Abstract
Background
Panax ginseng is an important crop in Asian countries given its pharmaceutical uses. It is usually harvested after 4–6 years of cultivation. However, various abiotic stresses have led to its quality reduction. One of the stress causes is high content of heavy metal in ginseng cultivation area. Plant growth–promoting rhizobacteria (PGPR) can play a role in healthy growth of plants. It has been considered as a new trend for supporting the growth of many crops in heavy metal occupied areas, such as Aluminum (Al).
Methods
In vitro screening of the plant growth promoting activities of five tested strains were detected. Surface-disinfected 2-year-old ginseng seedlings were dipping in Rhizobium panacihumi DCY116T suspensions for 15 min and cultured in pots for investigating Al resistance of P. ginseng. The harvesting was carried out 10 days after Al treatment. We then examined H2O2, proline, total soluble sugar, and total phenolic contents. We also checked the expressions of related genes (PgCAT, PgAPX, and PgP5CS) of reactive oxygen species scavenging response and pyrroline-5-carboxylate synthetase by reverse transcription polymerase chain reaction (RT-PCR) method.
Results
Among five tested strains isolated from ginseng-cultivated soil, R. panacihumi DCY116T was chosen as the potential PGPR candidate for further study. Ginseng seedlings treated with R. panacihumi DCY116T produced higher biomass, proline, total phenolic, total soluble sugar contents, and related gene expressions but decreased H2O2 level than nonbacterized Al-stressed seedlings.
Conclusion
R. panacihumi DCY116T can be used as potential PGPR and “plant strengthener” for future cultivation of ginseng or other crops/plants that are grown in regions with heavy metal exposure.
Keywords: Aluminum resistance, Aluminum stress, Panax ginseng, Plant growth–promoting rhizobacteria, Rhizobium panacihumi
1. Introduction
Panax ginseng is one of the most valuable oriental herbs and popular medicinal plants in Asian countries. Ginsenoside is considered to be one of the most important secondary metabolites of ginseng [1]. Ginsenoside has multiple pharmaceutical applications, including its roles in improving blood circulation, anticancer activity, antiinflammatory activity, and antiaging properties [2]. In general, producing good quality ginseng root requires 4 to 6 years of cultivation. Long-term monoculture of ginseng increased the prevalence of diseases, which subsequently reduced the yield by up to 30-60% during the first cultivation [3]. Cultivated ginseng is well known to be very vulnerable to abiotic stresses (such as salt, drought, and heavy metal stress). These stressors reduce the ginseng quality over its 4-6 years of cultivation [4]. One particular abiotic stress that affects ginseng cultivation is Aluminum (Al) exposure.
In many lands of the world, agricultural soils are contaminated with heavy metals that pose a serious health hazard to humans, animals, plants, and soil microorganisms [5]. Al is a heavy metal that is known to inhibit plant growth [6]. Plants have evolved in a soil environment where the roots may be exposed to high levels of Al. Total Al concentration in the soil and the speciation of Al depend on the pH and the solution's chemical environment [7]. Fortunately, phytotoxic forms of Al are relatively insoluble in alkaline, neutral, or mild acidic soil. At low concentrations, Al has several beneficial effects including stimulating plant growth, promoting nutrient uptake, and increasing metabolism [8]. However, at soil pH values ≤5, the rhizotoxic Al species, Al3+, is soluble in the soil solution. Once solubilized, the concentration of Al increases, which can have toxic effects on plants, including inhibition of root growth and water/nutrient uptake, which ultimately results in reduced crop yields [9]. Therefore, phytotoxic forms of Al may stimulate the formation of free radicals and reactive oxygen species (ROS), resulting in oxidative stress [10]. Proline and some antioxidant chemicals (such as phenolics and sugars) in plants are important indicators of a plant's defense against Al stress and are considered to have important protective roles [11].
Plant growth–promoting rhizobacteria (PGPR) were first defined by Kloepper in 1978 [12]. These bacteria were studied in the evaluation of particular soil bacteria that colonize plant roots (after inoculation onto the seed) and enhance growth by direct and indirect mechanisms [13,14]. Direct promotions between PGPR and their plant host include the following mechanisms: production of 1-aminocyclopropane-1-carboxylate deaminase and plant growth regulators (auxins, gibberellins, and cytokines) and nitrogen fixation and solubilization of mineral-like phosphorus [15]. Indirect promotion is related to the plant defense responses against biotic and abiotic stressors [16]. The following are genera of plant growth–promoting bacteria: Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Flavobacterium, Micrococcous, Paenibacillus, Pseudomonas, and some members of the family Rhizobiaceae including Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, and Rhizobium [17,18]. Such selective microbial isolates are called PGPRs that compose a group of bacteria present in the rhizosphere. These microbes can minimize abiotic stress by inducing the production of antioxidant compounds/ROS scavenging enzymes and hormones and metabolites that play important roles in reducing the adverse effects of abiotic stresses [19]. Some studies indicated that PGPRs can play a role in plant health and growth. They have been considered a new trend for supporting the growth of many crops in areas of abiotic stress, including salt, drought, or heavy metal stress [20,21]. To the best of our knowledge, very few studies have examined P. ginseng Al resistance by PGPRs. In this study, PGPRs were isolated from ginseng-cultivated soil via in vitro screening of the plant growth–promoting activities. Ultimately, this study sought to use PGPRs to enhance ginseng seedling resistance to Al stress.
