], [39],[41], [42], [43], [44]]. The duration of the CUMS procedure varied from three weeks [44], four weeks [26,33,41,42], five weeks [31,34,[36], [37], [38], [39]], six weeks [24], seven weeks [28,43], and eight weeks [23]. The chronic immobilization stress (CIS) model was also used, which lasted ten days [40]. Two studies used a 2-week-long chronic social defeat stress model [25,27] and one study used a 5-week-long socially isolated depression model [32]. Some studies adopted chemicals to establish a depression model. Of the 3 studies which used lipopolysaccharide (LPS), 2 used an intraperitoneal (ip) route [29,30], while the other used an intracerebroventricular (icv) route [35]. One study used an ip injection of reserpine, which depletes amines in neurons and induces a depressive-like state [31]. Furthermore, an intra-prefrontal cortex (PFC) injection of L-alpha-aminoadipic acid (L-AAA), gliotoxin was also adopted to ablate astrocytes in the PFC which resembles the state of patients with MDD [22].
3.2.2. Interventions
Fourteen species of ginsenosides were examined in the included studies. Rg1 was the most frequently investigated [23,28,[34], [35], [36], [37], [38], [39],[41], [42], [43], [44]]. In addition, ginsenoside Rb1 (Rb1) [28,33], ginsenoside Rb3 (Rb3) [31], ginsenoside Rd (Rd) [28], ginsenoside Re (Re) [28,40], ginsenoside Rg2 (Rg2) [24], ginsenoside Rg3 (Rg3) [25,26,29], ginsenoside Rg5 (Rg5) [27], and ginsenoside Rh2 (Rh2) [30] were used in the studies. Ginsenoside Rf (Rf) [22], a unique ingredient of P. ginseng was also investigated, while notoginsenoside R1 [28] from P. notoginseng and Majonoside-R1 [32], Majonoside-R2 [32], and vina-ginsenoside-R2 [32] from P. vietnamensis were also studied.
The duration of ginsenoside administration varied. In 5 studies, the duration was less than seven days [22,[29], [30], [31],35]. Those of 6 studies were less than fourteen days [[23], [24], [25],27,32,40] and those of 7 studies were less than thirty days [26,31,33,[41], [42], [43], [44]]. The duration of administration lasted for more than thirty days in 7 studies [28,34,[36], [37], [38], [39],41].
Drugs were administered via the per os (po) route [22,26,[28], [29], [30], [31],33,[41], [42], [43], [44]] and ip route [[23], [24], [25],27,32,[34], [35], [36], [37], [38], [39], [40]]. All the studies set the control group as the group receiving the vehicle treatment.
3.2.3. Outcome measures
3.2.3.1. Behavioral change
Every included study assessed behavioral changes after the administration of ginsenosides. The FST, SPT, and tail suspension test (TST) are representative tests for evaluating antidepressant efficacy. The FST [22,24,26,[28], [29], [30],[32], [33], [34],[36], [37], [38], [39], [40], [41], [42], [43]] and SPT [[23], [24], [25], [26], [27], [28],31,[34], [35], [36], [37], [38], [39],41,[43], [44], [45]] were equally used in 17 studies. Seven studies used the TST [22,24,26,[28], [29], [30],32]. The open field test [38,39,44], elevated plus maze [40], and novelty-suppressed feeding test [31] were used to assess anxiety. The active avoidance conditioning test [40] was used to measure associative learning and memory. Social interaction was also examined [25,27].
3.2.3.2. Physiological change
As depression alters physiological parameters such as body weight, food intake, and sleep, some studies evaluated changes in these parameters. Eight studies measured weight gain [23,24,26,29,35,40,41,43] and 2 of them simultaneously confirmed a change in food intake [29,35]. Alteration in sleep structure was measured using pentobarbital-induced sleep test [41].
3.2.3.3. Histological analysis
Brain regions of interest were as follows. Most studies focused on the hippocampus [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31],35,40,41,44]. The PFC was frequently studied [22,31,34,37,38,[41], [42], [43]] and some studies examined broader area such as the cortex [35] or frontal cortex [28]. In addition, the amygdala [31,36,39], striatum [28], and locus coeruleus [40] were evaluated. Two studies performed biochemical analysis using the whole brain [32,33].
Some studies sought to identify changes in astrocytes by assessing glial fibrillary acidic protein (GFAP) levels [22,37], neurons by neuronal nuclear protein (NeuN) levels [22,37], and microglia by Iba-1 levels [29,35,37]. Cui, Jiang [31] examined the weight of the hippocampus and Wu, Zhu [44] assessed the amino acids changes in the hippocampus.
3.3. Underlying mechanisms
Neuroprotective effects were the most reported results in the included studies. Brain-derived neutrophic factor (BDNF) [[23], [24], [25], [26], [27],30,31,34,36,39,40], extracellular-signal-regulated kinase (ERK) phosphorylation [23,34], cAMP response element-binding protein (CREB) phosphorylation [[23], [24], [25], [26], [27],34,36,39], tropomyosin-related kinase B (TrkB) phosphorylation [[23], [24], [25],27,30], protein kinase A (PKA) phosphorylation [36], sirtuin type 1 (Sirt1), nuclear-related factor 2 (Nrf2) [30], NF-κBp65 phosphorylation, and inhibitor of κB-α (IκB-α) phosphorylation [29,30] were studied.
Plasma concentration of interleukin (IL)-6 [29,35], tumor necrosis factor-alpha (TNF-α) [29,35], IL-10 [35], and their mRNAs [35] were measured. Hippocampal or cortical concentration of IL-1β [35,37], IL-6 [30,35], TNF-α [30,35,37], IL-10 [35], interferon (IFN)-γ [37], and their mRNAs [29,37] were measured. Indoleamine 2,3-dioxygenase (IDO) activity was also evaluated [29].