2. Materials and methods
2.1. Isolation and molecular characterization
Five different ginseng soil samples in Yeoncheon (38° 04′ 00″ N 126° 57′ 00″ E) and Gochang (35° 26′ 89″ N 126° 42′ 740″ E) County, Korea, were obtained. The bacteria strains from those soil samples were isolated using trypticase soy agar (TSA), nutrient agar (NA), or yeast mannitol agar (YMA) medium. Several purified isolates' genomic DNA was extracted and purified using GeneAll Exgene Clinic SV (Gene All Biotechnology, Korea). The 16S rRNA gene sequences were amplified using the previously described methods of Lane [22] and Weisburg et al. [23] via GenoTech (Daejeon, Korea). The 16S rRNA gene sequence was compiled using Seq-Man software version 4.1 (DNASTAR, Inc.). These sequences were compared with 16S rRNA gene sequences available in the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the EzTaxon-e server (http://eztaxon-e.ezbiocloud.net).
2.2. In vitro evaluation of plant growth promotion
In vitro indole-3-acetic acid (IAA) production test was performed using King B with and without additional L-tryptophan (3 mg/mL), as previously described [24]. After 6 days of incubation, a colorimetric analysis (Salkowski reagent: 1 ml (v/v) of 0.5 M ferric chloride solution to 50 ml of 35% perchloric acid) was used to measure the production of IAA. The IAA concentration was calculated using a standard curve prepared with IAA as the standard. For a siderophore production activity test, Pseudomonas agar F medium [25] was mixed with chrome azurol S following methods described by Schwyn and Neilands [26]. The siderophore activity can be determined by observing the appearance of yellow zone surrounding colonies in the blue-green–colored medium. The phosphate solubilizing ability test was performed using the plate screening methods as described by Pikovskaya [27]. The solubilization of phosphate test demonstrated positive results from the appearance of a clear region around the colonies in the Pikovskaya medium.
2.3. Determination of heavy metal resistant bacteria
The ability of five strains to resist heavy metal was established according to the following description. Six analytical grade salts (CdCl2·2.5H2O, CuSO4·5H2O, CoCl2·6H2O, HgCl2, FeCl3, and AlCl3·6H2O) were selected as described previously [4,28]. From an overnight grown culture of a single colony, 1% (v/v) was transferred to 5 mL of nutrient broth (NB; MB cell) media supplemented with each different concentration (from 25 μM to 150 μM) of metal. All of the strains were cultured in the same media without the addition of metals, as a control. The resistance was measured on the basis of growth observed (at 600nm) every 6 h until 54 h culture. If growth was observed, the inoculum was added to the media with increasing concentration (until 50mM) of selected metals. The inhibitory concentrations were measured using a spectrophotometer at an absorbance of 600nm against a NB broth (blank) containing the same amount of heavy metal [29]. The resistance of the bacteria isolated from ginseng-cultivated soil was evaluated according to its minimum inhibitory concentration (MIC) and the inhibitory concentration at 50% values (IC50 values) based on all test concentration results [30].
2.4. Compatibility test with P. ginseng
Two-year-old P. ginseng roots were surface-disinfected with 70% EtOH for 5 min, 2% NaOCl for 5 min, and rinsed twice with sterile distilled water (SDW). Twenty-four hours–cultured DCY116T suspensions were centrifuged at 10000 rpm/min for 5 min and resuspended in the sterilized 0.85% NaCl solution after twice washing for further inoculation. A ginseng pot assay was started by dipping the ginseng roots (purchased from a private field during February 2018) into different bacterial suspensions of various ODs (indicating variation in CFUs/ml) for 15 min. Then ginseng seedlings were cultivated on sterilized artificial soils (vermiculite:perlite:peat moss = 3:1:1), with additional sterilized tap water [25% (v/v)] in pots (11 cm high and 11 cm diameter). Each pot contained five roots. Each treatment was replicated on three pots. The photoperiod was adjusted to 16h of daytime and 8h of nighttime with cool white fluorescent lamps (Philips TLD-RS-FLR32SSEX-D 865K, 32 W) equal to 9500 lux for each covered area. The temperature was controlled at 25 ± 2 oC with moisture levels maintained at 60 ± 5%. The plants were watered once weekly with sterilized tap water, which was applied to the bottom of the pots. After 10 days of bacteria inoculation, sampling and morphological observations were conducted between inoculated seedlings and control seedlings.
2.5. Assessment of the Al tolerance level of P. ginseng
Based on a previous report [4], several concentrations of Al2(SO4)3 solutions (250 mM, 500 mM, and 1000 mM) were given by watering the ginseng from top of the soil when the roots sprouted (after 10 days). As a control, SDW was given instead of the Al2 (SO4)3 solution. After 10 days of Al stress application, the roots were harvested, and the morphological appearance was recorded. Each treatment was replicated three times.
2.6. R. panacihumi DCY116T application for inducing Al resistance of P. ginseng
According to previous optimization, strain DCY116T that successfully enhanced ginseng growth (to up to 108 CFU/mL) was selected for P. ginseng treatment. For Al stress treatment, the 500 mM Al concentration was selected. Twenty-four hours–cultured bacterial suspension of DCY116T was centrifuged and resuspended in the sterilized 0.85% NaCl solution after twice washing. Seedlings were inoculated with bacteria suspension or SDW as control for 10 min (1mL/one root). The ginseng pot assay is described previously. After 10 days of bacterial inoculation, Al stress was conducted by watering P. ginseng plants with 500 mM Al2 (SO4)3 solution. The harvesting was carried out 10 days after Al stress application. There were four different types of treatments as follows: control (no inoculation and no Al stress), bacteria treatment (DCY116T inoculation with no given Al stress), Al stress treatment (no inoculant with Al stress), and bacteria + Al stress treatment (DCY116T inoculation with given Al stress). Each treatment was replicated three times. On the day of sampling, some ginseng seedlings were sampled by separating the shoots and roots. After sampling, the seedlings were immediately frozen with liquid nitrogen and stored at −70 oC in a deep freezer until RNA isolation was performed. Another kind of sample was for proline and H2O2 determination. These measurements require appropriate buffer rinsing or solvent extraction on fresh roots or shoots. After morphological observation, measurements regarding other growth parameters were made. To make other measurements, such as the dry weight of the samples or the total phenolic and total soluble sugar (TSS) measurements, the ginseng samples must be dried at 50oC to obtain a constant weight for biomass determination.
2.6.1. RNA extraction and reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from frozen samples using TRI Reagent® (1 mL/50-100 mg tissue, Molecular Research Center, Inc, USA) according to the manufacturer's instructions. One microgram of extracted RNA from each sample was reverse transcribed with RevertAid™ H Minus M-MuLV Reverse Transcriptase (200 U/µL) (Fermentas, USA) according to the manufacturer's instructions. RT-PCR was performed in a reaction volume of 15 μL consisting of 2 μL of the synthesized cDNA, 10 pmol of forward and reverse primers of each target gene (Table 1), and 7.5 of RbTaq™ PCR 2X PreMIX (SYBR Green with high ROX) (Life Technologies, India) and water up to the final volume using MyCycler™ thermal cycler PCR machine (BioRad, USA). The reaction was started by initial denaturation at 95°C for 5 min, followed by 30 cycles at 95°C for 45 s, 56-62°C for 60 s, 72°C for 90 s and final elongation at 72°C for 5 min. The PCR products were applied on 1.5% agarose gel for visual analysis. The housekeeping gene, GAPDH (glyceraldehyde 3-phosphate dehydrogenase), was used as a control, as recommended by previous reports [31].
Table 1.
List of the RT-PCR primers in this study
| Primer | Sequence (5′- 3′) | Annealing temperature (°C) |
|---|---|---|
| Primers of pyrroline-5-carboxylate synthetase related genes | ||
| PgP5CS-F | TTTTGAGTCGCGTCCAGAA | 62 |
| PgP5CS-R | GCCAACACTTTTCGGGATAG | |
| Primers of ROS-scavenging system–related genes | ||
| PgCAT-F | GAGCGTGGAAGTCCTGAGAC | 62 |
| PgCAT-R | AATGTGAGACTTCGGGTTGG | |
| PgAPX-F | ATGGGAAAGTGCTACCCG | 60 |
| PgAPX-R | TGAACATGCTCACCCTTAATTCT | |
| Primers of expression patterns of housekeeping gene | ||
| PgGAPDH-F | GAGAAGGAATACACACCTGACC | 56 |
| PgGAPDH-R | CAGTAGTCATAAGCCCCTCAAC | |
RT-PCR, reverse transcription polymerase chain reaction; ROS, reactive oxygen species.
2.6.2. Plant growth parameter measurement
H2O2 content was detected using the protocol of Alexieva et al. [32] with trichloroacetic acid and calculated using a standard curve with a concentration ranging from 0.05 to 0.1 mM. Proline extraction was performed by using 50 times diluted fresh weight (w/v) in 70:30 ethanol:water (v/v). The extract was mixed with a reaction mix [ninhydrin 1% (w/v) in ethanol: acetic acid: water = 20:60:20 (v/v)] with equal volume under light-protected conditions. The reaction mixture was placed in a 95°C water bath for 20 min. After cooling to room temperature, the mixture was spun down for 1 min at 2500 rpm. The mixture was then processed according to the absorbance read at 520 nm. Proline solutions ranging from 0.04 to 1 mM were prepared in the same medium as the one that was used for the extraction [33]. The TSS content of the dried plant material was performed using Irigoyen's method [34]. Briefly, 0.5g of dried plant material was treated in a boiling water bath for 20 min. After centrifugation at 5000 rpm for 10 min, the supernatant was collected. One milliliter of the supernatant was mixed with 5 mL anthrone reagent and allowed to react in boiling water. The optical density of the mixture was measured at 625 nm. At first, the standard curve was made using glucose at various concentrations. Total phenolic content was determined using the Folin–Ciocalteu reagent method [35]. The total phenolic content was calculated from a calibration curve using gallic acid as a standard.
2.7. Statistical analysis
All data are shown as the means ± standard deviation from three replicates. Statistical analysis was performed using SPSS Software (IBM SPSS Statistics, SPSS inc., V18.0). Analysis of variance was used to test for significance, and significant differences (p < 0.05) between treatments were determined using the Tukey test. Different letters are used to indicate significantly different means.
3. Results and discussion
3.1. Molecular identification of the bacteria
The following five PGPR candidate strains were isolated from ginseng-cultivated soil: DCY87T, DCY104T, DCY113T, DCY114T, and DCY116T. Based on 16S rRNA gene sequence analysis, the isolates belonged to the following genera: Phycicoccus, Paralcaligenes, Paraburkholderia, Paenibacillus, and Rhizobium (Table 2).