Serum corticosterone [23,40,41], corticotrophin-releasing factor (CRF) in hypothalamus [40] and glucocorticoid receptor (GR) [41] in the brain was observed. Alterations in serum testosterone levels were studied [41].
In terms of monoamine neurotransmitters, the concentration of 5-hydroxytryptamine [28,31,33,35], dopamine [31,33], and norepinephrine [28,31,33] were measured.
Regarding oxidative stress, superoxide dismutase (SOD) [30], inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, myeloperoxidase (MPO), matrix metalloproteinase (MMP)-2, MMP-9, intercellular adhesion molecule (ICAM)-1 [35], reactive oxygen species (ROS), 4-hydroxynonenal (HNE) [37], malondialdehyde (MDA) [32,37], glutathione (GSH) [32] were examined. Neurogenesis was evaluated using doublecortin X (DCX) [23] and Ki-67 [22].
Rg1 especially caused changes in synaptic ultrastructure [[36], [37], [38], [39],42] and spine density [23,37,38]. Rg1 regulated synaptic-related protein expression such as miR-134 [38,39], Limk1 [38], p-cofilin [38], PSD-95 [37], and synaptophysin [37]. Rg1 decreased ultrastructural changes in the astrocyte gap junction and prevented the decrease in connexin 43 (Cx43) protein expression [43]. The effect of Rg1 against apoptosis was also assessed using a TUNEL assay, and apoptosis-related proteins such as cleaved caspase-3, caspase-9, Bcl-2, phosphorylated p38, p65, and Nrf2 [37].
3.4. Methodological quality of studies
The quality of methodologies used in the studies were assessed by a check list from CAMARADES. The average of the quality score was 6.91, the lowest score was 5, and the highest was 8. All studies had undergone a peer review and adopted appropriate animal models without relevant comorbidities. No study reported blinded induction of depression and calculation of the sample size (Table 2).
Table 2.
Quality assessment of studies included in systematic review of antidepressant effects of ginsenosides following modified scale of CAMARADES
| First author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2019 [30] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Cui 2012 [31] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Duong 2016 [32] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Fan 2018a [37] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Fan 2018b [38] | √ | √ | √ | √ | √ | 5 | |||||
| Huang 2013 [42] | √ | √ | √ | √ | √ | 5 | |||||
| Jiang 2012 [23] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Jin 2017 [43] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Kang 2017 [29] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Kim 2019 [22] | √ | √ | √ | √ | √ | √ | 6 | ||||
| Lee 2012 [40] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Liu 2016 [36] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Mou 2017 [41] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Ren 2017 [24] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Wang 2017 [33] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Wu 2012 [44] | √ | √ | √ | √ | √ | 5 | |||||
| Xu 2017 [27] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Yao 2012 [28] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| You 2017 [25] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Yu 2018 [39] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Zhang 2017 [26] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Zheng 2014 [35] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Zhu 2016 [34] | √ | √ | √ | √ | √ | √ | √ | √ | 8 |
Studies fulfilling the criteria of: (1) peer reviewed publication; (2) control of temperature; (3) random allocation to groups; (4) blinded induction of depression; (5) blinded assessment of behavioral outcome; (6) use of anesthetic without significant intrinsic neuroprotective activity; (7) calculation of the sample size necessary to achieve sufficient power; (8) appropriate animal model which uses animals without relevant comorbidities (aged, diabetic, or hypertensive); (9) compliance with animal welfare regulations; (10) statement of potential conflict of interest.
3.5. Meta-analysis on antidepressant effect of ginsenoside Rg1
As one of the most studied ginsenoside, Rg1 was studied in various doses and its effects were assessed by a number of outcome measures. We pooled the results from the FST and SPT, which were the most commonly used tests among the included studies. A total of 12 papers studied the antidepressant effect of Rg1 and they were included in the meta-analysis [23,28,[34], [35], [36], [37], [38], [39],[41], [42], [43], [44]]. Of the 12 papers included, 9 of the articles used FST [28,34,[36], [37], [38], [39],[41], [42], [43]] and 11 of the articles used SPT [23,[34], [35], [36], [37], [38], [39],[41], [42], [43], [44]]. All the studies assessed the effect of Rg1 treatment in comparison with a vehicle treatment. A random effects analysis was conducted to compute an MD between the two groups in both syntheses.