Table 2.
In vitro assessment of plant growth–promoting traits of the novel strains and reference-type strains, All data were from this study, +, positive; -, negative
| Bacteria name | IAA concentration (mg/L) |
Siderophore Production | Phosphate Solubilization | |
|---|---|---|---|---|
| King B without L-tryptophan | King B with L-tryptophan | |||
| Phycicoccus ginsengisoli DCY87T | 23.3 ± 1.25 | 24.7 ± 2.66 | + | + |
| Paralcaligenes ginsengisoli DCY104T | - | 0.1 ± 1.79 | + | - |
| Paraburkholderia panacisoli DCY113T | - | 0.3 ± 3.66 | ++ | + |
| Paenibacillus panacihumi DCY114T | 27.1 ± 2.55 | 42.4 ± 2.55 | + | - |
| Rhizobium panacihumi DCY116T | 28.0 ± 1.54 | 56.5 ± 1.56 | ++ | + |
IAA, indole-3-acetic acid
3.2. In vitro evaluation of plant growth promotion
Plant growth promotion properties were assessed in vitro including their IAA production, siderophore production, and phosphate solubilization (Table 2). In the case of IAA production assay, negative results were seen for Paralcaligenes ginsengisoli DCY104T and Paraburkholderia panacisoli DCY113T. These results indicated that the isolates did not have the ability to produce IAA. Most strains produced lower IAA in the absence of L-tryptophan (L-tryptophan is generally considered as an IAA precursor and enhances bacterial IAA biosynthesis activity) than they did in the presence of L-tryptophan. Based on the results in the presence of L-tryptophan, among all tested isolates, the highest IAA production was detected in Rhizobium panacihumi DCY116T (56.5 ± 1.56 mg/L), followed by Paenibacillus panacihumi DCY114T (42.4 ± 2.55 mg/L) and Phycicoccus ginsengisoli DCY87T (24.7 ± 2.66 mg/L). The siderophore production by those isolates was determined by a yellow zone surrounding colonies in the blue-green–colored medium. All of the isolates were capable of siderophore production. In particular, Paraburkholderia panacisoli DCY113T and Rhizobium panacihumi DCY116T were recorded as strong producers (Table 2). With regard to phosphate solubilization, three isolates demonstrated the appearance of clear zone after 7 days of incubation, except Paralcaligenes ginsengisoli DCY104T and P. panacihumi DCY114T (Table 2).
3.3. Determination of heavy metal resistant bacteria
The microbial turbidity decreased with increased concentration of heavy metals. The bacteria in this study demonstrated different ranges of heavy metal resistance using various concentrations of each metal. Based on the MIC values (Table 3) for each bacterium of six selected heavy metals, all strains had complete inhibition at 32mM concentration. The heavy metal resistance of all tested bacteria in decreasing order was Al > Fe > Cu > Cd > Co > Hg. These results were supported by previously described findings [29,36]. The strains DCY113T and DCY116T had higher heavy metal resistance than did other strains, according to the MICs results. Subsequently, the IC50 values of each bacterium were evaluated (Table 3). Among all of the tested strains isolated from ginseng-cultivated soil, DCY116T was considered to have the highest resistance at the 50% inhibitory concentration of 288.1 ± 5.49 μM in Cd, 2.9 ± 0.02 mM in Cu, 76.3 ± 0.58 μM in Co, 72.4 ± 0.08 μM in Hg, 10.6 ± 0.02 mM in Fe, and 8.2 ± 0.06 mM in Al. Resistance to heavy metals was correlated with siderophore production [37]. DCY113T and DCY116T in the present study were higher siderophore producers than the other strains. Therefore, we suspect that resistance to heavy metals is related to siderophore production.
Table 3.
In vitro assessment of heavy metal resistance of bacteria isolated from ginseng-cultivated soil
| Strain | Cd |
Cu |
Co |
Hg |
Fe |
Al |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | |
| DCY87T | 50 μM | 5.2 ± 1.02 μM | 4 mM | 2.5 ± 0.05 mM | 200 μM | 39.7 ± 0.18 μM | 50 μM | 30.2 ± 0.38 μM | 0.5 mM | 0.1 ± 0.02 mM | 0.5 mM | 0.1 ± 0.01 mM |
| DCY104T | 200 μM | 51.3 ± 2.29 μM | 2 mM | 2.7 ± 0.02 mM | 100 μM | 46.9 ± 0.89 μM | 50 μM | 7.7 ± 0.47 μM | 4 mM | 4.0 ± 0.02 mM | 16 mM | 0.5 ± 0.04 mM |
| DCY113T | 500 μM | 242.4 ± 3.09 μM | 4 mM | 2.6 ± 0.03 mM | 300 μM | 65.8 ± 1.07 μM | 25 μM | 0.5 ± 0.02 μM | 32 mM | 9.2 ± 0.08 mM | 32 mM | 5.9 ± 0.08 mM |
| DCY114T | 300 μM | 114.6 ± 4.01 μM | 2 mM | 1.03 ± 0.01 mM | 200 μM | 59.3 ± 0.46 μM | 25 μM | 1.9 ± 0.39 μM | 16 mM | 7.4 ± 0.10 mM | 16 mM | 3.8 ± 0.03 mM |
| DCY116T | 1000 μM | 288.1 ± 3.58 μM | 4 mM | 2.9 ± 0.02 mM | 150 μM | 76.3 ± 0.58 μM | 150 μM | 72.4 ± 0.08 μM | 32 mM | 10.6 ± 0.02 mM | 32 mM | 8.2 ± 0.06 mM |
MIC: Minimum inhibitory concentration; IC50: inhibitory concentration at 50%. The minimal inhibitory concentration (MIC) is defined as the lowest concentration that causes no visible growth. The IC50 value was expressed as the mean ± standard deviation of three independent experiments
3.4. Compatibility of R. panacihumi DCY116T with P. ginseng
The compatibility of strain DCY116T with P. ginseng using a pot assay was observed by morphological alterations after 10 days of bacterial inoculation (Fig. 1a). The root rot symptoms did not appear during the pot test. However, the root did expose some stress symptoms in the 1012 CFU/mL treatment. After sampling, the root and shoot parts were divided by fresh and dry conditions, and the biomass (fresh and dry weight) was detected (Fig. 1b). The 108 CFU/mL DCY116T strain was found to be have the largest weight and to be the best grown ginseng. For this reason, 108 CFU/mL DCY116T was selected for further experiments.
Fig. 1.
In planta compatibility testing of DCY116T on P. ginseng and biomass of P. ginseng seedlings by DCY116T using various CFU/mL inoculants. (A) Morphological appearance. (B) Fresh weight. (C) Dry weight. Error bars represent the standard deviation (n = 3). White scale bar indicates 2 cm.
3.5. Assessment of the aluminum tolerance level of P. ginseng
The morphological appearances of different Al-treated (0, 250, 500, and 1000 mM) ginseng seedlings given by watering from top of soil are shown in Fig. S1. Ginseng seedling growth was fully inhibited when they were exposed to 1000 mM Al (via watering). With this exposure, root rusty symptoms appeared. Ginseng seedlings that were exposed to <500 and 1000 mM Al stress gradually developed yellowing on the leaves and eventually complete wilting of the foliage. In contrast, leaves of the control (treated SDW) group and from the 250 mM Al stress ground remained green for the same period of time. Therefore, 500 mM Al stress given by watering was found to be enough Al exposure to damage ginseng seedlings and was, therefore, selected for further study. Farh et al. [4] reported that 1M Al solution treatment inhibited gingsing seedling growth and significantly decreased the dry weight and the number of fine roots. These effects were more pronounced than those when the same exposures were applied to mock seedlings' roots. Based on these results and those of previous reports, 500 mM Al was selected and used for further study.
3.6. R. panacihumi DCY116T application for inducing P. ginseng aluminum resistance
The morphological appearance of seedling growth after sampling is shown in Fig. 2A. To determine the disease severity index (DSI), all of the roots were rated using the scale described in Table S1 and categorized into six grades. The DSI was normalized for each isolate using the following equation:DSI=[(X1 × 1) +(X2 × 2)+ (X3 × 3)+ (X4 × 4)+(X5 × 5) + (X6 × 6)]/(X1 + X2 + X3 + X4 + X5 + X6) where X1, X2, X3, X4, X5, and X6 are the number of plants with disease severity scales of 1, 2, 3, 4, 5, and 6, respectively. Based on the described disease scales in Table S1, the DSI of each group was calculated and exhibited in Fig. 2B. As expected, Al stress inhibited the growth of two-year-old ginseng seedlings. The seedlings were protected against Al stress by treatment with DCY116T. These results confirmed that DCY116T promotes ginseng growth and protects the plants against Al stress. In addition, shoot and root biomass (fresh and dry weight) was detected and is shown in Fig. 3. Under nonstressful conditions, the groups that were treated with DCY116T had higher seedling biomass than did the control groups (noninoculated, nonstress). This result suggests that DCY116T promoted ginseng growth. The fresh and dry weight of the shoots and roots were decreased by Al stress. However, this biomass improved under Al stress when the seedlings were treated with DCY116T. Therefore, strain DCY116T prevented biomass decrease by Al stress and ensured seedling growth.
Fig. 2.
Al stress treatment to P. ginseng preinoculated with or without DCY116T. (A) Morphological appearance, scale: 2cm. (B) Severity scale. Values represent the means ± SDs from three independent experiments. Analysis of variance (ANOVA) was used to test for significance, and significant differences (p < 0.05) between treatments were determined using the Tukey test. Different letters are used to indicate significantly different means. SD, standard deviation.
Fig. 3.
Root and shoot biomass under Al stress treatment to P. ginseng. (A) Fresh weight. (B) Dry weight.