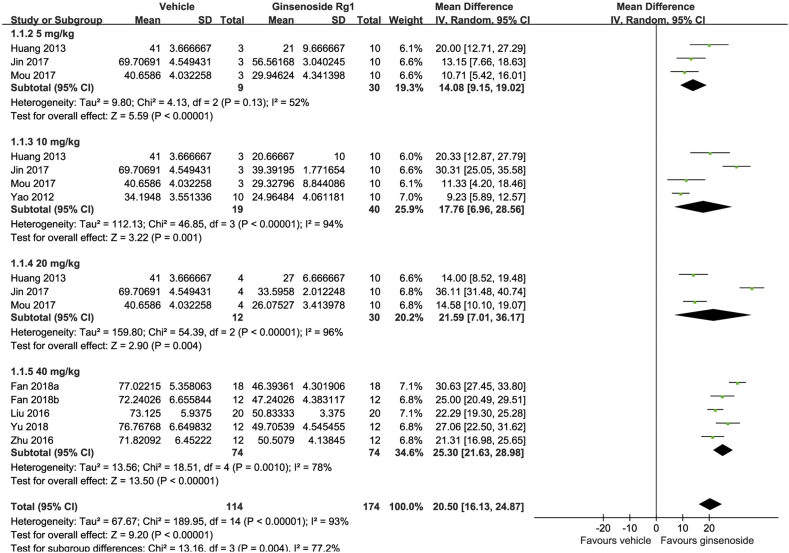
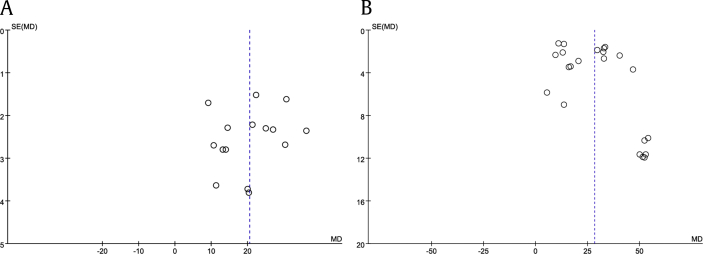
Regarding FST, the results from 9 studies were included in the analysis and the total sample size was 288, of which 174 received Rg1 and 114 received vehicle. We pooled the whole data which were normalized by total time and found significant difference with Rg1 treatment when compared with the control group (MD: 20.50, 95% CI: 16.13-24.87, Z = 9.20, p < 0.00001) (Fig. 2). There was heterogeneity across studies (I2 = 93%, p < 0.00001). However, the funnel plot showed no major asymmetry (Fig. 4(A)).
Fig. 2.
Forest plot for antidepressant effects of ginsenoside Rg1 on the forced swimming test. Global effect estimate comparing immobility time between ginsenoside Rg1 and vehicle treatments and its subgroup analysis according to dosage.
Fig. 4.
Funnel plot for (A) the forced swimming test and (B) the sucrose preference test to determine publication bias.
In the subgroup analysis of dosage subgroups, the mean difference of the immobility time increased in a dose-dependent manner. The pooled estimate for the MD of the immobility time was 14.08 (95% CI: 9.15-19.02) in the 2.5 mg/kg group, 17.76 (95% CI: 6.96-28.56) in the 10 mg/kg group, 21.59 (95% CI: 7.01-36.17) in the 20 mg/kg group, and 25.30 (95% CI: 21.63-28.98) in the 40 mg/kg group. In all groups, Rg1 was significantly associated with decreased immobility time (Fig. 2).
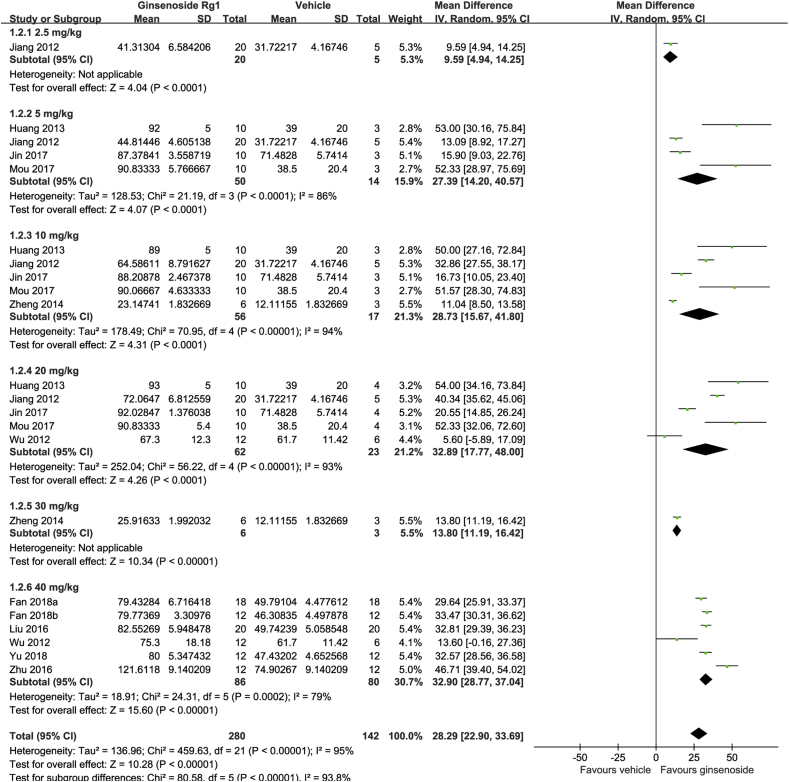
The analysis of SPT included a total of 11 studies that had a total sample size of 422, of which 280 animals received Rg1 and 142 received a vehicle treatment. The analysis showed that Rg1 was significantly more effective than the vehicle in increasing sucrose preference (MD: 28.29, 95% CI: 22.90-33.69, Z = 10.28, p < 0.00001) (Fig. 3). We found significantly evident heterogeneity across the studies (I2 = 95%, p < 0.00001) and the funnel plot showed asymmetry (Fig. 4(B)).
Fig. 3.
Forest plot for antidepressant effect of ginsenoside Rg1 on the sucrose preference test. Global effect estimate comparing sucrose preference between ginsenoside Rg1 and vehicle treatments and its subgroup analysis according to dosage.
A subgroup analysis according to dosage showed that Rg1 was significantly effective in every dosage. The pooled estimate for the MD was 9.59 (95% CI: 4.94-14.25) in the 2.5 mg/kg group, 27.39 (95% CI: 14.20-40.57) in 5 mg/kg group, 28.73 (95% CI: 15.67-41.80) in the 10 mg/kg group, 32.89 (95% CI: 17.77-48.00) in the 20 mg/kg group, 13.80 (95% CI: 11.19-16.42) in the 30 mg/kg group, and 32.90 (95% CI: 28.77-37.04) in the 40 mg/kg group. The mean difference of sucrose preference also increased in a dose-dependent manner, except in one subgroup which only included one study (Fig. 3).