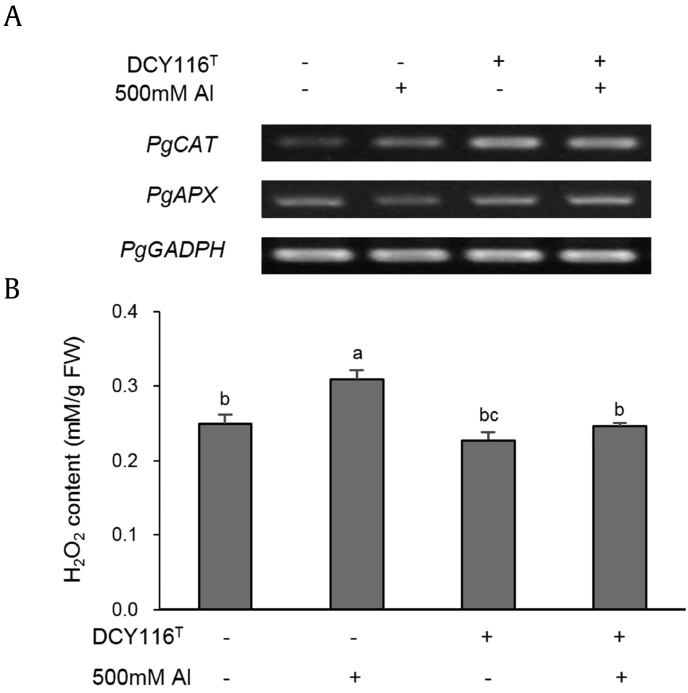
In the only Al stress treatment group, H2O2 content was higher than that in the control groups. This content reflects oxidative stress in the seedlings (Fig. 4). Previous reports have indicated that ROS can cause irreversible damage to growing tissues, in part because of Al-induced metabolic changes [38]. Therefore, Al stress can increase H2O2 level and inhibit seedling growth. However, DCY116T exhibited a significant decrease in the H2O2 production under Al stress compared with that in the only Al stress–treated group (Fig. 4B). This result suggests that DCY116T reduced oxidative stress in the seedlings and showed its protective potential. The transcription of antioxidant genes, including PgAPX and PgCAT (Fig. 4A), was also found to increase significantly in the DCY116T-treated groups under Al stress compared with that of the group treated with Al stress only. Transcription level reflects that the ginseng seedlings had increased antioxidant activities. Higher expression of ROS-scavenging system–related genes usually enhances Al resistance, which suggests that plants reduced Al-induced ROS damage to induce Al resistance [39]. These results suggest that the induction of a low amount of H2O2 by DCY116T and ROS scavenging activity as defense against oxidative stress enhanced Al resistance.
Fig. 4.
Antioxidant activities induced by DCY116T against Al stress on P. ginseng. (A) Expression of their ROS-scavenging system–related genes. (B) H2O2 content. Values represent the means ± SDs from three independent experiments. Analysis of variance (ANOVA) was used to test for significance, and significant differences (p < 0.05) between treatments were determined using the Tukey test. Different letters are used to indicate significantly different means. FW, fresh weight; SD, standard deviation; ROS, reactive oxygen species.
The proline analysis from each group is shown in Fig. 5. The proline content was higher in samples that were inoculated with DCY116T under Al stress than it was in the other groups (Fig. 5B). Meanwhile, the expression of the related gene PgP5CS was also higher than that in other groups (Fig. 5A). These results suggest that DCY116T induced proline production to strengthen the antioxidant activity against Al-induced oxidative stress and then protected the seedlings against Al stress. Some reports have suggested that an increasing accumulation of proline can reduce stress-related damage to plant cells [40]. Proline also decreases the amount of free radicals and ROS [41]. In this study, we show that proline accumulation triggered by DCY116T enhanced ROS scavenging activity against Al stress.
Fig. 5.
Proline level of seedlings induced by DCY116T under Al stress. (A) Expression of proline synthesis–related genes. (B) Proline content. Values represent the means ± SDs from three independent experiments. Analysis of variance (ANOVA) was used to test for significance, and significant differences (p < 0.05) between treatments were determined using the Tukey test. Different letters are used to indicate significantly different means. FW, fresh weight; SD, standard deviation.
Analysis of total phenolic content was evaluated and is shown in Fig. 6A and B. In nonstressful conditions, the DCY116T-treated group had significantly higher total phenolic content than the control group. This group also showed greater seedling growth than the control group. After exposure to Al stress, there was a significant decrease in total phenolic content. However, inoculation by DCY116T increased total phenolic content in Al-stressed seedlings than in noninoculated seedlings. However, there was no significant difference between control seedlings (nonstress, noninoculation). A number of studies have shown that the degree of oxidative cellular damage in plants exposed to abiotic or biotic stress is controlled by their antioxidative systems. Phenolic content is one of the primary components of the antioxidant system [42]. In this study, DCY116T helped to activate the antioxidant system by increasing the total phenolic contents, and ultimately enhanced seedling resistance to Al.
Fig. 6.
Estimation of the total phenolic (TP) and total soluble sugar (TSS) contents. (A) TP content of shoot. (B) TP content of root. (C) TSS conetnt of shoot. (D) TSS content of root. Values represent the means ± SDs from three independent experiments. Analysis of variance (ANOVA) was used to test for significance, and significant differences (p < 0.05) between treatments were determined using the Tukey test. Different letters are used to indicate significantly different means. SD, standard deviation.
The content of TSSs was detected and is shown in Fig. 6C and D. Seedlings that were inoculated with DCY116T and exposed to Al could increase their soluble sugar content better than noninoculated Al stress seedlings. Soluble sugar content in plants also played an important role when plants were under abiotic stress [43]. Soluble sugars are key osmolytes contributing toward osmotic adjustment. Soluble sugar accumulation enhanced proline content and also reduced oxidative damage under abiotic stress [[44], [45], [46], [47]]. Therefore, results in this study indicated that DCY116T increased the content of soluble sugars to decrease ROS accumulation and reduce Al toxicity of ginseng seedlings, which suggest DCY116T is a potential plant growth–promoting bacterium to resist Al toxicity and promote ginseng growth.