4. Discussion
The aim of this study was to systemically review the antidepressant effects of various types of ginsenosides in animal models of depression. This is the first systematic review and meta-analysis of existing studies supporting the use of ginsenosides on depression in animal models. The results suggest that ginsenosides are able to improve depression-related symptoms such as helplessness, anxiety, weight gain, and insomnia by modulating a wide range of biological mechanisms such as neuroinflammation, neurotransmitter disruption, synaptic dysfunction, oxidative stress, and apoptosis. Rg1 dose-dependently improved behavioral changes related to depressive symptoms in FST and SPT.
We assessed the efficacy of ginsenosides on depression using 23 studies. All the studies were published in the 2010s. We chose the CAMARADES checklist with modifications for depression to assess the risk of bias in studies. Considering previous studies which used CAMARADES or its modified form [46,47], the score was moderate for most studies. However, none of the studies reported a blinded induction of depression and calculation of the sample size necessary to achieve sufficient power. Furthermore, no study measured baseline values of the behavioral tests. Even though the assessors in most studies were blinded to the subjects, there is still a lack of confidence that the samples were consistent across groups at initiation. The studies included did not discuss the sample size calculation necessary to achieve sufficient power; therefore, the results of the original articles may not be sufficient to ensure a therapeutic effect of ginsenosides.
The molecular and biological mechanisms of the antidepressant effects of ginsenosides are under investigation. Ginsenosides widely induced effects, from the cortex to the limbic system, including the hippocampus which was the most studied. They altered neurons, astrocytes, and microglia, which are basic elements of brain. These changes were influenced by the following mechanisms. This review showed that most studies focused on neuroinflammation by assessing BDNF, ERK, CREB, TrkB, and their phosphorylation. Immunomodulation was also detected both in the brain and serum. It is reported that ginsenosides and their metabolites/derivatives such as Rb1, Rb2, Rd, Re, Rg1, Rg3, Rg5, Rh1, Rh2, Rp1, and compound K have anti-inflammatory effects and they can be used for a diverse set of diseases which are related to inflammatory responses [6]. Even though metabolites produced after digestion were excluded from this review, their effects have been previously investigated. The intestinal metabolites of P. ginseng, 20(S)-Protopanaxadiol, 20(S)-Protopanaxatriol, and compound K decreased depressive-like behavior and was shown to have anti-inflammatory effects [15,16]. Since the most frequently used antidepressants were developed focused on regulation of hyperactivity of the hypothalamic-pituitary-adrenal-axis and changes of relevant neurotransmitter levels, it is important to investigate whether ginsenosides modulate these. Rb1 and Rg1 was able to alter serotonin, dopamine, and norepinephrine levels, while Rd and Re affected both serotonin and norepinephrine levels and Rg1 only affected serotonin levels. Compound K altered serotonin and dopamine levels [16] and 20(S)-Protopanaxadiol altered serotonin and norepinephrine levels [17]. These results confirm the potential that other protopanaxadiol type ginsenosides can modulate monoamine neurotransmitter levels. Furthermore, changes in oxidative stress, neurogenesis, and endocrinological aspect were also caused by ginsenosides.
The included studies mostly examined despair, anhedonia and abnormalities in eating behavior. The effects of ginsenosides on despair was evaluated the most, using FST and TST which are common screening tests for antidepressants [48]. Rb1, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh2, majonoside-R1, majonoside-R2 and vina-ginsenoside-R2 were shown to decrease despair [22,24,26,[28], [29], [30],[32], [33], [34],[36], [37], [38], [39], [40], [41], [42], [43]]. PFC [22,28,34,37,38,[41], [42], [43]], hippocampus [22,24,26,[28], [29], [30],40,41], amygdala [36,39], striatum [28], and locus coeruleus [40] were studied. SPT, active avoidance conditioning test, and social interaction test are comprehensively associated with anhedonia [49]. 37% of patients with depression suffer from anhedonia but serotonin reuptake inhibitors failed to fully treat anhedonia [50]. The anhedonia was consistently suggested to be related to dopaminergic pathways. Included studies which assessed dopamine did not show difference in sucrose preference after administration of Rb1, Rb3, Rd, Rd, and Re [28,31,33]. Meanwhile, Re, Rg1, Rg2, Rg3, and Rg5 showed significant difference in anhedonia-related parameters. Hippocampus [[24], [25], [26], [27],29,35,40,41], PFC [34,35,37,38,[41], [42], [43]], amygdala [36,39] and locus coeruleus [40] were the areas of interest and neuroplasticity were mostly investigated. Even though the studies did not limit to dopamine neurons, the changes in dopaminergic mechanism by these ginsenosides could be investigated further since the studied areas are known to be associated with anhedonia [51,52]. Re, Rg1, Rg2, and Rg3 influenced a change of appetite [23,24,29,35,40,43]. Collectively, Re, Rg1, Rg2, and Rg3 are thought to be able to cover diverse symptomatic dimensions of depression.
In the present analysis, Rg1 was the most studied ginsenoside. The most abundant compounds in P. ginseng are Rb3 and Rh1 in the leaves, and Rb1 and Rc in the roots [53]. Despite being an active ingredient in P. ginseng, the number of studies on Rg1 overwhelms those of other ginsenosides despite its relatively low content. Rg1 was tested in CUMS model and LPS injection model, and showed antidepressant effect on global dimension of depressive symptoms including despair [28,34,[36], [37], [38], [39],[41], [42], [43]], anhedonia [23,[34], [35], [36], [37], [38], [39],[41], [42], [43], [44]], anxiety [38,39,44], and change of appetite [23,35,43]. Sleep was solely examined in Rg1 [41]. Protective effect against neuroinflammation was investigated extensively with a variety of factors. Especially, proinflammatory cytokines were examined in PFC and hippocampus of both CUMS model and LPS injection model. Rg1 regulated 5-hydroxytryptamine [35] and its anxiolytic effect was also verified by γ-aminobutyric acid [44]. The modulatory effect of HPA and HPG axis was associated [23,41]. Rg1 was solely found to have an effect on apoptosis and synapse- or astrocyte gap junction-related alterations in the studies included in this review, which studied synapse number, spine density, synaptic plasticity. This result is consistent with an in vitro study which showed that Rg1 ameliorated dysfunction of the astrocyte gap junction induced by corticosterone in primary cortical and hippocampal astrocytes [21]. Therefore, a ginseng processing method to enhance the content of Rg1 should be developed. Research regarding the antidepressant effects and mechanisms of action of the remaining ginsenosides should be further followed.
The antidepressant effect of Rg1 was dose dependent, as evaluated in both the FST and SPT. In this study, the SPT results from some included studies were statistically insignificant while the FST results were not. Although more than half of the studies of the lower dose subgroups reported a higher mean difference that those of the 40 mg/kg subgroup, the pooled mean difference almost increased in a dose-dependent manner. The 30 mg/kg subgroup deviated from this tendency, but this is thought to be due to the small number of studies. Some studies reported insignificant results in subgroups of dose 20mg/kg and 40mg/kg. If we simply interpret the results from respective studies, it can be quite controversial regarding its effect on behavioral change as there is a criticism that FST usually reports positive results [54]. However, the pooled results of the SPT in meta-analysis were statistically significant and dose dependent as well. Considering these results, the statistical insignificance might have been caused by the small sample size of each study and could be complemented by the meta-analysis.
These values should be interpreted cautiously, however, because the differences between each strain should be considered. In the meta-analysis of FST, Sprague Dawley rats were used in the studies with the low doses (2.5, 10, 20mg/kg), and Wistar rats were used in the studies with the high dose (40mg/kg). As Wistar rats and Sprague Dawley rats did not show significant difference in immobility time of the FST in the previous study comparing the strain difference, there is low risk of bias [55]. In the meta-analysis of SPT, Sprague Dawley rats and Wistar rats were used, except one study which used C57BL/6. In the studies with the low doses (2.5, 5, 10, 20 mg/kg), one used C57BL/6 and the others used Sprague Dawley rats. The results of C57BL/6 mice showed homogeneous with those of Sprague Dawley rats. In the studies with the high doses (30, 40 mg/kg), one used Sprague Dawley rats and its result was heterogeneous with and the others which used Wistar rats. In the previous study, Sprague Dawley rats showed lower sucrose preference than Wistar rats [55]. Considering this, the lower response of Sprague Dawley rats in the dose of 40mg/kg can be attributable to the strain difference. Even though the mean differences of intervention from control were pooled, their value could be susceptible to strain difference.
The second most studied ginsenoside was Rg3 but each study adopted different models of depression, which are CSDS, CUMS, and LPS injection. Since its effect was confirmed using three different models respectively mimicking different causes like socio-environmental stressors and inflammation, Rg3 can be another promising candidate for antidepressant [48]. However, the changes were only limited to hippocampus so the changes could be investigated in other potential brain regions such as PFC and amygdala [56,57].
Although they did not meet inclusion criteria, there were some studies which explored the effect of ginsenosides on secondary depression. The antidepressant effect of Rb1 [9,58], Rg1 [9], and Ro [9] on female menopausal depression, Rg1 on male menopausal depression [41], Rb1 on post-traumatic stress disorder [59], and Rf [60] and Rg2 [61] on depressive symptoms in neuropathic pain and Rh2 on those in cancer [62] were studied. As currently used antidepressants, ginsenosides can be exploited for use in patients with a depressive state in diverse diseases as well as depressive disorder itself.
There were few clinical trials which examined the effect of ginseng or ginsenoside on depression. The effect of ginseng extract and fermented red ginseng on the depressive symptoms was not assessed in patients with primary depression, but assessed only in postmenopausal women [[63], [64], [65]] and cancer patients [66]. One study confirmed that Korean Red Ginseng could improve the sympathetic nervous system and cognition in subjects with high stress, but they excluded clinically significant depression as participants [67]. However, considering the results from subsequent preclinical studies, a clinical trial on the effects of either ginseng or single ginsenosides on primary depression are worth researching. The results of this study can be used as a reference for conducting clinical trials in the future. The dose of Rg1 administration in humans could be inferred from the results of this meta-analysis.
There are some limitations of this study. First, animal experiments which yielded neutral or negative results that went against their hypothesis are not likely to be published; therefore, the possibility of bias in the included studies remain. Second, there was statistically significant heterogeneity as measured by a significant Q statistic and high I2. Inspection of the funnel plots showed asymmetry, but this was expected given the heterogeneity of the included studies in terms of factors such as dosage, species, duration of establishing the animal model. Third, no study was blinded to the induction of depression in animal models or measured baseline values of behavioral tests. The studies did not mention calculations on the sample size necessary to achieve sufficient power. For these reasons, the results of the original articles may have a low possibility of demonstrating the therapeutic potential of ginsenosides; thus the results of this study are susceptible to these drawbacks. Fourth, this study included primary depression, and the result can differ in secondary depression as mentioned above.