4. Conclusion
We isolated five PGPR candidate strains from ginseng-cultivated soil in Yeoncheon and Gochang County, Korea. Among these isolates, R. panacihumi DCY116T was chosen for further study given its growth-promoting activities and ability to tolerate heavy metal exposure. We found that Al-500 mM was sufficient to induce Al stress in two-year-old ginseng seedlings. Incubation with 108 CFU/mL DCY116T for 15 minutes can be used to prime ginseng seedlings against Al stress. DCY116T increased proline, phenolic, and sugar contents to induce ROS scavenging activity in Al-stressed seedlings. It also induced higher expression of ROS scavenging genes, which prevent oxidative stress and promote seedling growth. In conclusion, DCY116T can be used to prime ginseng seedlings and enhance Al resistance. Based on these results, strain DCY116T can be used as a potential plant growth–promoting bacterium and “plant strengthener” for future cultivation of ginseng or other crops/plants that are grown in regions with heavy metal exposure.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was supported by a grant from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (KIPET NO: 317007-3), Korea.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.01.001.
Contributor Information
Jong-Pyo Kang, Email: kangjongpyo@naver.com.
Deok-Chun Yang, Email: dcyang@khu.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wu L., Jin Y., Yin C., Bai L. Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C–K and F1 by Cladosporium cladosporioides. J Ind Microbiol Biotechnol. 2012;39:521–527. doi: 10.1007/s10295-011-1058-9. [DOI] [PubMed] [Google Scholar]
- 2.Keum Y.S., Park K.K., Lee J.M., Chun K.S., Park J.H., Lee S.K., Kwon H., Surh Y.J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.S., Lee M.S., Yeom J.H., Song J.G., Lee I.K., Yeo W.H., Yun B.S. Screening of antagonistic bacteria for biological control of ginseng root rot. Kor J Mycol. 2012;40:44–48. [Google Scholar]
- 4.Farh M.E.A., Kim Y.J., Sukweenadhi J., Singh P., Yang D.C. Aluminium resistant, plant growth promoting bacteria induce overexpression of Aluminium stress related genes in Arabidopsis thaliana and increase the ginseng tolerance against Aluminium stress. Microbiol Res. 2017;200:45–52. doi: 10.1016/j.micres.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Ghnaya A.B., Hourmant A., Cerantola S., Kervarec N., Cabon J.Y., Branchard M., Charles G. Influence of zinc on soluble carbohydrate and free amino acid levels in rapeseed plants regenerated in vitro in the presence of zinc. Plant Cell Tiss Org. 2010;102:191–197. [Google Scholar]
- 6.Emamverdian A., Ding Y., Mokhberdoran F., Xie Y. Heavy metal stress and some mechanisms of plant defense response. Sci World J. 2015:756120. doi: 10.1155/2015/756120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisnierienė V., Lapeikaitė I. When chemistry meets biology: the case of aluminium–a review. Chemija. 2015;26:148–158. [Google Scholar]
- 8.Bojórquez-Quintal E., Escalante-Magaña C., Echevarría-Machado I., Martínez-Estévez M. Aluminum, a friend or foe of higher plants in acid soils. Front Plant Sci. 2017;8:1767. doi: 10.3389/fpls.2017.01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezaki B., Suzuki M., Motoda H., Kawamura M., Nakashima S., Matsumoto H. Mechanism of gene expression of Arabidopsis glutathione S-transferase, AtGST1, and AtGST11 in response to aluminum stress. Plant Physiol. 2004;134:1672–1682. doi: 10.1104/pp.103.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz K.J., Baier M., Krämer U. Heavy metal stress in plants. Springer; 1999. Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants; pp. 73–97. [Google Scholar]
- 11.Zengin F.K., Munzuroglu O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov Ser Bot. 2005;47:157–164. [Google Scholar]
- 12.Kloepper J.W. Plant growth-promoting rhizobacteria on radishes. Proc. of the 4th Internet. Proceedings of the 4th International Conference on Plant Pathogenic Bacteria. 1978;2:879–882. [Google Scholar]
- 13.Bashan Y., Holguin G., De-Bashan L.E. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997-2003) Can J Microbiol. 2004;50:521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- 14.Yang J., Kloepper J.W., Ryu C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Forchetti G., Masciarelli O., Alemano S., Alvarez D., Abdala G. Endophytic bacteria in sunflower (Helianthus annuus L.): isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl Microbiol Biotechnol. 2007;76:1145–1152. doi: 10.1007/s00253-007-1077-7. [DOI] [PubMed] [Google Scholar]
- 16.Dimkpa C., Merten D., Svatoš A., Büchel G., Kothe E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol. 2009;107:1687–1696. doi: 10.1111/j.1365-2672.2009.04355.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayat R., Ali S., Amara U., Khalid R., Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60:579–598. [Google Scholar]
- 18.Ahemad M., Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26:1–20. [Google Scholar]
- 19.de Oliveira D.M., de Lima A.L.A., Diniz N.B., Santos C., da Silva S.L.F., Simões A. Inoculation of plant-growth-promoting rhizobacteria in Myracrodruon urundeuva Allemão supports in tolerance to drought stress. J Plant Interact. 2018;13:91–99. [Google Scholar]
- 20.Kohler J., Hernández J.A., Caravaca F., Roldán A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol. 2008;35:141–151. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- 21.Sukweenadhi J., Kim Y.J., Choi E.S., Koh S.C., Lee S.W., Kim Y.J., Yang D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol Res. 2015;172:7–15. doi: 10.1016/j.micres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic acid techniques in bacterial systematics. Wiley; Chichester: 1991. pp. 115–176. [Google Scholar]
- 23.Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shokri D., Emtiazi G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr Microbiol. 2010;61:217–225. doi: 10.1007/s00284-010-9600-y. [DOI] [PubMed] [Google Scholar]
- 25.Glickmann E., Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwyn B., Neilands J. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 27.Pikovskaya R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 28.Gupta K., Chatterjee C., Gupta B. Isolation and characterization of heavy metal tolerant Gram-positive bacteria with bioremedial properties from municipal waste rich soil of Kestopur canal (Kolkata), West Bengal, India. Biologia. 2012;67:827–836. [Google Scholar]
- 29.Oaikhena E.E., Makaije D.B., Denwe S.D., Namadi M.M., Haroun A.A. Bioremediation potentials of heavy metal tolerant bacteria isolated from petroleum refinery effluent. Am J Environ Protect. 2016;5:29–34. [Google Scholar]
- 30.Egler M., Grosse C., Grass G., Nies D.H. Role of the extracytoplasmic function protein family sigma factor RpoE in metal resistance of Escherichia coli. J Bacteriol. 2005;187:2297–2307. doi: 10.1128/JB.187.7.2297-2307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J., Wang Q., Sun M., Zhu L., Yang M., Zhao Y. Selection of reference genes for quantitative real-time PCR normalization in Panax ginseng at different stages of growth and in different organs. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexieva V., Sergiev I., Mapelli S., Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. [Google Scholar]
- 33.Carillo P., Mastrolonardo G., Nacca F., Parisi D., Verlotta A., Fuggi A. Nitrogen metabolism in durum wheat under salinity: accumulation of proline and glycine betaine. Funct Plant Biol. 2008;35:412–426. doi: 10.1071/FP08108. [DOI] [PubMed] [Google Scholar]
- 34.Irigoyen J., Einerich D., Sánche-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol Plant. 1992;84:55–60. [Google Scholar]
- 35.Jin Y., Kim Y.J., Jeon J.N., Wang C., Min J.W., Noh H.Y., Yang D.C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Food Hum Nutr. 2015;70:141–145. doi: 10.1007/s11130-015-0470-0. [DOI] [PubMed] [Google Scholar]
- 36.Dziewit L., Pyzik A., Szuplewska M., Matlakowska R., Mielnicki S., Wibberg D., Schlüter A., Pühler A., Bartosik D. Diversity and role of plasmids in adaptation of bacteria inhabiting the Lubin copper mine in Poland, an environment rich in heavy metals. Front Microbiol. 2015;6:152. doi: 10.3389/fmicb.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal S., Meyer J.M., Goel R. Isolation and characterization of aluminium and copper Resistant 'P' Solubilizing alkalophilic bacteria. Indian. J Biotechnol. 2003;2:583–586. [Google Scholar]
- 38.Daspute A.A., Sadhukhan A., Tokizawa M., Kobayashi Y., Panda S.K., Koyama H. Transcriptional regulation of aluminum-tolerance genes in higher plants: clarifying the underlying molecular mechanisms. Front Plant Sci. 2017;8:1358. doi: 10.3389/fpls.2017.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kusunoki K., Nakano Y., Tanaka K., Sakata Y., Koyama H., Kobayashi Y. Transcriptomic variation among six Arabidopsis thaliana accessions identified several novel genes controlling aluminium tolerance. Plant Cell Environ. 2017;40:249–263. doi: 10.1111/pce.12866. [DOI] [PubMed] [Google Scholar]
- 40.Ashraf M., Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59:206–216. [Google Scholar]
- 41.Chen C., Wanduragala S., Becker D.F., Dickman M.B. Tomato QM-like protein protects Saccharomyces cerevisiae cells against oxidative stress by regulating intracellular proline levels. Appl Environ Microbiol. 2006;72:4001–4006. doi: 10.1128/AEM.02428-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali A., Alqurainy F. Activities of antioxidants in plants under environmental stress. In: Motohashi N., editor. The lutein-prevention and treatment for diseases. Transworld Research Network; India: 2006. pp. 187–256. [Google Scholar]
- 43.Vardharajula S., Zulfikar-Ali S., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact. 2011;6:1–14. [Google Scholar]
- 44.Hellmann H., Funck D., Rentsch D., Frommer W.B. Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol. 2000;122:357–368. doi: 10.1104/pp.122.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu M., Shi Z., Zhang Z., Zhang Y., Li H. Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress. Plant Growth Regul. 2012;68:177–188. [Google Scholar]
- 46.Sami F., Yusuf M., Faizan M., Faraz A., Hayat S. Role of sugars under abiotic stress. Plant Physiol Biochem. 2016;109:54–61. doi: 10.1016/j.plaphy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Sukweenadhi J., Balusamy S.R., Kim Y.J., Lee C.H., Kim Y.J., Koh S.C., Yang D.C. A growth promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front Plant Sci. 2018;9:813. doi: 10.3389/fpls.2018.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.