In conclusion, our study provides a collective review of the preclinical studies assessing antidepressant effects of a variety of ginsenosides by appraising them with rigorous strategies. Their effects were evaluated by several behavioral tests and a number of mechanisms in different brain regions connected to mood regulation. Rg1, the most studied ginsenoside, was shown to decrease depressive-like symptoms in a dose-dependent manner in the pooled results from the meta-analysis. As depression has a high recurrence rate and long-term morbidity, an effective treatment that is stable and has a low risk of side effects during long-term intake is necessary. Ginsenosides are components of P. ginseng, which has a long therapeutic history; therefore, the results from this review can provide feasibility to the development of new drugs. Rg1 is especially a promising drug candidate for depression based on their different mechanisms of action, which has been revealed in previous bench studies, which could be used to develop therapeutics from bench to bedside.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
This work was supported by a grant from Kyung Hee University in 2016. (KHU-20161702)
References
- 1.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2013;2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; 2008. The global burden of disease: 2004 update. [Google Scholar]
- 3.Read J.R., Sharpe L., Modini M., Dear B.F. Multimorbidity and depression: a systematic review and meta-analysis. J Affect Disord. 2017;221:36–46. doi: 10.1016/j.jad.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Global data . 2015. Major depressive disorder – global drug forecast and market assessment to 2025. [Google Scholar]
- 5.Yu S.E., Mwesige B., Yi Y.-S., Yoo B.C. Ginsenosides: the need to move forward from bench to clinical trials. J Ginseng Res. 2019;43:361–367. doi: 10.1016/j.jgr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.H., Yi Y.-S., Kim M.-Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang H., Chen Y., Liu X., Wang Q., Wang L., Jia W., Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatr. 2009;33:1417–1424. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Choi J.H., Lee M.J., Jang M., Kim H.-J., Lee S., Lee S.W. Panax ginseng exerts antidepressant-like effects by suppressing neuroinflammatory response and upregulating nuclear factor erythroid 2 related factor 2 signaling in the amygdala. J Ginseng Res. 2018;42:107–115. doi: 10.1016/j.jgr.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada N., Araki H., Yoshimura H. Identification of antidepressant-like ingredients in ginseng root (Panax ginseng C.A. Meyer) using a menopausal depressive-like state in female mice: participation of 5-HT2A receptors. Psychopharmacology (Berl) 2011;216:589–599. doi: 10.1007/s00213-011-2252-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee B., Kim H., Shim I., Lee H., Hahm D.-H. Wild ginseng attenuates anxiety-and depression-like behaviors during morphine withdrawal. J Microbiol Biotechnol. 2011;21:1088–1096. doi: 10.4014/jmb.1106.06027. [DOI] [PubMed] [Google Scholar]
- 11.Macleod M.R., O’Collins T., Howells D.W., Donnan G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 12.Jiang N., Zhang B.Y., Dong L.M., Lv J.W., Lu C., Wang Q., Fan L.X., Zhang H.X., Pan R.L., Liu X.M. Antidepressant effects of dammarane sapogenins in chronic unpredictable mild stress-induced depressive mice. Phytother Res. 2018;32:1023–1029. doi: 10.1002/ptr.6040. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Luo Y., Zhang R., Guo J. Effects of ginsenosides on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor in rats exposed to chronic unpredictable mild stress. Zhongguo Zhongyao Zazhi. 2011;36:1342–1347. [PubMed] [Google Scholar]
- 14.Kim N.-H., Kim K.-Y., Jeong H.-J., Kim H.-M. Antidepressant-like effect of altered Korean red ginseng in mice. Behav Med. 2011;37:42–46. doi: 10.1080/08964289.2011.566591. [DOI] [PubMed] [Google Scholar]
- 15.Oh H.A., Kim D.-E., Choi H.J., Kim N.J., Kim D.-H. Anti-stress effects of 20(S)-Protopanaxadiol and 20(S)-Protopanaxatriol in immobilized mice. Biolog Pharmaceut Bullet. 2015;38:331–335. doi: 10.1248/bpb.b14-00669. [DOI] [PubMed] [Google Scholar]
- 16.Song W., Guo Y., Jiang S., Wei L., Liu Z., Wang X., Su Y. Antidepressant effects of the ginsenoside metabolite compound K, assessed by behavioral despair test and chronic unpredictable mild stress model. Neurochem Res. 2018;43:1371–1382. doi: 10.1007/s11064-018-2552-5. [DOI] [PubMed] [Google Scholar]
- 17.Xu C., Teng J., Chen W., Ge Q., Yang Z., Yu C., Yang Z., Jia W. 20(S)-protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Progr Neuro-Psychopharmacol Biolog Psychiatr. 2010;34:1402–1411. doi: 10.1016/j.pnpbp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Li Z., Zhou Z., Yang H., Zhong Z., Lou C. Antidepressant-like effects of ginsenosides: a comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacol Biochem Behav. 2016;140:17–26. doi: 10.1016/j.pbb.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H., Zhang H., Cui J., Liu Y., Wu R., Xiang H. Protopanaxadiol saponins in the caudexes and leaves of panax notoginseng coul d be the main constituents that contribute to its antidepressant effects. Int J Pharm Pharmaceut Sci. 2014;6:301–311. [Google Scholar]
- 20.Lee S., Rhee D.-K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic–pituitary–adrenal axis. J Ginseng Res. 2017;41:589–594. doi: 10.1016/j.jgr.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia C.Y., Chu S.F., Zhang S., Gao Y., Ren Q., Lou Y.X., Luo P., Tian M.T., Wang Z.Q., Du G.H. Ginsenoside Rg1 alleviates corticosterone-induced dysfunction of gap junctions in astrocytes. J Ethnopharmacol. 2017;208:207–213. doi: 10.1016/j.jep.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y., Lee H.-Y., Choi Y.-J., Cho S.-H. Antidepressant effects of ginsenoside Rf on behavioral change in the glial degeneration model of depression by reversing glial loss. J Ginseng Res. 2020;44:603–610. doi: 10.1016/j.jgr.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B., Xiong Z., Yang J., Wang W., Wang Y., Hu Z.L., Wang F., Chen J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol. 2012;166:1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y., Wang J.L., Zhang X., Wang H., Ye Y., Song L., Wang Y.J., Tu M.J., Wang W.W., Yang L. Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res Bullet. 2017;134:211–219. doi: 10.1016/j.brainresbull.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 25.You Z., Yao Q., Shen J., Gu Z., Xu H., Wu Z., Chen C., Li L. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J Nat Med. 2017;71:367–379. doi: 10.1007/s11418-016-1066-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Zhou Z., Chen Z., Zhong Z., Li Z. Ginsenoside Rg3 exerts anti-depressive effect on an NMDA-treated cell model and a chronic mild stress animal model. J Pharmacol Sci. 2017;134:45–54. doi: 10.1016/j.jphs.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Xu D., Wang C., Zhao W., Gao S., Cui Z. Antidepressant-like effects of ginsenoside Rg5 in mice: involving of hippocampus BDNF signaling pathway. Neurosci Lett. 2017;645:97–105. doi: 10.1016/j.neulet.2017.02.071. [DOI] [PubMed] [Google Scholar]
- 28.Yao Y., Sang W., Yang X.-s., Zhai M.-j., Wang L.-l., Qin P.-y., Wu L., Zhou X.-r., Wang L.-j., Li J.-y. Antidepressant effects of ginsenosides from panax notoginseng. J Integr Agri. 2012;11:483–488. [Google Scholar]
- 29.Kang A., Xie T., Zhu D., Shan J., Di L., Zheng X. Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J Agri Food Chem. 2017;65:6861–6869. doi: 10.1021/acs.jafc.7b02386. [DOI] [PubMed] [Google Scholar]
- 30.Chen L., Qi Z., Shao Z., Li S., Qi Y., Gao K., Liu S.X., Li Z., Sun Y.S., Li P.Y. vol. 24. Molecules; Basel, Switzerland): 2019. (Study on antidepressant activity of pseudo-ginsenoside HQ on depression-like behavior in mice). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J., Jiang L., Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012;26:697–713. doi: 10.1177/0269881111415735. [DOI] [PubMed] [Google Scholar]
- 32.Duong Q.H.T., Nguyen P.T.V., Nguyen H.T.T., Nguyen D.M. Effects of ocotillol-type saponins majonoside-R1 and vina-ginsenoside-R2 on abrogating depression and neuronal oxidative stress in socially isolated depression mouse model. Int J Appl Res Nat Prod. 2016;9:27–32. [Google Scholar]
- 33.Wang G.-L., He Z.-M., Zhu H.-Y., Gao Y.-G., Zhao Y., Yang H., Zhang L.-X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng CA Meyer. J Ethnopharmacol. 2017;204:118–124. doi: 10.1016/j.jep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X., Gao R., Liu Z., Cheng Z., Qi Y., Fan C., Yu S.Y. Ginsenoside Rg1 reverses stress-induced depression-like behaviours and brain-derived neurotrophic factor expression within the prefrontal cortex. Eur J Neurosci. 2016;44:1878–1885. doi: 10.1111/ejn.13255. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X., Liang Y., Kang A., Ma S.J., Xing L., Zhou Y.Y., Dai C., Xie H., Xie L., Wang G.J. Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats. Neuroscience. 2014;256:210–222. doi: 10.1016/j.neuroscience.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z., Qi Y., Cheng Z., Zhu X., Fan C., Yu S.Y. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience. 2016;322:358–369. doi: 10.1016/j.neuroscience.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 37.Fan C., Song Q., Wang P., Li Y., Yang M., Yu S.Y. Neuroprotective effects of ginsenoside-rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Fan C., Zhu X., Song Q., Wang P., Liu Z., Yu S.Y. MiR-134 modulates chronic stress-induced structural plasticity and depression-like behaviors via downregulation of Limk1/cofilin signaling in rats. Neuropharmacology. 2018;131:364–376. doi: 10.1016/j.neuropharm.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Yu H., Fan C., Yang L., Yu S., Song Q., Wang P., Mao X. Ginsenoside Rg1 prevents chronic stress-induced depression-like behaviors and neuronal structural plasticity in rats. Cell Physiol Biochem. 2018;48:2470–2482. doi: 10.1159/000492684. [DOI] [PubMed] [Google Scholar]
- 40.Lee B., Shim I., Lee H., Hahm D.H. Effect of ginsenoside re on depression- and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J Microbiol Biotechnol. 2012;22:708–720. doi: 10.4014/jmb.1112.12046. [DOI] [PubMed] [Google Scholar]
- 41.Mou Z., Huang Q., Chu S.F., Zhang M.J., Hu J.F., Chen N.H., Zhang J.T. Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed Pharmacother. 2017;92:962–971. doi: 10.1016/j.biopha.2017.05.119. [DOI] [PubMed] [Google Scholar]
- 42.Huang Q., Chu S.F., Zhang J.T., Chen N.-h. Effects of Ginsenoside Rg1 on anti-depression and synaptic ultrastructure. Chin Pharmacol Bull. 2013;29:1124–1127. [Google Scholar]
- 43.Jin C., Wang Z.Z., Zhou H., Lou Y.X., Chen J., Zuo W., Tian M.T., Wang Z.Q., Du G.H., Kawahata I. Ginsenoside Rg1-induced antidepressant effects involve the protection of astrocyte gap junctions within the prefrontal cortex. Progr Neuro-Psychopharmacol Biolog Psychiatr. 2017;75:183–191. doi: 10.1016/j.pnpbp.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Wu H., Zhu C., Guo J. [Effect of ginsenoside Rg1 on behaviors and hippocampal amino acids in depressive-like rats] Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J Chin Materia Medica. 2012;37:3117–3121. [PubMed] [Google Scholar]
- 45.Huang J., Huang X.N., Zhang S., Yang D.L., Wu Q., Deng J., Gao Y. Effect of total ginsenosides on the cell cycle of rat vascular smooth muscle cell proliferation induced by PDGF-BB. Chin Pharmacol Bullet. 2010;26:787–791. [Google Scholar]
- 46.Song L., Xu M.-B., Zhou X.-L., Zhang D.-p., Zhang S.-l., Zheng G.-q. A preclinical systematic review of ginsenoside-Rg1 in experimental Parkinson’s disease. Oxid Med Cell Long. 2017 doi: 10.1155/2017/2163053. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheng C., Peng W., Xia Z.-a., Wang Y., Chen Z., Su N. The impact of ginsenosides on cognitive deficits in experimental animal studies of Alzheimer’s disease: a systematic review. BMC Compl Alter Med. 2015;15:386. doi: 10.1186/s12906-015-0894-y. https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-015-0894-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Planchez B., Surget A., Belzung C. Animal models of major depression: drawbacks and challenges. J Neural Transm (Vienna) 2019;126:1383–1408. doi: 10.1007/s00702-019-02084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheggi S., De Montis M.G., Gambarana C. Making sense of rodent models of anhedonia. Int J Neuropsychopharmacol. 2018;21:1049–1065. doi: 10.1093/ijnp/pyy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelizza L., Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunlop B.W., Nemeroff C.B. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatr. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 53.Kang O.-J., Kim J.-S. Comparison of ginsenoside contents in different parts of Korean ginseng (panax ginseng C.A. Meyer) Prev Nutr Food Sci. 2016;21:389–392. doi: 10.3746/pnf.2016.21.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos-Hryb A.B., Bahor Z., McCann S., Sena E., MacLeod M.R., Lino de Oliveira C. Protocol for a systematic review and meta-analysis of data from preclinical studies employing forced swimming test: an update. BMJ Open Sci. 2019;3 doi: 10.1136/bmjos-2017-000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nam H., Clinton S., Jackson N., Kerman I. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hultman R., Mague S.D., Li Q., Katz B.M., Michel N., Lin L., Wang J., David L.K., Blount C., Chandy R. Dysregulation of prefrontal cortex-mediated slow-evolving limbic dynamics drives stress-induced emotional pathology. Neuron. 2016;91:439–452. doi: 10.1016/j.neuron.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill M.N., Hellemans K.G.C., Verma P., Gorzalka B.B., Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao K., Gong P., Sun S.Q., Hao H.P., Wang G.J., Dai Y., Liang Y., Xie L., Li F.Y. Beneficial estrogen-like effects of ginsenoside Rb1, an active component of Panax ginseng, on neural 5-HT disposition and behavioral tasks in ovariectomized mice. Eur J Pharmacol. 2011;659:15–25. doi: 10.1016/j.ejphar.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Lee B., Sur B., Cho S.-G., Yeom M., Shim I., Lee H., Hahm D.-H. Ginsenoside Rb1 rescues anxiety-like responses in a rat model of post-traumatic stress disorder. J Nat Med. 2016;70:133–144. doi: 10.1007/s11418-015-0943-3. [DOI] [PubMed] [Google Scholar]
- 60.Li Y., Chen C., Li S., Jiang C. Ginsenoside Rf relieves mechanical hypersensitivity, depression-like behavior, and inflammatory reactions in chronic constriction injury rats. Phytother Res. 2019;33:1095–1103. doi: 10.1002/ptr.6303. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q.L., Li S.Y., Li P. Effects of ginsenoside-Rg2 on mechanical allodynia, heat hyperalgeia, depressive state of rats with chronic sciatic nerve constriction injury. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chinese Journal of Applied Physiology. 2019;35:228–231. doi: 10.12047/j.cjap.5763.2019.049. [DOI] [PubMed] [Google Scholar]
- 62.Wang J., Chen Y., Dai C., Shang Y., Xie J. Ginsenoside Rh2 alleviates tumor-associated depression in a mouse model of colorectal carcinoma. Am J Transl Res. 2016;8:2189–2195. [PMC free article] [PubMed] [Google Scholar]
- 63.Lee K.J., Ji G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr Neurosci. 2014;17:7–15. doi: 10.1179/1476830513Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 64.Wiklund I.K., Mattsson L.A., Lindgren R., Limoni C. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group. Int J Clin Pharmacol Res. 1999;19:89–99. [PubMed] [Google Scholar]
- 65.Kim M.S., Lim H.J., Yang H.J., Lee M.S., Shin B.C., Ernst E. Ginseng for managing menopause symptoms: a systematic review of randomized clinical trials. J Ginseng Res. 2013;37:30–36. doi: 10.5142/jgr.2013.37.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pourmohamadi K., Ahmadzadeh A., Latifi M. Investigating the effects of oral ginseng on the cancer-related fatigue and quality of life in patients with non-metastatic cancer. Int J Hematol-Oncol Stem Cell Res. 2018;12:312–316. [PMC free article] [PubMed] [Google Scholar]
- 67.Baek J.H., Heo J.-Y., Fava M., Mischoulon D., Choi K.W., Na E.J., Cho H., Jeon H.J. Effect of Korean Red Ginseng in individuals exposed to high stress levels: a 6-week, double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2019;43:402–407. doi: 10.1016/j.jgr.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]